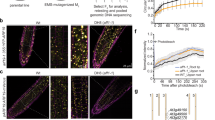
Abstract
The plant hormone auxin has crucial roles in almost all aspects of plant growth and development. Concentrations of auxin vary across different tissues, mediating distinct developmental outcomes and contributing to the functional diversity of auxin. However, the mechanisms that underlie these activities are poorly understood. Here we identify an auxin signalling mechanism, which acts in parallel to the canonical auxin pathway based on the transport inhibitor response1 (TIR1) and other auxin receptor F-box (AFB) family proteins (TIR1/AFB receptors)1,2, that translates levels of cellular auxin to mediate differential growth during apical-hook development. This signalling mechanism operates at the concave side of the apical hook, and involves auxin-mediated C-terminal cleavage of transmembrane kinase 1 (TMK1). The cytosolic and nucleus-translocated C terminus of TMK1 specifically interacts with and phosphorylates two non-canonical transcriptional repressors of the auxin or indole-3-acetic acid (Aux/IAA) family (IAA32 and IAA34), thereby regulating ARF transcription factors. In contrast to the degradation of Aux/IAA transcriptional repressors in the canonical pathway, the newly identified mechanism stabilizes the non-canonical IAA32 and IAA34 transcriptional repressors to regulate gene expression and ultimately inhibit growth. The auxin–TMK1 signalling pathway originates at the cell surface, is triggered by high levels of auxin and shares a partially overlapping set of transcription factors with the TIR1/AFB signalling pathway. This allows distinct interpretations of different concentrations of cellular auxin, and thus enables this versatile signalling molecule to mediate complex developmental outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data that support the findings of this study are available within the paper and its Supplementary Information. RNA sequencing raw data associated with Fig. 3c, d are available at the Gene Expression Omnibus under accession number GSE111716. Source Data (gels and graphs) for Figs. 1, 3, 4 and Extended Data Figs. 1–6, 8–10 are provided with the paper.
References
Salehin, M., Bagchi, R. & Estelle, M. SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell 27, 9–19 (2015).
Weijers, D. & Wagner, D. Transcriptional responses to the auxin hormone. Annu. Rev. Plant Biol. 67, 539–574 (2016).
Kicheva, A., Bollenbach, T., Wartlick, O., Jülicher, F. & Gonzalez-Gaitan, M. Investigating the principles of morphogen gradient formation: from tissues to cells. Curr. Opin. Genet. Dev. 22, 527–532 (2012).
Bargmann, B. O. et al. A map of cell type-specific auxin responses. Mol. Syst. Biol. 9, 688 (2013).
Friml, J. et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108, 661–673 (2002).
Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 69, 417–435 (2018).
Adamowski, M. & Friml, J. PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27, 20–32 (2015).
Raz, V. & Ecker, J. R. Regulation of differential growth in the apical hook of Arabidopsis. Development 126, 3661–3668 (1999).
Zádníková, P. et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137, 607–617 (2010).
Liao, C. Y. et al. Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods 12, 207–210, 2, 210 (2015).
Li, H., Johnson, P., Stepanova, A., Alonso, J. M. & Ecker, J. R. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev. Cell 7, 193–204 (2004).
Žádníková, P. et al. A model of differential growth-guided apical hook formation in plants. Plant Cell 28, 2464–2477 (2016).
Zhao, Y. et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306–309 (2001).
Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008).
Tao, Y. et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176 (2008).
Fendrych, M., Leung, J. & Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife 5, e19048 (2016).
Xu, T. et al. Cell surface ABP1–TMK auxin-sensing complex activates ROP GTPase signaling. Science 343, 1025–1028 (2014).
Vandenbussche, F. et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137, 597–606 (2010).
Nishimura, T. et al. Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J. 77, 352–366 (2014).
Hayashi, K. et al. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc. Natl Acad. Sci. USA 105, 5632–5637 (2008).
Overvoorde, P. J. et al. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17, 3282–3300 (2005).
Wu, Y. et al. Genome-wide expression pattern analyses of the Arabidopsis leucine-rich repeat receptor-like kinases. Mol. Plant 9, 289–300 (2016).
Ramos, J. A., Zenser, N., Leyser, O. & Callis, J. Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13, 2349–2360 (2001).
Dharmasiri, N., Dharmasiri, S. & Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 (2005).
Kepinski, S. & Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451 (2005).
Ulmasov, T., Hagen, G. & Guilfoyle, T. J. ARF1, a transcription factor that binds to auxin response elements. Science 276, 1865–1868 (1997).
Mazzella, M. A., Casal, J. J., Muschietti, J. P. & Fox, A. R. Hormonal networks involved in apical hook development in darkness and their response to light. Front. Plant Sci. 5, 52 (2014).
Bielach, A. et al. Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 24, 3967–3981 (2012).
Gray, W. M., Kepinski, S., Rouse, D., Leyser, O. & Estelle, M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414, 271–276 (2001).
Yan, L. et al. High-efficiency genome editing in Arabidopsis using YAO promoter-driven CRISPR/Cas9 system. Mol. Plant 8, 1820–1823 (2015).
Pasternak, T. et al. Protocol: an improved and universal procedure for whole-mount immunolocalization in plants. Plant Methods 11, 50 (2015).
Uhrig, R. G., She, Y. M., Leach, C. A. & Plaxton, W. C. Regulatory monoubiquitination of phosphoenolpyruvate carboxylase in germinating castor oil seeds. J. Biol. Chem. 283, 29650–29657 (2008).
Yoo, S. D., Cho, Y. H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Cox, M. P., Peterson, D. A. & Biggs, P. J. SolexaQA: at-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics 11, 485 (2010).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Acknowledgements
We thank J. Sheen and Z. Yang for critical comments and suggestions; J. Du for providing the pDu vector. This work was supported by National Key R&D Program of China (2016YFA0503200) and National Natural Science Foundation of China (Grant 31422008), CAU State Key Laboratory of Plant Physiology and Biochemistry open funds, start-up funds from PSC and FAFU to T.X. and the European Union’s Horizon 2020 program (ERC grant agreement no. 742985) to J.F.
Reviewer information
Nature thanks Dolf Weijers and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
T.X., M.C. and R.C. initiated the project and designed the experiments; M.C., R.C. and P.L. carried out most of the experiments; J.H., W.Z. and Z.G. did TMK1 immunolocalization; Y.Y. and R.Z. conducted protoplast and yeast two-hybrid assays; X.W. and Z.G. analysed the apical-hook phenotype; Y.G. did most of the protein purifications; H.Z. conducted the whole-genome and RNA sequencing; D.G. and R.L. analysed the sequencing data; T.X., M.C., R.C. and J.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Disrupting the asymmetric distribution of auxin causes apical-hook development defect in Arabidopsis.
a, Schematic showing cells on the concave and convex sides of the apical hook that were used for cell-length quantification. b, n3×Venus (green) and ntdTomato (magenta) fluorescence signal in the apical hooks of R2D2 seedlings at the maintenance stage. c, Quantification of the ratio of the ntdTomato to n3×Venus signals (mDII/DII, mutated domain II-to-domain II ratio) at the concave and convex sides of R2D2 apical hooks. n = 8; two-sided t-test; dots show data distribution; data are mean ± s.d. d, Representative images of apical hooks in Col-0, yuc1-D and wei8-3 tar2-1 seedlings at 45 h after germination. e, Quantification of apical-hook curvature in d. Box plots show the first and third quartiles, split by the median and extended to minimum and maximum values. One-way ANOVA with Dunnett’s multiple-comparisons test. f, Epidermis cell images of the apical hook in Col-0 plants, and yuc1-D and wei8-3 tar2-1 mutants. g, Quantification of cell length at both concave and convex sides of the apical hook. Col-0 (n = 17), yuc1-D (n = 13), wei8-3 tar2-1 (n = 22); two-sided t-test; dots show data distribution; data are mean ± s.e.m. h, Representative images of apical hooks in Col-0, tmk1-1 and complementing gTMK1-FLAG;tmk1-1 lines at the time points of formation (12 h), early (21 h) and late (45 h) maintenance, and opening (69 h) phases. Quantification is shown in Fig. 1b. n denotes the number of biologically independent seedlings; x denotes cell numbers. Scale bars, 50 μm (b,f); 500 μm(d,h).
Extended Data Fig. 2 TMK1 specifically regulates apical-hook maintenance.
a, Description of tmk transfer DNA (T-DNA) insertion mutants. Lines represent introns; black and grey boxes represent exons and untranslated regions, respectively. b, Western blot of the proteins extracted from the apical hook of Col-0 and different tmk1 alleles by TMK1C-terminus antibody. Asterisk indicates a non-specific band. Three biological repeats. The same membrane was stripped and blotted with anti-actin antibodies as a loading control. c, Representative images of apical hooks in different tmk1 alleles 45 h after germination. d, Quantification of apical-hook curvature in c. n = 11. e, Representative images of apical hooks in the mutants of different TMK family members (tmk1-1, tmk2-1, tmk3-1 and tmk4-1) 48 h after germination. f, Quantification of apical-hook curvature in e. Col-0 (n = 12), tmk1-1 (n = 12), tmk2-1 (n = 12), tmk3-1 (n = 17) and tmk4-1 (n = 15). g, Representative images of apical hooks in Col-0, wei8-3 tar2-1 and tmk1-1 on medium supplemented with or without 0.5 μM IAA at 50 h after germination. h, Quantification of apical-hook curvature in g. n = 20. Box plot show the first and third quartiles, split by the median and extended to minimum and maximum values. One-way ANOVA with Dunnett’s multiple-comparisons test (d, f) or two-sided t-test (h). n denotes the number of biologically independent seedlings. All scale bars, 500 μm.).
Extended Data Fig. 3 Immunolocalization of TMK1 during apical-hook development.
a, Immunolocalization of TMK1 protein and DAPI staining at the apical-hook maintenance stage in tmk1-1 mutants. The immunostaining was performed using same condition as those in Fig. 1d. Scale bars, 20 μm. Three biological repeats. b, Schematic showing the region (dashed frame) across the cells used for the quantification of fluorescence signal. c, Quantification of the relative intensity of TMK1C-terminus immunofluorescence and DAPI across a representative cell from both concave (top) or convex (bottom) sides of apical hook in Col-0 (left) and the tmk1-1 (right) mutant. Dashed lines define the nucleus by the enriched DAPI signal. Fluorescence intensity was calculated using ImageJ, and normalized by the maximum intensity at 255. The position across the cell was normalized as 0–1, as shown in b. Twenty cells from four biologically independent seedlings showed a similar pattern. d, Representative images of immunolocalization of TMK1 in the apical hook at three different stages (formation, maintenance and opening). Scale bars, 20 μm. Three biological repeats. e, Quantification of the relative intensity of TMK1C-terminus immunofluorescence and DAPI across representative cells from concave and convex sides of the apical hook, at three different stages. Normalization was done as in c. Twenty cells from four biologically independent seedlings showed a similar pattern.
Extended Data Fig. 4 Cleavage of TMK1C-terminus is independent of both the auxin–TIR1 and the ethylene pathways.
a, Identification of TMK1 C terminus. Full-length TMK1 and TMK1C-terminus were immunoprecipitated from gTMK1-GFP seedlings and separated by SDS–PAGE. Three biological repeats were performed with similar results. b, Mass spectrometry analysis of the TMK1C-terminus band. Results are displayed as spectral counts of the peptide fragment (y axis) along the TMK1 amino acid position (x axis). c, Quantification of western blotting results shown in Fig. 1f. d, Treatment with yucasin and auxin affects TMK1 cleavage. e, Quantification of western blotting results shown in d. f, Quantification of western blotting results shown in Fig. 1g. n = 3. g, The effect of treatment with PEO-IAA was tested by the inhibition of auxin-mediated IAA28 degradation by 35S-IAA28-MYC transgenic line. Col-0 was used as a negative control; the asterisk indicates a non-specific band. h, The effect of treatment with PEO-IAA on auxin-mediated TMK1 cleavage. Three biological repeats. i, The effect on TMK1 cleavage of treatment with 10 μM ACC for a short period. Three biological repeats. For d, g–i, the same membrane was stripped and blotted with anti-actin antibodies as a loading control. j, Apical-hook development in the presence and absence of ACC in both Col-0 and tmk1-1 plants. n = 20. Data are mean ± s.e.m.; dots show data distribution. n denotes the number of biologically independent seedlings.
Extended Data Fig. 5 TMK1C-terminus partially rescues the phenotype associated with the tmk1 mutant.
a, Representative images of TMK1C-terminus–GFP localization in epidermal cells of the apical hook in the pTMK1-TMK1C-terminus-GFP transgenic line. The 35S-GFP line was used as a control. Scale bars, 50 μm. Three individual lines showed a similar pattern. b, Representative images of pTMK1-TMK1C-terminus-GFP plants, which partially complemented the tmk1-1 phenotype at 45 h after germination. Three out of three T3 individual homozygous lines showed similar phenotypes. Scale bars, 500 μm. c, Quantification of apical-hook curvature in b. Measurements displayed as box plots, showing the first and third quartiles, split by the median and extended to minimum and maximum values. Col-0 (n = 25), tmk1-1 (n = 28), pTMK1-TMK1C-terminus-GFP;tmk1-1 (n = 32). n denotes the number of biologically independent seedlings. Two-sided t-test.
Extended Data Fig. 6 TMK1C-terminus specifically interacts with IAA32 and IAA34.
a, Quantification of protein–protein interactions between TMK family members and IAA family members in yeast, by β-galactosidase assay. Three biologically independent experiments were performed for quantification. Data are mean ± s.e.m., one-way ANOVA with Dunnett’s multiple-comparisons test compared to activation domain. Dots show data distribution. b, Protein expression verification for yeast two-hybrid interactions by western blotting. Experiment was repeated twice with similar results. c, Bayesian phylogenetic tree of the 29 IAA proteins in A. thaliana. This phylogenetic tree was generated through Bayesian inference, using the JTT model with 100,000 generations. The posterior probabilities are indicated (in per cent) at the base of each bipartition. d, TMK1C-terminus interacts with IAA32 and IAA34 in protoplasts. 35S–IAA32–GFP, 35S–IAA34–GFP and 35S–TMK1C-terminus–HA were co-expressed in Arabidopsis protoplasts for co-immunoprecipitation (IP). 35S–IAA14–GFP was used as a control. Three biological repeats with similar results. e, TMK1C-terminus interacts with IAA32 and IAA34, shown by in vitro pull-down assay. TMK1C-terminus–GFP protein from pTMK1-TMK1C-terminus-GFP transgenic plants was incubated with E. coli-purified IAA32 and IAA34 recombinant protein. IAA14 was used as a control. Three biological repeats with similar results.
Extended Data Fig. 7 IAA32 and IAA34 express in the apical-hook bending region, and localize in both the cytosol and nucleus.
a, Expression patterns of IAA32 and IAA34 in three-day-old seedlings of pIAA32-GUS and pIAA34-GUS lines. Four independent T2 heterozygous plants are displayed. Scale bars, 500 μm. Representative of eight out of eight lines (pIAA32-GUS), or seven out of eight lines (pIAA34-GUS). b, Expression pattern of IAA32–GFP and IAA34–GFP proteins in three-day-old seedlings of gIAA32-GFP and gIAA34-GFP lines. Four independent T2 heterozygous plants are displayed. Scale bars, 50 μm. Representative of 11 out of 12 lines (gIAA32-GFP) or 9 out of 11 lines (gIAA34-GFP). c, Temporal and spatial expression patterns of IAA32 and IAA34 protein in both wild-type and tmk1-1 plants during apical-hook development. Scale bars, 50 μm. Three individual lines of T3 homozygous plants showed a similar pattern. d, Representative images of subcellular localization of 35S–IAA32–GFP and 35S–IAA34–GFP at the apical hook of wild-type plants and tmk1-2 mutant. Scale bars, 25 μm. Three individual lines of T3 homozygous plants showed a similar subcellular localization pattern.
Extended Data Fig. 8 The iaa32 iaa34 mutant shows cell-elongation defects at the apical hook.
a, Schematic gene structure of IAA32 and IAA34, with iaa32 and iaa34 mutants indicated. Lines represent introns; black and grey boxes represent exons and untranslated regions, respectively. Asterisk indicates the early stop codon in the iaa32 mutant. Dashes indicate the deleted DNA base pairs. Arrows indicate primers that were used for quantitative PCR with reverse transcription analysis in b. Bar indicates the respective DNA lengths. b, Gene expression analysis of IAA32 and IAA34 transcripts in the iaa32 iaa34 double mutant. Three biological repeats. Dots show data distribution. Two-sided t-test. Data are mean ± s.d. The expression levels were standardized as 1 in Col-0. UBQ10 was used as an internal control. c, Representative images of apical hooks in Col-0, iaa32 iaa34, complementing gIAA32-GFP;iaa32 iaa34 and complementing gIAA34-GFP;iaa32 iaa34 lines. The growth conditions are comparable to those of experiments shown in Extended Data Fig. 1h. Scale bar, 500 μm. d, Images of the apical-hook epidermal cell in Col-0, iaa32 iaa34, gIAA32-GFP;iaa32 iaa34 and gIAA34-GFP;iaa32 iaa34 lines. Scale bars, 50 μm. Three biological repeats were performed with similar results. e, Quantification of cell length at the concave and convex sides of the apical hook, shown in d. Number of biologically independent seedlings: Col-0, n = 10; iaa32 iaa34, n = 12; gIAA32-GFP;iaa32 iaa34, n = 11; gIAA34-GFP;iaa32 iaa34, n = 10. x denotes cell numbers. Dots show data distribution. Two-sided t-test. Data are mean ± s.e.m.
Extended Data Fig. 9 IAA32 and IAA34 interact with ARFs, and suppress ARF activity.
a, Auxin-response element (AuxRE) analyses in genes that are co-regulated by TMK1, IAA32 and IAA34. The auxin-response element motif (TGTCTC) within the 2-kb upstream promoter of differentially regulated genes was analysed. Twenty-five out of forty-seven genes that are upregulated by TMK1, IAA32 and IAA34 contained the auxin-response element; 84 out of 186 genes that are downregulated by TMK1, IAA32 and IAA34 contained the auxin-response element. Venn diagrams are used to present the comparison and overlaps of the gene lists. b, Protein–protein interactions between IAA proteins (IAA32, IAA34 and IAA14), and ARF family members in yeast two-hybrid analysis. Three biological repeats were performed with similar results. c, IAA32- and IAA34-inhibited ARF2 activity in Arabidopsis protoplasts. d, IAA32- and IAA34-inhibited ARF7 activity in Arabidopsis protoplasts. Top, relative luciferase (LUC) activity of DR5–LUC with co-expression of ARF and IAA proteins, as indicated. Bottom, protein expression by western blot. For c, d, the same membrane was stripped and blotted with anti-actin antibodies as a loading control. Luciferase activities were normalized by both GUS activity and the empty-vector control. n = 3 independent biological repeats. Two-sided t-test. Dots show data distribution, data are mean ± s.d.
Extended Data Fig. 10 Transcriptional regulation of IAA32 and IAA34 depends on TIR1, but protein stability depends on the kinase activity of TMK1.
a, Western blotting analysis of etiolated 35S-IAA32-GFP line in either wild-type or tmk1, treated with auxin for indicated time points. Three biological repeats. b, Western blotting analysis of 35S-IAA28-MYC line treated with auxin for indicated time points; asterisk indicates non-specific band. Three biological repeats. c, The TIR1 inhibitor PEO-IAA did not affect auxin-mediated accumulation of IAA32 and IAA34. Three biological repeats. d, Level of IAA32 and IAA34 transcription was downregulated in tir1 afb2 afb3 mutant. Three biological repeats. Dots show data distribution. Two-sided t-test. Data are mean ± s.d. The expression level was standardized to 1 in wild-type seedlings. UBQ10 was used as internal control. e, The protein stability of IAA32 with or without co-expression of 35S–TMK1C-terminus–HA. CHX treatment for indicated time periods. 35S–sGFP was used as a control. Three biological repeats. f, Western blot of proteins in protoplasts co-transfected with HBT–TMK1C-terminus–HA and HBT–IAA14–GFP. Three repeats showed similar results. g, The protein stability of IAA32 with or without co-expression of 35S–TMK1C-terminus–HA. CHX treatment for indicated time periods. 35S–sGFP was used as a control. Three biological repeats. h, Confocal microscopy of IAA32–GFP protein in the apical hook of a tmk1 mutant with gTMK1-FLAG or gTMK1(K616E)-FLAG. Scale bars, 50 μm. Three biological repeats. i, Representative images of apical hooks in Col-0, tmk1-1, gTMK1-FLAG;tmk1-1 and gTMK1(K616E)-FLAG;tmk1-1 at indicated time points after germination, Scale bars, 500 μm. Three independent repeats showed similar results. j, Quantification of apical-hook curvature at the time points corresponding to those shown in i. n = 15 biologically independent seedlings; data are mean ± s.e.m. For western blot images a–c and g, the same membrane was stripped and blotted with anti-actin antibodies as a loading control. For f, IAA14–GFP and TMK1C-terminus–HA were run separately (with the same amount of protein per 10-μl sample) owing to the similar molecular mass of these two proteins. The same membrane of IAA14–GFP was stripped and blotted by anti-actin antibodies as a loading control.
Supplementary information
Supplementary Figure
This file contains the uncropped geld for the main figures and Extended Data Figures.
Supplemental Table 1 | List of primers used in this study
The purposes of these primers were listed in left. The sequence of these primers were displayed in right. F, forward; R, reverse.
Supplemental Table 2 | List of TMK1-IAA32/34 target genes
Data in Table 2 include both up- and down-regulated TMK1-IAA32/34 target genes, which were used for hierarchical clustering analysis (Fig. 3c). The significance cutoffs for Differentially expressed genes (DEGs) were at least two-fold change in expression (signal log2 ratio ≥1 for up-regulated; signal log2 ratio ≤ -1 for down-regulated).
Supplemental Table 3 | List of TMK1-IAA32/34 target genes harboring AuxRE element
Data in Table 3 include both up- and down-regulated TMK1-IAA32/34 target genes that contain AuxRE (TGTCTC) element, which were used for analyzing AuxRE element in TMK1-IAA32/34 co-regulated genes presented by venn diagram (Extended Fig. 9a). prt, promoter.
Supplemental Table 4 | GO enrichment analysis of TMK1-IAA32/34 target genes
Data in Table 4 show the functional classification of TMK1-IAA32/34 target genes based on GO analyses, commonly up-regulated gene in tmk1 and iaa32iaa34 were shown in Fig. 3e.
Source data
Rights and permissions
About this article
Cite this article
Cao, M., Chen, R., Li, P. et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568, 240–243 (2019). https://doi.org/10.1038/s41586-019-1069-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1069-7
This article is cited by
-
Heat Stress Responsive Aux/IAA Protein, OsIAA29 Regulates Grain Filling Through OsARF17 Mediated Auxin Signaling Pathway
Rice (2024)
-
Spatial IMA1 regulation restricts root iron acquisition on MAMP perception
Nature (2024)
-
The new horizon of plant auxin signaling via cell-surface co-receptors
Cell Research (2024)
-
Lighting up plants with near-infrared fluorescence probes
Science China Chemistry (2024)
-
Mechanisms controlling plant proteases and their substrates
Cell Death & Differentiation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.