Abstract
Antibodies secreted into mucosal barriers serve to protect the host from a variety of pathogens, and are the basis for successful vaccines1. In type I mucosa (such as the intestinal tract), dimeric IgA secreted by local plasma cells is transported through polymeric immunoglobulin receptors2 and mediates robust protection against viruses3,4. However, owing to the paucity of polymeric immunoglobulin receptors and plasma cells, how and whether antibodies are delivered to the type II mucosa represented by the lumen of the lower female reproductive tract remains unclear. Here, using genital herpes infection in mice, we show that primary infection does not establish plasma cells in the lamina propria of the female reproductive tract. Instead, upon secondary challenge with herpes simplex virus 2, circulating memory B cells that enter the female reproductive tract serve as the source of rapid and robust antibody secretion into the lumen of this tract. CD4 tissue-resident memory T cells secrete interferon-γ, which induces expression of chemokines, including CXCL9 and CXCL10. Circulating memory B cells are recruited to the vaginal mucosa in a CXCR3-dependent manner, and secrete virus-specific IgG2b, IgG2c and IgA into the lumen. These results reveal that circulating memory B cells act as a rapidly inducible source of mucosal antibodies in the female reproductive tract.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
RNA sequencing data are available at BioProject, under accession number PRJNA524497. All datasets generated and/or analysed during the current study are available in the Letter, the accompanying Source Data or Supplementary Information, or are available from the corresponding author upon reasonable request.
Code availability
All R and Python codes used in this analysis are available from the corresponding author upon request.
References
Plotkin, S. A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17, 1055–1065 (2010).
Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 25, 381–418 (2007).
Franco, M. A., Angel, J. & Greenberg, H. B. Immunity and correlates of protection for rotavirus vaccines. Vaccine 24, 2718–2731 (2006).
Patel, M. et al. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J. Infect. Dis. 208, 284–294 (2013).
Parr, E. L. & Parr, M. B. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J. Virol. 71, 8109–8115 (1997).
Whaley, K. J., Zeitlin, L., Barratt, R. A., Hoen, T. E. & Cone, R. A. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J. Infect. Dis. 169, 647–649 (1994).
Sherwood, J. K., Zeitlin, L., Whaley, K. J., Cone, R. A. & Saltzman, M. Controlled release of antibodies for long-term topical passive immunoprotection of female mice against genital herpes. Nat. Biotechnol. 14, 468–471 (1996).
Nardelli-Haefliger, D. et al. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl Cancer Inst. 95, 1128–1137 (2003).
Crowley-Nowick, P. A. et al. Rectal immunization for induction of specific antibody in the genital tract of women. J. Clin. Immunol. 17, 370–379 (1997).
Bouvet, J. P., Bélec, L., Pirès, R. & Pillot, J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect. Immun. 62, 3957–3961 (1994).
Iwasaki, A. Exploiting mucosal immunity for antiviral vaccines. Annu. Rev. Immunol. 34, 575–608 (2016).
Li, Q. et al. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J. Immunol. 193, 3113–3125 (2014).
Parr, E. L. & Parr, M. B. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J. Virol. 72, 5137–5145 (1998).
Iijima, N. & Iwasaki, A. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346, 93–98 (2014).
Beura, L. K. et al. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 216, 1214–1229 (2019).
Schenkel, J. M. et al. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346, 98–101 (2014).
Zuccarino-Catania, G. V. et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 15, 631–637 (2014).
Parr, M. B. & Parr, E. L. Interferon-γ up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology 99, 540–545 (2000).
Gill, N., Deacon, P. M., Lichty, B., Mossman, K. L. & Ashkar, A. A. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J. Virol. 80, 9943–9950 (2006).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Benner, R., Hijmans, W. & Haaijman, J. J. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin. Exp. Immunol. 46, 1–8 (1981).
Takatsuka, S. et al. IL-9 receptor signaling in memory B cells regulates humoral recall responses. Nat. Immunol. 19, 1025–1034 (2018).
Wang, Y. et al. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat. Immunol. 18, 921–930 (2017).
Zhu, M. et al. Negative regulation of lymphocyte activation by the adaptor protein LAX. J. Immunol. 174, 5612–5619 (2005).
Mueller, J., Matloubian, M. & Zikherman, J. Cutting edge: An in vivo reporter reveals active B cell receptor signaling in the germinal center. J. Immunol. 194, 2993–2997 (2015).
Gupta, N. T. et al. Hierarchical clustering can identify B cell clones with high confidence in Ig repertoire sequencing data. J. Immunol. 198, 2489–2499 (2017).
Iwasaki, A. The role of dendritic cells in immune responses against vaginal infection by herpes simplex virus type 2. Microbes Infect. 5, 1221–1230 (2003).
Awasthi, S. & Friedman, H. M. Status of prophylactic and therapeutic genital herpes vaccines. Curr. Opin. Virol. 6, 6–12 (2014).
Iijima, N. et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 205, 3041–3052 (2008).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108, 5354–5359 (2011).
Iijima, N., Mattei, L. M. & Iwasaki, A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl Acad. Sci. USA 108, 284–289 (2011).
Iijima, N., Linehan, M. M., Saeland, S. & Iwasaki, A. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc. Natl Acad. Sci. USA 104, 19061–19066 (2007).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Ye, J., Ma, N., Madden, T. L. & Ostell, J. M. IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 41, W34–W40 (2013).
Acknowledgements
We thank M. Linehan and H. Dong for technical assistance, Yale Flow Cytometry Facility, especially G. Lyon, for cell sorting, Yale Center for Genome Analysis (YCGA), especially G. Wang and C. Castaldi, for 10x Chromium library preparations and help with sequencing, P. Wong and T. Mao for helpful discussions regarding the single-cell RNA-seq data, Y. Kong for helpful advice in statistical analysis, and K. B. Hoehn for advice on BCR lineage tree drawing. This study was supported by awards from NIH AI054359, AI062428, AI064705, AI102625 (to A.I.), NIH AI104739 (to S.H.K.), Basic Science Research Program through the National Research Foundation of Korea (NRF-2016R1A6A3A03010349) funded by the Ministry of Education (to J.E.O.), the Japan Agency for Medical Research and Development (AMED) JP18fm0208011h0002, the Japan Society for the Promotion of Science (JSPS) JP18H02857, Takeda Science Foundation (to N.I.), and NIH T32GM007205 (MSTP training Grant) (to E.S. and R.J.). A.I. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.E.O., N.I., E.S. and A.I. designed the experiments; J.E.O., N.I., E.S., R.J. and A.I. prepared the manuscript; J.E.O., N.I., P.L. and J.K. performed experiments; J.E.O., N.I. and A.I. analysed data; and E.S., R.J. and S.H.K. analysed single-cell RNA-seq data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Circulating antibodies are incapable of entering the vaginal mucosal lumen in the absence of minor abrasion.
a, C57BL/6 mice treated intravaginally or intravenously with HSV-gD-specific monoclonal antibody were infected with intravaginal wild-type HSV-2. Survival, disease severity and virus titre in vaginal wash were analysed (n = 6 mice). The dashed line indicates the limit of detection. b, C57BL/6 mice treated with Depo-Provera were injected intravenously with FITC-conjugated mouse IgG antibody. Frozen sections of vagina, spleen and lung were stained with anti-CD31 (red), anti-FITC (green) antibodies, and DAPI (blue). L indicates vaginal lumen. Scale bars, 100 μm. c–e, C57BL/6 mice were immunized subcutaneously with TK− HSV-2. Five weeks later, the vaginal tract of immunized mice with Depo-Provera treatment was brushed with a cervical brush to make a breach, or to create minor abrasions of the epithelial barrier of the vaginal mucosa. HSV-specific antibodies in vaginal wash before and after cervical brush (c; naive; n = 3 mice; subcutaneous (SQ) TK− HSV-2, n = 9 mice) and in serum (d; naive; n = 3 mice; subcutaneous TK− HSV-2, n = 15 mice) were measured by ELISA. Sample dilution for ELISA was 1:2 (vaginal wash) or 1:103 (serum). e, H&E stain of the vagina before and after breach was performed in mice with Depo-Provera treatment. f, g, C57BL/6 mice were immunized intravaginally with TK− HSV-2 or intranasally (i.n.) with influenza A strain PR8 (H1N1) virus. Five weeks later, virus-specific antibodies in bronchoalveolar lavage (BAL) fluid, vaginal wash (f; naive, n = 4 mice; intravaginal TK− HSV-2, n = 6 mice; intranasal PR8, n = 4 mice) and blood (g; naive mice, n = 4 mice; intravaginal TK− HSV-2, n = 13 mice; intranasal PR8, n = 8 mice) were measured by ELISA. Data are mean ± s.e.m. Data are representative of two independent experiments (a, b, e) or are pooled from two independent experiments (c, d, f, g). Statistical significance was analysed by two-way analysis of variance (ANOVA) (a; disease score and virus titre), log-rank (Mantel–Cox) test (a; survival) or two-tailed Mann–Whitney U test (c, d).
Extended Data Fig. 2 The vagina is devoid of virus-specific antibodies and tissue-resident B cells even after boosting local inflammation after immunization with HSV-2.
C57BL/6 mice were immunized intravaginally with TK− HSV-2. On day 5 after immunization, CpG1826 was injected into the vagina to boost local inflammation of the vaginal mucosa. a, Five weeks later, the number of CD138+CD19+, CD138+CD19− and CD138−CD19+ cells in vaginal tissues was analysed by flow cytometry (naive, n = 4 mice; immune, n = 6 mice; immune and CpG1826, n = 5 mice). b, c, HSV-2-specific antibodies in vaginal wash (b; naive, n = 4 mice; immune, n = 6 mice; immune and CpG1826, n = 5 mice) and in serum (c; naive, n = 3 mice; immune, n = 3 mice; immune and CpG1826, n = 5 mice) were measured by ELISA. Sample dilution for the vaginal wash ELISA was 1:5. Data are mean ± s.e.m. Data are representative of two independent experiments. Statistical significance was analysed by two-tailed Mann–Whitney U test.
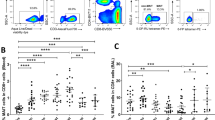
Extended Data Fig. 3 B cells do not establish residency in the upper reproductive tract following immunization and challenge with HSV-2.
C57BL/6 mice were immunized intravaginally with TK− HSV-2. Five weeks later, mice were challenged with intravaginal wild-type HSV-2. One day after challenge, the number of CD138+CD19+, CD138+CD19− and CD138−CD19+ cells in cervix and uterus was analysed by flow cytometry (naive, n = 4 mice; immune, n = 6 mice; immune and wild-type HSV-2, n = 6 mice). Data are mean ± s.e.m. Data are pooled from two independent experiments. Statistical significance was analysed by two-tailed Mann–Whitney U test.
Extended Data Fig. 4 Surface-marker profiles of recruited memory B cells in the vagina.
C57BL/6 mice immunized with TK− HSV-2 five weeks previously were challenged with wild-type HSV-2. a, Eighteen hours later, CD80 and PD-L2 expression on both IgD+ and IgD− B cells in vaginal tissues was analysed by flow cytometry. b, The number of CD80+PD-L2+ memory B cells in the vagina at 18 h after secondary challenge was analysed by flow cytometry (naive, n = 2 mice; immune, n = 4 mice). Data are mean ± s.e.m. Data are representative of two independent experiments. Statistical significance was analysed by two-tailed Mann–Whitney U test.
Extended Data Fig. 5 Migrated B cells rapidly activate in the vagina and remain for several weeks.
a, Naive C57BL/6 mice or C57BL/6 mice immunized with TK− HSV-2 five weeks previously were challenged with wild-type HSV-2. Eight, sixteen and twenty-four hours after challenge, CD86 and CD69 expression on IgD−IgG+ memory B cells in vaginal tissues was analysed by flow cytometry. b, C57BL/6 mice were immunized vaginally with TK− HSV-2 five weeks prior. After challenge, frozen sections of vagina were stained with antibodies against IgG (red), CD4, HSV, CD31 and VCAM-1 (green), and DAPI (blue). Scale bars, 100 μm. c, C57BL/6 mice immunized with TK− HSV-2 five weeks previously were challenged with wild-type HSV-2. At the indicated days after challenge, the number of memory B cells in vaginal tissues was analysed by flow cytometry (n = 2–4 mice in each time point). Data are mean ± s.e.m. Data are representative of two independent experiments.
Extended Data Fig. 6 Local intravaginal immunization with HSV-2 is required for the recruitment of memory B cells.
C57BL/6 mice were immunized intravaginally or subcutaneously with TK− HSV-2. Five weeks later, mice were challenged with wild-type HSV-2. a, One day after challenge, the number of memory and naive B cells in vaginal tissues was analysed by flow cytometry (naive, n = 4 mice; intravaginal immune, n = 6 mice; subcutaneous immune and wild-type HSV-2, n = 5 mice; intravaginal immune and wild-type HSV-2, n = 5 mice). b, HSV-2-specific IgG2b antibody in serum was measured by ELISA (naive, n = 3 mice; intravaginal immune, n = 3 mice; subcutaneous immune and wild-type HSV-2, n = 5 mice; intravaginal immune and wild-type HSV-2, n = 3 mice). Data are mean ± s.e.m. Data are representative of two independent experiments. Statistical significance was analysed by two-tailed Mann–Whitney U test.
Extended Data Fig. 7 Antigen-specific recall response is necessary for the recruitment of memory B cells.
a, b, C57BL/6 mice immunized with intravaginal TK− HSV-2 five weeks previously were challenged with wild-type HSV-2 or influenza A strain PR8 virus intravaginally (immune, n = 6 mice; immune and wild-type HSV-2, n = 4 mice; immune and PR8, n = 4 mice). a, One day after secondary challenge, the number of memory B cells in vaginal tissues was analysed by flow cytometry. b, Indicated virus titre in vaginal wash was measured one day after secondary challenge. c, C57BL/6 mice immunized intravaginally with TK− HSV-2 five weeks previously were challenged with wild-type HSV-2 or CpG1826 intravaginally. One day after secondary challenge, the number of memory B cells in vaginal tissues was analysed by flow cytometry (immune, n = 5 mice; immune and wild-type HSV-2, n = 6 mice; immune and CpG1826, n = 6 mice). d, e, C57BL/6 mice immunized intravaginally with TK− HSV-2 five weeks previously were intravaginally challenged with active or heat-inactivated (HI) wild-type HSV-2. d, One day after secondary challenge, the number of memory B cells in vaginal tissues was analysed by flow cytometry (immune, n = 5 mice; immune and wild-type HSV-2, n = 7 mice; immune and heat-inactivated HSV-2, n = 14 mice). e, One day after challenge, HSV-2-specific antibodies in vaginal wash were measured by ELISA (immune, n = 19 mice; immune and wild-type HSV-2, n = 10 mice; immune and heat-inactivated HSV-2, n = 9 mice). Sample dilution for ELISA was 1:5. Data are mean ± s.e.m. Data are representative of two independent experiments (a–c) or are pooled from three independent experiments (d, e). Statistical significance was analysed by two-tailed Mann–Whitney U test.
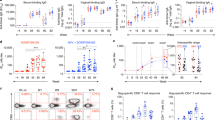
Extended Data Fig. 8 FTY720 treatment has no effect on circulating antibody levels.
C57BL/6 mice were infected vaginally with TK− HSV-2. Six weeks later, immunized mice were given drinking water containing 4 μg ml−1 of FTY720. Two weeks later, FTY720-treated mice were challenged intravaginally with wild-type HSV-2 (104 pfu). At the indicated days after challenge, HSV-2-specific antibodies (a) and total antibodies (b) in the blood were measured by ELISA. Sample dilution for virus-specific antibody ELISA was 1:140. Data are mean ± s.e.m. Data are representative of two independent experiments with 3 mice per group.
Extended Data Fig. 9 scRNA-seq analysis reveals unique features associated with memory B cells recruited to the FRT.
Spleens and FRT of C57BL/6 mice were collected for 10x Genomics scRNA-seq one day after (re-)challenge with HSV-2; spleens were sorted for B cells and FRTs were sorted for B and T cells (n = 8 pooled mice). a, t-distributed stochastic neighbour-embedding (t-SNE) plot associated with 5′ gene-expression profiling of 13,763 cells captured on the 10x Chromium platform. B-cell identities were determined by re-clustering CD19+ populations after initial clustering, and reassigning them according to the analysis in b (see Methods). Violin plots depict kernel density estimates to show the distribution of expression values, and were generated using the default VlnPlot function in Seurat (n = 13,763 cells). b, t-SNE plot associated with CD19+ clusters identified from a after re-clustering (n = 5,390 cells). c, Relative cell-population frequency in the different compartments and conditions are shown in pie charts, and the gene expression of each cluster is shown in violin plots. Violin plots depict kernel density estimates to show the distribution of expression values, and were generated using the default VlnPlot function in Seurat (n = 5,390 cells). d, Heat maps of genes in B cells associated with activation and chemokine or cytokine receptors (n = 5,390 cells). e, t-SNE plots of B cells found in the FRT of naive infected and immunized re-challenged mice after re-clustering only B cells from the FRT (n = 517 cells). f, Heat map of the top differentially expressed genes in each cluster in e. g, Clonal overlap between the spleen and the FRT of mice after primary or secondary challenge was measured using single-cell BCR sequencing data, confidence intervals for clonal overlap were computed by bootstrap analysis and significance was assessed for the null hypothesis of zero overlap after secondary challenge (P = 0.003). Clonal overlap was computed from all productive BCR sequences from the 5,390 cells. Box plots depict the number of clonal overlaps calculated from bootstrap analysis with whiskers depicting upper percentile of 95th and lower percentile of 5th, and box depicting 80th and 20th percentiles. h. Lineage tree of clones that were shared in the spleen and FRT compartment; colours of cell types for splenic B cells and FRT B cells correspond to legends in c and e, respectively.
Extended Data Fig. 10 Surface chemokine receptors on circulating memory B cells, and validation of CXCR3 deficiency and its effect on the number of circulating memory B cells.
a, C57BL/6 mice were immunized intravaginally with TK− HSV-2. Five weeks later, the expression of various chemokine receptors on circulating IgD−IgG+ memory B cells in blood was analysed by flow cytometry. b, c, C57BL/6 mice with (n = 6) or without (n = 5) immunization with TK− HSV-2 five weeks previously were challenged intravaginally with wild-type HSV-2. At the indicated days following challenge, CXCR3 expression on IgD−IgG+ memory B cells was analysed by flow cytometry (b) and CXCL9 secretion in vaginal wash was measured by ELISA (c). d, Strategy for generating mixed bone-marrow chimeric mice. e, f, Wild-type mice and chimeric mice that lacked CXCR3 only on B cells (CXCR3-B cell KO) were immunized intravaginally with TK− HSV-2 (n = 10 mice). e, Five weeks later, CXCR3 expression on IgD−IgG+ memory B cells, CD4 T cells and CD8 T cells were analysed by flow cytometry. f, Five weeks later, the number of circulating memory B cells, CD4 T cells and CD8 T cells was analysed by flow cytometry. Data are mean ± s.e.m. Data are representative of four (a) and two (b, c) independent experiments or are pooled from two independent experiments (e, f). Statistical significance was analysed by two-tailed Mann–Whitney U test.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Oh, J.E., Iijima, N., Song, E. et al. Migrant memory B cells secrete luminal antibody in the vagina. Nature 571, 122–126 (2019). https://doi.org/10.1038/s41586-019-1285-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1285-1
This article is cited by
-
B cell memory: from generation to reactivation: a multipronged defense wall against pathogens
Cell Death Discovery (2024)
-
Establishment of isotype-switched, antigen-specific B cells in multiple mucosal tissues using non-mucosal immunization
npj Vaccines (2023)
-
Surface phenotypes of naive and memory B cells in mouse and human tissues
Nature Immunology (2022)
-
Blood monocyte-derived CD169+ macrophages contribute to antitumor immunity against glioblastoma
Nature Communications (2022)
-
Mucosal vaccines — fortifying the frontiers
Nature Reviews Immunology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.