Abstract
In vitro differentiation of human stem cells can produce pancreatic β-cells; the loss of this insulin-secreting cell type underlies type 1 diabetes. Here, as a step towards understanding this differentiation process, we report the transcriptional profiling of more than 100,000 human cells undergoing in vitro β-cell differentiation, and describe the cells that emerged. We resolve populations that correspond to β-cells, α-like poly-hormonal cells, non-endocrine cells that resemble pancreatic exocrine cells and a previously unreported population that resembles enterochromaffin cells. We show that endocrine cells maintain their identity in culture in the absence of exogenous growth factors, and that changes in gene expression associated with in vivo β-cell maturation are recapitulated in vitro. We implement a scalable re-aggregation technique to deplete non-endocrine cells and identify CD49a (also known as ITGA1) as a surface marker of the β-cell population, which allows magnetic sorting to a purity of 80%. Finally, we use a high-resolution sequencing time course to characterize gene-expression dynamics during the induction of human pancreatic endocrine cells, from which we develop a lineage model of in vitro β-cell differentiation. This study provides a perspective on human stem-cell differentiation, and will guide future endeavours that focus on the differentiation of pancreatic islet cells, and their applications in regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw and processed single-cell RNA sequencing data have been deposited in the Gene Expression Omnibus under accession number GSE114412. Any other relevant data are available from the corresponding author upon reasonable request.
Code availability
The analysis code is available at https://github.com/meltonlab/scbeta_indrops.
References
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014).
Russ, H. A. et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34, 1759–1772(2015).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Baron, M. et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst. 3, 346–360.e4 (2016).
Segerstolpe, Å. et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 (2016).
Xin, Y. et al. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 24, 608–615 (2016).
Muraro, M. J. et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 3, 385–394.e3 (2016).
Enge, M. et al. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell 171, 321–330.e14 (2017).
Byrnes, L. E. et al. Lineage dynamics of murine pancreatic development at single-cell resolution. Nat. Commun. 9, 3922 (2018).
Scavuzzo, M. A. et al. Endocrine lineage biases arise in temporally distinct endocrine progenitors during pancreatic morphogenesis. Nat. Commun. 9, 3356 (2018).
Sharon, N. et al. A peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets. Cell 176, 790–804 (2019).
Xie, R. et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell 12, 224–237 (2013).
Hrvatin, S. et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proc. Natl Acad. Sci. USA 111, 3038–3043 (2014
Petersen, M. B. K. et al. Single-cell gene expression analysis of a human ESC model of pancreatic endocrine development reveals different paths to β-cell differentiation. Stem Cell Reports 9, 1246–1261 (2017).
Rutter, G. A., Pullen, T. J., Hodson, D. J. & Martinez-Sanchez, A. Pancreatic beta-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 466, 203–218 (2015).
Thurmond, D. C. in Mechanisms of Insulin Action (eds Pessin, J. E. & Saltiel, A. R.) 52–70 (Springer, New York, 2007).
Aslamy, A. & Thurmond, D. C. Exocytosis proteins as novel targets for diabetes prevention and/or remediation? Am. J. Physiol. 312, R739–R752 (2017).
Arda, H. E. et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab. 23, 909–920 (2016).
Blum, B. et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 30, 261–264 (2012).
Thorrez, L. et al. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Res. 21, 95–105 (2011).
Kelly, O. G. et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol. 29, 750–756 (2011).
Riedel, M. J. et al. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia 55, 372–381 (2012).
Spijker, H. S. et al. Loss of β-cell identity occurs in type 2 diabetes and is associated with islet amyloid deposits. Diabetes 64, 2928–2938 (2015).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198.e16 (2017).
Haber, A. L. et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017).
Grün, D. et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525, 251–255 (2015).
Martin, A. M. et al. The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterol. Motil. 29, e13046 (2017).
Gupta, S. K. et al. NKX6.1 induced pluripotent stem cell reporter lines for isolation and analysis of functionally relevant neuronal and pancreas populations. Stem Cell Res. 29, 220–231 (2018).
Almaça, J. et al. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Reports 17, 3281–3291 (2016).
Goyvaerts, L., Schraenen, A. & Schuit, F. Serotonin competence of mouse beta cells during pregnancy. Diabetologia 59, 1356–1363 (2016).
Ohta, Y. et al. Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes 60, 3208–3216 (2011).
Lu, T. T.-H. et al. The polycomb-dependent epigenome controls β cell dysfunction, dedifferentiation, and diabetes. Cell Metab. 27, 1294–1308.e7 (2018).
Britt, L. D., Stojeba, P. C., Scharp, C. R., Greider, M. H. & Scharp, D. W. Neonatal pig pseudo-islets: a product of selective aggregation. Diabetes 30, 580–583 (1981).
Agulnick, A. D. et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl. Med. 4, 1214–1222 (2015).
Tsoukalas, N. et al. Pancreatic carcinoids (serotonin-producing pancreatic neuroendocrine neoplasms): report of 5 cases and review of the literature. Medicine (Baltimore) 96, e6201 (2017).
Hilderink, J. et al. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J. Cell. Mol. Med. 19, 1836–1846 (2015).
Ramachandran, K., Peng, X., Bokvist, K. & Stehno-Bittel, L. Assessment of re-aggregated human pancreatic islets for secondary drug screening. Br. J. Pharmacol. 171, 3010–3022 (2014).
Spijker, H. S. et al. Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes 62, 2471–2480 (2013).
Zuellig, R. A. et al. Improved physiological properties of gravity-enforced reassembled rat and human pancreatic pseudo-islets. J. Tissue Eng. Regen. Med. 11, 109–120 (2017).
Zilionis, R. et al. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 12, 44–73 (2017).
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018).
Traag, V., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Preprint at https://arxiv.org/abs/1810.08473 (2018).
Haghverdi, L., Büttner, M., Wolf, F. A., Buettner, F. & Theis, F. J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13, 845–848 (2016).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Zhang, J. M., Kamath, G. M. & Tse, D. N. Towards a post-clustering test for differential expression. Preprint at https://www.biorxiv.org/content/10.1101/463265v1 (2018).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Smyth, G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, article3 (2004).
Acknowledgements
We thank A. Ratner, R. Zilionis, S. Wolock, J. Guo and L. Ye for technical support; A. Klein, D. Kotliar, E. Hodis, Y. Reshef, M. A. Nagy, the CGTA discussion group, R. Pop, C. Kayatekin and L. Schissler for discussions and feedback on the manuscript; and the Bauer Core Facility at Harvard University and the BPF Next-Gen Sequencing Core Facility at Harvard Medical School for their sequencing support. D.A.M. is an Investigator of the Howard Hughes Medical Institute. A.V. is funded by the Harvard University Presidential Scholar fund, Harvard Stem Cell Institute Medical Scientist MD/PhD Training Fellowship and Harvard/MIT MD/PhD program. A.L.F. is supported by NIH T32GM007226. This work was supported by grants from the Harvard Stem Cell Institute, Helmsley Charitable Trust, JDRF and the JPB Foundation. This research was performed using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (HIRN, RRID:SCR_014393; https://hirnetwork.org; UC4 DK104165-04 and UC4 DK104159-03).
Reviewer information
Nature thanks Peter Butler, Heiko Lickert and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.V. and D.A.M. conceived the study and analysed the results. A.V., A.L.F., H.L.B., E.N.E. and Q.P.P. conducted directed differentiations, immunofluorescence and flow cytometry experiments. A.V., A.L.F. and H.L.B. performed inDrops experiments and all computational analyses. J.H.-R.K. conducted β-cell function and transplantation experiments. G.H., Y.-C.P., M.G. and F.W.P. designed and performed endocrine re-aggregation experiments. A.V., A.L.F., H.L.B. and E.S. characterized and validated β-cell purification sorting. All authors helped to write the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.A.M. is a founder and advisor of Semma Therapeutics. G.H., Y.-C.P., F.W.P. and M.G. are employees of Semma Therapeutics. D.A.M., F.W.P., Q.P.P., M.G. and A.V. are inventors on patents and patent applications related to β-cell-directed differentiation and purification strategies. All other authors declare no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
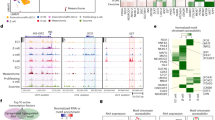
Extended Data Fig. 1 Comparison of two SC-β-cell protocol variants and resulting cell types.
a–c, Immunofluorescence imaging of differentiated (v8, stage 6, day 13) SC-islets showing staining of relevant markers. a, SC-β-cells, which are typically positioned in the periphery, are positive for both NKX6.1 and C-peptide (fragment of proinsulin). b, SC-EC cells are positive for SLC18A1, an enterochromaffin cell marker. These cells are also present in the periphery. c, Non-endocrine cells, which are marked by SOX9, are most commonly found near the centre of SC-islets. Scale bars, 100 μm. d, e, Summary of changes in stages 3 and 4 in protocols x1 (d) and x2 (e) (see Extended Data Table 1 for protocol summaries), and representative flow cytometry results at the end of stages 4 and 6. f–i, t-SNE projection of cells sampled from the ends of stages 3–6 of protocol x2. Cells in f–i are coloured according to their assigned cluster. Horizontal bars indicate cell-type proportions. Related to Fig. 1d–g. j, Comparison of cell populations from protocols x1 and x2. Correlation is computed using the z-scores of mean TPM values (for each cluster) of 2,000 high-variance genes. Rows and columns are ordered using hierarchical clustering. Cells are labelled as in f–i and Fig. 1d–g. k, l, t-SNE projection of stage 6 from three differentiations, coloured by cell type (k) and by differentiation (l). m, Correlation of cell populations derived from HUES8 (embryonic stem cells, v4 and x3) and iPS1016/31 (v4). Colours are the same as in k. Correlation is computed as in j.
Extended Data Fig. 2 Functional assay of GSIS during stage-6 time course.
a, Design for sequential GSIS assay. b, Complete data for three independent flasks, assayed across several weeks. Circles are individual technical triplicates and bars show mean of those triplicates. c, Complete data for cadaveric human islets (seven donors), run alongside samples from b.
Extended Data Fig. 3 Stage-6 SC-β-cells express characteristic β-cell markers.
a, b, t-SNE projection of stage-6 time-course data shaded by sampling time (a) and by representative marker genes (b). Expression is normalized relative to maximum value and smoothed over neighbouring cells. c, Expression profiles for key genes necessary for β-cell function. Shading displays mean expression (TPM, log-scaled) and diameter denotes fractional expression. d, e, Comparison of global expression between human islet β-cells and in vitro progenitors (d) and SC-β-cells (e). Note the shift in gene expression from progenitors to SC-β-cells. All genes shown in all panels from c are circled in red. f, Results from GSEA show that gene sets from c are significantly upregulated during differentiation. Value plotted is −log10 of the GSEA-reported FDR q-value (capped at 10), with sign (positive or negative) showing the direction of the effect (that is, purple positive values are upregulated in SC-β-cells compared to NKX6.1 progenitors).
Extended Data Fig. 4 Comparison of SC-β and SC-α-cells to each other, and their islet counterparts.
a, Insulin and glucagon expression in SC-β- (purple distributions) and SC-α-cells (red distributions) during several weeks of stage 6, shown as violin plots of SC-β or SC-α-cells from that particular time point. The connected lines connect the medians of each population at each time point. b, Identification of genes enriched in cadaveric-islet α-cells and islet β-cells, from previously published data5. c, Heat map of expression level of genes from b, shown for islet α, SC-α, SC-β and islet β-cells. d, Genes enriched in islet β-cells are upregulated in SC-β-cells, and genes enriched in α-cells are upregulated in SC-α-cells. The displayed P value is computed using a two-sided Wilcoxon rank-sum test. In the box plot, boxes extend from first to third quartiles, whiskers extend from 5th to 95th percentiles, central line indicates median and box notching indicates 95th percentile confidence interval for median.
Extended Data Fig. 5 SC-EC cells secrete serotonin and exist in other protocols.
a, Schematic of serotonin synthesis from tryptophan. Enterochromaffin cells use TPH1, whereas serotoninergic neurons use TPH2 for the first and rate-limiting synthesis step. b, Serotonin release during sequential challenges of low and high glucose, followed by KCl depolarization. Top, clusters from three independent SC-β-cell protocol differentiations. Bottom, human cadaveric islets from two donors. Symbols show values of individual replicates for each sample (different clusters from the same sample are split and measured separately). P values computed using two-sided Wilcoxon rank-sum test. NS, non-significant with P > 0.05. c, d, Expression of enterochromaffin marker genes (shown in blue) is detectable in bulk RNA-sequencing data (from a previous publication29), and enriched via sorting of NKX6.1–GFP + cells, shown as fold change, mean expression and differential expression q-values. Positive fold change indicates higher expression in NKX6.1–GFP + cells. Enrichment of SC-EC-cell markers is comparable to β-cell markers (shown in purple) and opposite of α-cell markers (shown in red). All values shown are directly reproduced from results that were previously computed and deposited29. e, Flow cytometry shows that SLC18A1 is co-expressed with NKX6.1+ in SC-EC cells of v8 SC-β-cell protocol differentiations. This example is representative across more than 100 independent differentiations. f, Comparison of gene expression between wild-type mouse islets and mouse islets 25 weeks after β-cell-specific polycomb repressive complex 2 ablation via Eed knockout. Purple genes are examples of downregulated β-cell-identity genes, blue genes represent serotonin–enterochromaffin signature. q-values are FDR-corrected (α = 0.05) P values from Limma differential expression analysis.
Extended Data Fig. 6 Characterization of non-endocrine cells from stage-6 time course.
a, b, t-SNE projection of non-endocrine cells from stage-6 time course, shaded by collection day (a) or by genes relevant to cell identity (b). Expression is normalized relative to maximum value, and smoothed over neighbouring cells. c, t-SNE projection shaded by assigned cluster and bar charts of cellular fraction in each cluster by week of differentiation. d, Gene expression of population-specific markers for each subpopulation of non-endocrine cells. Shading displays mean expression (z-normalized TPM) and diameter denotes fractional expression.
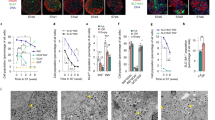
Extended Data Fig. 7 Re-aggregation is a scalable, function-preserving method to enrich for endocrine cells.
a, Schematic of re-aggregation procedure to remove non-endocrine cells. Cells are enzymatically dissociated and re-aggregated during continued suspension culture. Non-endocrine cells fail to adhere and are removed by filtration. b, Schematic of CD49a enrichment procedure to produce SC-β-cell enriched clusters. Dissociated cells are stained with anti-CD49a PE-conjugated antibody, incubated with anti-PE magnetic microbeads and magnetically separated. The enriched cells are re-aggregated in six-well plates on a rocker. c, t-SNE projection of cells sequenced from native and re-aggregated clusters from a single differentiation show a strong depletion of the non-endocrine population. Cells in both panels were differentiated using protocol v8. d, Immunofluorescence staining for C-peptide, GCG and SLC18A1 shows distinct neighbourhoods in re-aggregated clusters (protocol v8). Images shown are maximum intensity projections from z-stacks. Each panel shows separate representative clusters stained for all markers. Scale bars, 100 μm. e, f, Representative flow cytometry analysis of endocrine cell abundance (from protocol v8), before and after re-aggregation. Endocrine cells express CHGA. g, Summary of population composition (as assayed by flow cytometry) in 60 re-aggregated and 41 native independent differentiations, carried out using protocol v8. Re-aggregations were carried out in spinner flasks. P value computed using two-sided Wilcoxon rank-sum test. In g, h box plots, boxes extend from first to third quartiles, whiskers extend from 5th to 95th percentiles, central line indicates median and box notching indicates 95th percentile confidence interval for median. h, Stimulation index (insulin released at 20 mM glucose versus insulin released at 2 mM) of 52 independent protocol v8 differentiations, with paired native versus re-aggregated comparisons. P value computed using two-sided Wilcoxon signed-rank test. i, Complete data for static GSIS assays, performed as in Extended Data Fig. 2, corresponding to stimulation indices shown in Fig. 4d. Circles are individual technical triplicates and bars show mean of those triplicates. j, Dynamic perifusion assay of glucose-responsive insulin secretion of human islets, native SC-β-cell clusters (stage 6, day 22, v8) and matched SC-β islets produced via magnetic sorting for CD49a. Each point is the mean of three technical replicates, and the vertical bar indicates the s.e. across those triplicates. k, Area under the curve comparing the first low-glucose stimulation and the high-glucose stimulation, normalized to equal effective time in each treatment.
Extended Data Fig. 8 Stage-5 time-course markers and progenitor population heterogeneity.
a, b, t-SNE projection of stage-5 time-course data shaded by collection day (a) and by population-marker genes (b). Expression is normalized relative to maximum value, and smoothed over neighbouring cells. c, Pseudotime analysis of day 0 (top) and day 1 (bottom) progenitor cells. Shading on each t-SNE shows assigned pseudotime value of each cell. d, Pseudotime ordering of progenitor cells from stage 5 day 0 (top row) and day 1 (bottom row), showing population heterogeneity among early progenitors. Individual cells are shown as dots, shaded as in c. Gene expression predicted from pseudotime regression shown as overlaid line. e, Summary of stage-5 (day 0) heterogeneity captured by pseudotime analysis. Fold change between start and end of pseudotime ordering. q-value from likelihood ratio test of model with and without pseudotime. f, Heat map of receptors, ligands and signalling effectors that are dynamically expressed across stage-5 populations. Shading displays mean expression (z-normalized TPM) and diameter denotes fractional expression.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-3 and Supplementary Table 2.
Supplementary Table 1
Complete specification of SC-beta differentiation protocols. Stage-by-stage and day-by-day description of cell culture media used for several versions of the SC-beta differentiation protocol (on separate sheets). Additional sheets detail base media composition, and differentiation factors aliquot stocks.
Supplementary Table 3
Comparison of gene expression in NKX6.1+ progenitors, Stage 6 SC-beta cells and human islet beta cells. Complete differential gene expression for all genes, as presented Extended Data Figures 3d,e and complete GSEA results (for Hallmark and custom gene sets) are presented in Extended Data Figures 3f.
Supplementary Table 4
Pseudotime regression analyses of Stage 6 SC-beta cells. Complete results from regression analysis of Stage 6 SC-beta pseudotime for all genes, as presented in Figure 2g-i. GSEA results are included on separate sheet, showing no relevant gene sets.
Supplementary Table 5
Pseudotime regression analyses of Stage 5 progenitors and SC-beta and SC-EC branching. Complete results from regression analyses of Stage 5 populations gene expression for all genes, as presented in Figure 5e-g (SC-beta and SC-EC branching) and Extended Data Figure 8c-e (Day 0 and Day 1 progenitors). Each regression analysis presented on a separate spreadsheet.
Rights and permissions
About this article
Cite this article
Veres, A., Faust, A.L., Bushnell, H.L. et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 569, 368–373 (2019). https://doi.org/10.1038/s41586-019-1168-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1168-5
This article is cited by
-
Comparative and integrative single cell analysis reveals new insights into the transcriptional immaturity of stem cell-derived β cells
BMC Genomics (2024)
-
Metabolic switching, growth kinetics and cell yields in the scalable manufacture of stem cell-derived insulin-producing cells
Stem Cell Research & Therapy (2024)
-
ISR inhibition reverses pancreatic β-cell failure in Wolfram syndrome models
Cell Death & Differentiation (2024)
-
FGF7 enhances the expression of ACE2 in human islet organoids aggravating SARS-CoV-2 infection
Signal Transduction and Targeted Therapy (2024)
-
The efficiency of stem cell differentiation into functional beta cells for treating insulin-requiring diabetes: Recent advances and current challenges
Endocrine (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.