Abstract
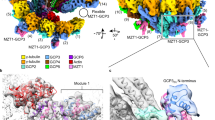
Microtubules are dynamic polymers of α- and β-tubulin and have crucial roles in cell signalling, cell migration, intracellular transport and chromosome segregation1. They assemble de novo from αβ-tubulin dimers in an essential process termed microtubule nucleation. Complexes that contain the protein γ-tubulin serve as structural templates for the microtubule nucleation reaction2. In vertebrates, microtubules are nucleated by the 2.2-megadalton γ-tubulin ring complex (γ-TuRC), which comprises γ-tubulin, five related γ-tubulin complex proteins (GCP2–GCP6) and additional factors3. GCP6 is unique among the GCP proteins because it carries an extended insertion domain of unknown function. Our understanding of microtubule formation in cells and tissues is limited by a lack of high-resolution structural information on the γ-TuRC. Here we present the cryo-electron microscopy structure of γ-TuRC from Xenopus laevis at 4.8 Å global resolution, and identify a 14-spoked arrangement of GCP proteins and γ-tubulins in a partially flexible open left-handed spiral with a uniform sequence of GCP variants. By forming specific interactions with other GCP proteins, the GCP6-specific insertion domain acts as a scaffold for the assembly of the γ-TuRC. Unexpectedly, we identify actin as a bona fide structural component of the γ-TuRC with functional relevance in microtubule nucleation. The spiral geometry of γ-TuRC is suboptimal for microtubule nucleation and a controlled conformational rearrangement of the γ-TuRC is required for its activation. Collectively, our cryo-electron microscopy reconstructions provide detailed insights into the molecular organization, assembly and activation mechanism of vertebrate γ-TuRC, and will serve as a framework for the mechanistic understanding of fundamental biological processes associated with microtubule nucleation, such as meiotic and mitotic spindle formation and centriole biogenesis4.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cryo-EM densities of the γ-TuRC filtered according to global or local resolution have been deposited in the Electron Microscopy Data Bank (EMDB) under accession code EMD-10491. Atomic coordinates for the γ-TuRC have been deposited at the PDB under accession code 6TF9. The original immunoblots and further source data from LFQ mass spectrometry (Fig. 2a), immunoblot quantification (Fig. 3c), geometric analysis of the atomic model (Figs. 3g, 4b–d), MT nucleation assays (Extended Data Figs. 1c, 6i, 9b, d), circular dichroism measurements (Extended Data Fig. 4e), unbiased structure-guided identification (Extended Data Fig. 6a–d), quantification of indirect immunofluorescence (Extended Data Fig. 6g) and actin polymerization (Extended Data Fig. 6h) are included in the Supplementary Information. The raw cryo-EM micrograph movie stacks are available from the corresponding authors upon request.
References
Borisy, G. et al. Microtubules: 50 years on from the discovery of tubulin. Nat. Rev. Mol. Cell Biol. 17, 322–328 (2016).
Zheng, Y., Wong, M. L., Alberts, B. & Mitchison, T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature 378, 578–583 (1995).
Kollman, J. M., Merdes, A., Mourey, L. & Agard, D. A. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 12, 709–721 (2011).
Schmidt-Cernohorska, M. et al. Flagellar microtubule doublet assembly in vitro reveals a regulatory role of tubulin C-terminal tails. Science 363, 285–288 (2019).
Guillet, V. et al. Crystal structure of γ-tubulin complex protein GCP4 provides insight into microtubule nucleation. Nat. Struct. Mol. Biol. 18, 915–919 (2011).
Aldaz, H., Rice, L. M., Stearns, T. & Agard, D. A. Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature 435, 523–527 (2005).
Moritz, M., Braunfeld, M. B., Guénebaut, V., Heuser, J. & Agard, D. A. Structure of the γ-tubulin ring complex: a template for microtubule nucleation. Nat. Cell Biol. 2, 365–370 (2000).
Kabsch, W., Mannherz, H. G., Suck, D., Pai, E. F. & Holmes, K. C. Atomic structure of the actin:DNase I complex. Nature 347, 37–44 (1990).
Farina, F. et al. The centrosome is an actin-organizing centre. Nat. Cell Biol. 18, 65–75 (2016).
Farache, D. et al. Functional analysis of γ-tubulin complex proteins indicates specific lateral association via their N-terminal domains. J. Biol. Chem. 291, 23112–23125 (2016).
Anders, A., Lourenço, P. C. & Sawin, K. E. Noncore components of the fission yeast γ-tubulin complex. Mol. Biol. Cell 17, 5075–5093 (2006).
Choi, Y. K., Liu, P., Sze, S. K., Dai, C. & Qi, R. Z. CDK5RAP2 stimulates microtubule nucleation by the γ-tubulin ring complex. J. Cell Biol. 191, 1089–1095 (2010).
Scrofani, J., Sardon, T., Meunier, S. & Vernos, I. Microtubule nucleation in mitosis by a RanGTP-dependent protein complex. Curr. Biol. 25, 131–140 (2015).
Kollman, J. M., Polka, J. K., Zelter, A., Davis, T. N. & Agard, D. A. Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature 466, 879–882 (2010).
Petry, S. & Vale, R. D. Microtubule nucleation at the centrosome and beyond. Nat. Cell Biol. 17, 1089–1093 (2015).
Zhang, L., Keating, T. J., Wilde, A., Borisy, G. G. & Zheng, Y. The role of Xgrip210 in γ-tubulin ring complex assembly and centrosome recruitment. J. Cell Biol. 151, 1525–1536 (2000).
Chinen, T. et al. The γ-tubulin-specific inhibitor gatastatin reveals temporal requirements of microtubule nucleation during the cell cycle. Nat. Commun. 6, 8722 (2015).
Bärenz, F. et al. The centriolar satellite protein SSX2IP promotes centrosome maturation. J. Cell Biol. 202, 81–95 (2013).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Schwanhäusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
van Heel, M., Harauz, G., Orlova, E. V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996).
Liu, X. & Wang, H. W. Single particle electron microscopy reconstruction of the exosome complex using the random conical tilt method. J. Vis. Exp. 49, 2574 (2011).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ. 6, 5–17 (2019).
Nakane, T., Kimanius, D., Lindahl, E. & Scheres, S. H. W. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. eLife 7, e36861 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Buchan, D. W. A. & Jones, D. T. The PSIPRED Protein Analysis Workbench: 20 years on. Nucleic Acids Res. 47, W402–W407 (2019).
Jones, D. T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 (1999).
Pei, J., Tang, M. & Grishin, N. V. PROMALS3D web server for accurate multiple protein sequence and structure alignments. Nucleic Acids Res. 36, W30–W34 (2008).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protocols 10, 845–858 (2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Trabuco, L. G., Villa, E., Schreiner, E., Harrison, C. B. & Schulten, K. Molecular dynamics flexible fitting: a practical guide to combine cryo-electron microscopy and X-ray crystallography. Methods 49, 174–180 (2009).
Ribeiro, J. V. et al. QwikMD — integrative molecular dynamics toolkit for novices and experts. Sci. Rep. 6, 26536 (2016).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Kidmose, R. T. et al. Namdinator - automatic molecular dynamics flexible fitting of structural models into cryo-EM and crystallography experimental maps. IUCrJ. 6, 526–531 (2019).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Delano, W. L. PyMOL: an open-source molecular graphics tool http://www.ccp4.ac.uk/newsletters/newsletter40/11_pymol.pdf (2018).
Waterhouse, A. M., Procter, J. B., Martin, D. M., Clamp, M. & Barton, G. J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Sievers, F. & Higgins, D. G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 27, 135–145 (2018).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Acknowledgements
We thank A. Boland for graphene oxide grids, G. Hofhaus and T. Mielke for technical cryo-EM support, S. Hata for suggestions, S. Kmiecik for assisting with circular dichroism measurements, L. Rohland for assisting with circular dichroism and actin polymerization measurements. We acknowledge the services SDS@hd and bwHPC supported by the Ministry of Science, Research and the Arts Baden-Württemberg, as well as the German Research Foundation (INST 35/1314-1 FUGG and INST 35/1134-1 FUGG). We also acknowledge support from the German Research Foundation to E.S. (Schi 295/4-3), O.J.G. (GR-1737/9-1) and T. Ruppert (Ru 747/1-1). P.L. received a HBIGS fellowship.
Author information
Authors and Affiliations
Contributions
P.L. optimized γ-TuRC purification, performed γ-TuRC activity measurements, salt treatments, CEP215-N analyses in vitro and in egg extracts, GCP6 insertion domain alignment, sucrose gradient and silver staining. P.L. and A.N. performed negative-stain electron microscopy and particle selection. E.S. and P.L. performed the DNaseI inhibition experiment. D.F. performed 2D classification of negatively stained γ-TuRC. J.L. and C.S. performed initial cryo-EM analysis of the γ-TuRC. P.L. and A.N. prepared, optimized and screened γ-TuRC cryo-EM grids and, together with E.Z., selected particles for initial model generation. P.L. performed immunoprecipitation experiments. A.B. performed circular dichroism measurements and actin polymerization assay. T. Ruppert performed LFQ mass spectrometry analysis. A.N. and A.B. detected actin in the γ-TuRC by immunofluorescence. C.P. prepared egg extracts. O.J.G. provided egg extracts and advised P.L. with the γ-TuRC purification. E.Z. analysed the negative-staining data of γ-TuRC and CEP215-N, acquired and analysed cryo-EM data, and, together with T. Rudack, prepared atomic models and pursued all related aspects of structure analysis. All authors discussed and interpreted the γ-TuRC cryo-EM density. E.S. and S.P. planned and supervised the experiments, analysed data and, together with E.Z. and P.L., wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Biochemical, functional and structural characterization of γ-TuRCs purified from X. laevis egg extracts.
a, Domain organization of GCP2–GCP6. Between the conserved GRIP1 and GRIP2 domains, GCP5 and GCP6 possess a 120- and 750-residue-long insertion domain, respectively. The GCP6 insertion domain contains 8 repeats of 27 amino acids. Domains are annotated according to the Pfam database. b, Schematic of γ-TuRC purification. The γ-TuRC was purified with γ-tubulin antibody-crosslinked Protein A Dynabeads, washed with CSF-XB buffer containing 250 mM salt (KCl), and then eluted by a short peptide corresponding to the C terminus of γ-tubulin. c, Purified γ-TuRCs showed basal MT nucleation activity. Experiment was carried out with (+) or without (−) purified γ-TuRCs, and 33 µM tubulin (5% Cy3-labelled tubulin for visualization). In the negative control, the same purification procedure was used with eluates from rabbit random IgG-crosslinked Protein A Dynabeads. Twenty random images were acquired with a light microscope, and representative overview images are shown. Right, the number of MTs was quantified by ImageJ and data are mean ± s.d. n = 4 biologically independent experiments. P value determined by unpaired two-sided t-test. Scale bar, 10 µm. d, e, After the affinity purification of γ-TuRCs, the eluted proteins were resolved by SDS–PAGE followed by silver staining (d) and immunoblotting (e). Representative images in d and e are from three biologically independent experiments. For gel source data, see Supplementary Fig. 1. f, Immunoblotting analysis of the purified γ-TuRCs after sucrose gradient. Purified γ-TuRCs were applied to a 5–40% sucrose gradient and fractionated after centrifugation. Fractions were resolved by SDS–PAGE and probed using γ-tubulin and GCP5 antibodies. Thyroglobulin (19.4 S) was used as a standard marker and run on a parallel gradient. Representative images were from three biologically independent experiments. For gel source data, see Supplementary Fig. 1. g, Confirmation of structural integrity of the purified γ-TuRCs by negative-staining electron microscopy. Representative micrograph is from five biologically independent experiments. Scale bar, 100 nm. Black arrowheads denote examples of particles used in 2D classification and averaging. h, i, γ-TuRC particles from the negative-stain electron microscopy were classified and averaged with a mask size of either 46.5 nm (h) or 14.5 nm (i). Representative classes of γ-TuRCs are from three biologically independent experiments. The number of particles contributing to each class is given. An example of the ‘asymmetric’ density inside the γ-TuRC is highlighted by a white arrow (h), which is more readily visible with a 14.5-nm mask focusing on the inner part of the γ-TuRC (i). Scale bars, 20 nm.
Extended Data Fig. 2 Cryo-EM data processing and resolution estimation.
a, A representative micrograph from four biologically independent experiments with manually selected particles (green circles) is shown. Three selected particles are shown below. b, Initial model of the γ-TuRC obtained from 3,000 manually selected particles (Methods). Scale bars are depicted in a. c, Four datasets were acquired and initially processed separately. Datasets 1, 2 and 3 were submitted to two consecutive rounds of 3D classification with a varying number of classes. All class averages obtained for dataset 1 are shown. Retained class averages are highlighted by a rectangular box. d, Dataset 4 was the largest dataset, and was therefore divided into four subsets. The higher-quality subsets were submitted to two rounds of 3D classification and particles encompassed in the high-quality classes from these two subsets were combined with all four original subsets of particles to nucleate high-quality classes in the two lower-quality subsets. Only class averages retained for further processing are shown. e, The final sets of particles from all four datasets were refined separately, submitted to CTF refinement and Bayesian polishing, and subsequently merged. The γ-TuRC density was split into several combinations of segments, as indicated. All combinations of segments were subjected to a multibody 3D refinement separately. Their output density segments were combined into one composite density for analysis. The angular distribution of particle views is shown. f, Comparison of local resolution estimation before (left) and after (right) 3D multibody refinement. Local resolution was markedly improved for the peripheral segments of the γ-TuRC density reconstruction upon 3D multibody refinement. The colour-coded resolution scale is identical for both panels. g, Local resolution estimation of the final γ-TuRC density map with a resolution range covering the entire spectrum. h, Mask-corrected FSC between the two independently refined half-set reconstructions (purple), and between the full reconstruction and the atomic model for the γ-TuRC (green).
Extended Data Fig. 3 Structural grouping and unique GCP-variant-specific features identify GCP proteins in the γ-TuRC.
a, Pairwise cross-correlation between isolated density segments, colour-coded from higher (red) to lower (blue) correlation. The actual correlation values are given. b, Atomic models of human GCP4 and γ-tubulin were fitted into the 14 γ-TuRC spokes domainwise (Methods). Pairwise r.m.s.d. between Cα atoms of atomic models representing the individual spokes, colour-coded from lower (red) to higher (blue) r.m.s.d. values. The actual r.m.s.d. values are given. Both approaches cluster the spokes into five classes, colour-coded in the left column and top row. c, d, Atomic models for human GCP4 (white) were fitted domainwise into the density. Density segments covered by the atomic model are depicted in transparent grey. Remaining unexplained segments are depicted in colour. Features are shown for all 14 individual spokes of the γ-TuRC. c, Characteristic density segments of the GRIP2 domain. Extended C-terminal α-helices (red model and density) are unique for group (ii) (spokes 2, 4, 6, 8, 14), and an extended loop between the GRIP2 β-strands (blue model and density) is present only in group (i) (spokes 1, 3, 5, 7, 13). d, Unexplained density segments N-terminal of the GRIP1 domain. Only group (iii) (spokes 9, 11) is devoid of a continuous density connecting to the N-terminal helix of GCP4 (position 9 and 11; lack of yellow extension). Colour code as in Fig. 1a.
Extended Data Fig. 4 Secondary structure prediction and biochemical characterization for GCP variant-distinguishing regions and the GCP5 and GCP6 insertion domains.
a, C-terminal segments of all GCP variants were aligned and the secondary structure was predicted. Confidence for α-helical secondary structure is colour-coded (blue denotes low confidence, red denotes high confidence). b, Multiple sequence alignment for the GRIP2 segments encompassing the inter-β-strand loop for all GCP variants. β-strands are highlighted in blue. c, Secondary structure prediction for the insertion domain of GCP5. The prediction confidence for α-helical secondary structure is colour-coded. d, Secondary structure prediction of xGCP6(546–794) showing highly α-helical character. e, Circular dichroism (CD) analysis of the purified xGCP6(546–794). Left, representative plots are from three biologically independent experiments. Comparison of the circular dichroism spectra for xGCP6(546–794) and BSA (containing only α-helices) confirms the predicted α-helical character of the N-terminal part of the GCP6 insertion domain. Right, Coomassie-blue-stained SDS–PAGE gel of the elution fraction of the xGCP6(546–794) shows the purity of the sample. Representative image is from three biologically independent experiments.
Extended Data Fig. 5 Gallery of bulky amino acid side chains resolved in the γ-TuRC density.
GCP-variant-specific bulky amino acid side chains are marked by an asterisk. Combinations of such side chains in close proximity are marked by two asterisks.
Extended Data Fig. 6 Actin is a bona fide γ-TuRC component involved in MT nucleation.
a–d, High-confidence homology models were docked with 10,000 randomly sampled starting positions and orientations into the envelope of the cryo-EM density. The number of fits is plotted against the respective correlation coefficients. a, γ-Tubulin and GCP4 served as positive controls. True positive fits correspond to redundant high-frequency fits with high correlation coefficients (dashed box). b, Ovalbumin served as negative control. No redundant high-frequency fits with high correlation coefficient indicative of a true positive match were observed. c, Unbiased fitting of NME7, NEDD1 and MOZART1 resulted in a distribution of correlation coefficients similar to the negative control, indicating no positive fit. d, Unbiased fitting of actin resulted in a distribution of correlation coefficients similar to the positive controls, clearly including a true positive fit (dashed box) shown in Fig. 1a. e, Purified γ-TuRCs from X. laevis egg extracts were resolved by SDS–PAGE and immunoblotted with anti-γ-tubulin, anti-GCP5 and anti-actin antibodies, which confirms that actin is associated with the purified γ-TuRC fraction. Representative blots are from three biologically independent experiments. For gel source data, see Supplementary Fig. 1. f, g, Indirect immunofluorescence of adsorbed γ-TuRC rings with antibodies directed against actin and GCP6 indicates colocalization of both proteins. Treatment with 1% SDS increased the antibody accessibility of actin located in the spatially confined interior of the γ-TuRC. f, Representative fluorescence images together with magnified views from three biologically independent experiments. Scale bars, 20 nm. g, Percentage of colocalization events of actin and GCP6 normalized to the GCP6 signal. After treatment with 1% SDS, 40.2% of GCP6 signals colocalized with actin signals. In the absence of 1% SDS, colocalization events decreased to 6.4% owing to inaccessibility of the epitope. Data are mean ± s.d. from n = 3 biologically independent experiments. P values were determined by unpaired two-sided t-test. h, Actin polymerization activity was tested for buffer with and without pyrene F-actin and 0.5 nM ARP2/3, 0.5 nM ARP2/3 with 15 nM VCA and 0.5 nM γ-TuRC with pyrene F-actin. The purified γ-TuRC is devoid of actin nucleation activity. The fluorescence intensity change was determined over time. Data are the mean of five independent experiments. i, γ-TuRC MT nucleation activity after pre-incubation with buffer, the actin-binding protein DNaseI and the preformed actin–DNaseI complex. n = 3 biologically independent experiments; data are mean ± s.e.m. P values were determined by unpaired two-sided t-test. j, After 3 h incubation on ice, before the MT nucleation assay, samples were analysed by immunoblotting to confirm that equal amounts γ-TuRC were present in different experimental groups. Three biologically independent experiments were performed with similar results. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 7 Co-immunoprecipitation of wild-type or mutant 3×Flag–GCP6 insertion domain fragments with the 3×Myc-tagged GCP2 and GCP5 N termini.
a, Co-immunoprecipitation of the GCP6 insertion domain (IDo; residues 606–1499), part 1 of the GCP6 insertion domain (IDo-P1; residues 606–1026), GCP6 insertion domain 9 repeats (IDo-9 repeats; residues 1027–1268) and part 2 of the GCP6 insertion domain (IDo-P2; residues 1269–1499) (as defined in Fig. 3a) with GCP2-N and GCP5-N. Immunoblotting was performed with indicated antibodies. Representative result is from three independent experiments. For gel source data, see Supplementary Fig. 1. b, Co-immunoprecipitation of the GCP6 insertion domain or part 1 of the GCP insertion domain with residues V644, F706, F732 and Q783 mutated to proline (GCP6-IDo4P and GCP6-IDo-P14P, respectively) with both GCP2-N and GCP5-N. Immunoblotting was performed with indicated antibodies. Three biologically independent experiments were performed with similar results. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 8 Sequence conservation of the GCP6 insertion domain region.
The GCP6 insertion domains from human, bovine, mouse, chicken, X. laevis, Anolis carolinensis and medaka (Oryzias latipes) were aligned with Clustal Omega (default settings) build in Jalview software42,43,44. The mutations in the 4P mutant (V644P, F706P, F732P and Q783P) are indicated by asterisks. The individual repeats of the nine-repeat structure in human GCP6 are marked by arrows.
Extended Data Fig. 9 The CM1 motif of CEP215-N is not sufficient to activate the MT nucleation activity of γ-TuRC.
a, γ-TuRC particles with or without a 600-fold excess of CEP215-N were analysed by negative-staining electron microscopy. The resulting 3D densities (grey, yellow) were superimposed and compared to a simulated density of the γ-TuRC in the extrapolated active conformation (red). Arrows indicate the most pronounced structural differences (transparent grey density not filled by red density) between the CEP215-N–γ-TuRC complex and the simulated density of the γ-TuRC in the extrapolated active conformation. b, γ-TuRC (0.5 nM) was incubated with an excess of CEP215-N or CEP215(F75A)-N (3 µM). In vitro MT nucleation activity of the γ-TuRC incubated with buffer, glutathione S-transferase (GST), GST–CEP215-N or GST–CEP215(F75A)-N. n = 3 biologically independent experiments; data are mean ± s.d. P values were determined by unpaired two-sided t-test. Scale bar, 10 µm. c, In vitro binding of γ-TuRC to purified and recombinant GST, CEP215-N and CEP215(F75A)-N. Immunoblots were probed with anti-γ-tubulin and anti-GST antibodies as shown. Three biologically independent experiments were performed with similar results. For gel source data, see Supplementary Fig. 1. d, MT nucleation activity in egg extracts induced by the addition of Ran(Q69L) and CEP215-N. The MT nucleation reaction was stopped after 15 min at 20 °C when Ran(Q69L) addition only induced a small number of MTs. Fold changes of total aster fluorescence intensity from ten random fields were quantified and normalized to the group adding Ran(Q69L) and without CEP215-N or CEP215(F75A)-N. n = 3 biologically independent experiments; data are mean ± s.e.m. P values were determined by unpaired two-sided t-test. Scale bars, 50 µm.
Supplementary information
Supplementary Figure 1
Uncropped gels and blots with size markers.
Supplementary Table 1
LFQ mass spectrometry of γ-TuRC.
Supplementary Table 2
Custom made database containing additional database entries for γ-TuRC.
Video 1
Top view conformational changes required for activation of the γ-TuRC. Top view. Colouring as in Fig. 1a.
Video 2
Side view conformational changes required for activation of the γ-TuRC. Side view. Colouring as in Fig. 1a.
Rights and permissions
About this article
Cite this article
Liu, P., Zupa, E., Neuner, A. et al. Insights into the assembly and activation of the microtubule nucleator γ-TuRC. Nature 578, 467–471 (2020). https://doi.org/10.1038/s41586-019-1896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1896-6
This article is cited by
-
Structure of the native γ-tubulin ring complex capping spindle microtubules
Nature Structural & Molecular Biology (2024)
-
Structure of the γ-tubulin ring complex-capped microtubule
Nature Structural & Molecular Biology (2024)
-
γ-TuRC asymmetry induces local protofilament mismatch at the RanGTP-stimulated microtubule minus end
The EMBO Journal (2024)
-
CAMSAPs and nucleation-promoting factors control microtubule release from γ-TuRC
Nature Cell Biology (2024)
-
Microtubule nucleation and γTuRC centrosome localization in interphase cells require ch-TOG
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.