Abstract
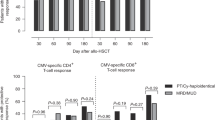
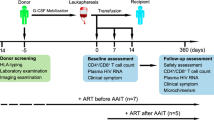
A cure for HIV-1 remains unattainable as only one case has been reported, a decade ago1,2. The individual—who is known as the ‘Berlin patient’—underwent two allogeneic haematopoietic stem-cell transplantation (HSCT) procedures using a donor with a homozygous mutation in the HIV coreceptor CCR5 (CCR5Δ32/Δ32) to treat his acute myeloid leukaemia. Total body irradiation was given with each HSCT. Notably, it is unclear which treatment or patient parameters contributed to this case of long-term HIV remission. Here we show that HIV-1 remission may be possible with a less aggressive and toxic approach. An adult infected with HIV-1 underwent allogeneic HSCT for Hodgkin’s lymphoma using cells from a CCR5Δ32/Δ32 donor. He experienced mild gut graft-versus-host disease. Antiretroviral therapy was interrupted 16 months after transplantation. HIV-1 remission has been maintained over a further 18 months. Plasma HIV-1 RNA has been undetectable at less than one copy per millilitre along with undetectable HIV-1 DNA in peripheral CD4 T lymphocytes. Quantitative viral outgrowth assays from peripheral CD4 T lymphocytes show no reactivatable virus using a total of 24 million resting CD4 T cells. CCR5-tropic, but not CXCR4-tropic, viruses were identified in HIV-1 DNA from CD4 T cells of the patient before the transplant. CD4 T cells isolated from peripheral blood after transplantation did not express CCR5 and were susceptible only to CXCR4-tropic virus ex vivo. HIV-1 Gag-specific CD4 and CD8 T cell responses were lost after transplantation, whereas cytomegalovirus-specific responses were detectable. Similarly, HIV-1-specific antibodies and avidities fell to levels comparable to those in the Berlin patient following transplantation. Although at 18 months after the interruption of treatment it is premature to conclude that this patient has been cured, these data suggest that a single allogeneic HSCT with homozygous CCR5Δ32 donor cells may be sufficient to achieve HIV-1 remission with reduced intensity conditioning and no irradiation, and the findings provide further support for the development of HIV-1 remission strategies based on preventing CCR5 expression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hütter, G. et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 (2009).
Allers, K. et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 117, 2791–2799 (2011).
UNAIDS. UNAIDS Data 2017 http://www.unaids.org/en/resources/documents/2017/20170720_Data_book_2017 (2017).
Gupta, R. K. et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect. Dis. 18, 346–355 (2018).
The TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect. Dis. 16, 565–575 (2016).
Barth, R. E., van der Loeff, M. F., Schuurman, R., Hoepelman, A. I. & Wensing, A. M. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect. Dis. 10, 155–166 (2010).
Avert. Funding for HIV and AIDS https://www.avert.org/professionals/hiv-around-world/global-response/funding (2017).
Simmons, G. et al. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol. 70, 8355–8360 (1996).
Kordelas, L. et al. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N. Engl. J. Med. 371, 880–882 (2014).
Verheyen, J. et al. Rapid rebound of a preexisting CXCR4-tropic human immunodeficiency virus variant after allogeneic transplantation with CCR5 Δ32 homozygous stem cells. Clin. Infect. Dis. 64, 684–687 (2019).
Symons, J. et al. Dependence on the CCR5 coreceptor for viral replication explains the lack of rebound of CXCR4-predicted HIV variants in the Berlin patient. Clin. Infect. Dis. 59, 596–600 (2014).
Henrich, T. J. et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann. Intern. Med. 161, 319–327 (2014).
Cummins, N. W. et al. Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: a case study. PLoS Med. 14, e1002461 (2017).
Bosman, K. J. et al. Development of sensitive ddPCR assays to reliably quantify the proviral DNA reservoir in all common circulating HIV subtypes and recombinant forms. J. Int. AIDS Soc. 21, e25185 (2018).
Yukl, S. A. et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 9, e1003347 (2013).
Ritchie, A. V. et al. Performance evaluation of the point-of-care SAMBA I and II HIV-1 Qual whole blood tests. J. Virol. Methods 237, 143–149 (2016).
Laird, G. M. et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog. 9, e1003398 (2013).
Fun, A., Mok, H. P., Wills, M. R. & Lever, A. M. A highly reproducible quantitative viral outgrowth assay for the measurement of the replication-competent latent HIV-1 reservoir. Sci. Rep. 7, 43231 (2017).
Jensen, M. A., Coetzer, M., van ’t Wout, A. B., Morris, L. & Mullins, J. I. A reliable phenotype predictor for human immunodeficiency virus type 1 subtype C based on envelope V3 sequences. J. Virol. 80, 4698–4704 (2006).
Lengauer, T., Sander, O., Sierra, S., Thielen, A. & Kaiser, R. Bioinformatics prediction of HIV coreceptor usage. Nat. Biotechnol. 25, 1407–1410 (2007).
Smith, N. M. et al. Proof-of-principle for immune control of global HIV-1 reactivation in vivo. Clin. Infect. Dis. 61, 120–128 (2015).
Watters, S. A. et al. Sequential CCR5-tropic HIV-1 reactivation from distinct cellular reservoirs following perturbation of elite control. PLoS ONE 11, e0158854 (2016).
Salgado, M. et al. Mechanisms that contribute to a profound reduction of the HIV-1 reservoir after allogeneic stem cell transplant. Ann. Intern. Med. 169, 674–683 (2018).
Hamilton, B. K. et al. Long-term follow-up of a prospective randomized trial comparing CYA and MTX with CYA and mycophenolate mofetil for GVHD prophylaxis in myeloablative sibling donor hematopoietic cell transplantation. Bone Marrow Transplant. 48, 1578–1580 (2013).
Duarte, R. F. et al. CCR5 Δ32 homozygous cord blood allogeneic transplantation in a patient with HIV: a case report. Lancet HIV 2, e236–e242 (2015).
Pavlů, J. et al. LACE-conditioned autologous stem cell transplantation for relapsed or refractory diffuse large B-cell lymphoma: treatment outcome and risk factor analysis from a single centre. Hematol. Oncol. 29, 75–80 (2011).
Bronnimann, M. P., Skinner, P. J. & Connick, E. The B-cell follicle in HIV infection: barrier to a cure. Front. Immunol. 9, 20 (2018).
Symons, J. et al. Impact of triplicate testing on HIV genotypic tropism prediction in routine clinical practice. Clin. Microbiol. Infect. 18, 606-612 (2012).
Edgar, R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Amendola, A. Standardization and performance evaluation of “modified” and “ultrasensitive” versions of the Abbott RealTime HIV-1 assay, adapted to quantify minimal residual viremia. J. Clin. Virol. 42, 17–22 (2011).
Acknowledgements
This study was funded by a Wellcome Trust Senior Fellowship in Clinical Science to R.K.G., research capability funding (RCF) from UCLH BRC to R.K.G., as well as funding from Oxford and Cambridge Biomedical Research Centres (BRC), amfAR (The Foundation for AIDS Research), through the amfAR Research Consortium on HIV Eradication (ARCHE) program (AmfAR 109858-64-RSRL), the MRC (MR/R008698/1 to L.E.M. and MRM008614/2 to D.P.). A.J.I. is supported by an NIHR Clinical Lectureship, and acknowledges support from the NIHR and Imperial Biomedical Research Centre (BRC). E.N. received funding from the University College London Hospitals NHS Trust (UCLH)/University College London (UCL) National Institute for Health Research (NIHR) Biomedical Research Centre and research funding through an independent grant by ViiV Healthcare UK as part of the EPIICAL Consortium. We thank the CHERUB (http://www.cherub.uk.net) and IciStem Consortia (https://www.icistem.org/) for support and continuous discussion of results; N. Parmahand, M. Bandara, I. Jarvis, A. Fun, M. Lee, L. Hedley, K. Ardeshna, A. Hill, N. Goel, R. Szydlo, D. Slade, S. Griffith and C. Gálvez, Á. Hernández Rodríguez, V. González Soler and B. Rivaya Sánchez, E. van Maarseveen, L. Huyveneers, P. Schipper and D. de Jong; J. Apperley, Z. Allwood and S. Loaiza and all the nurses in the BMT Unit that looked after the patient.
Reviewer information
Nature thanks Steven Deeks, Sarah J. Fidler, Timothy Henrich and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
R.K.G., S.A.-J., S.G.E., E.O., H.P.M. and I.H.G. conceived and designed the study. S.A.-J., J.M.-P., A.M.J.W., M.S., M.N., F.S., L.M., I.H.G., L.E.M., D.P., H.P.M., P.G., E.N., A.M.L.L., L.W., S.G.E., M.P. and C.M. designed and/or performed experiments. R.K.G., J.M.-P., A.M.J.W., S.A.-J., M.S., M.N., F.S., L.M., L.E.M., E.O., H.L., J.F., M.P., I.H.G., H.P.M., S.G.E., E.N., P.G., J.L., A.J.I. and C.M. performed analyses and interpreted data. R.K.G., M.N., A.M.J.W., J.M.-P., A.M.L.L., J.L., H.P.M., and A.J.I. wrote the draft of the manuscript. J.M.-P., A.M.J.W., M.S., M.N., F.S., S.A.-J., L.M., L.E.M., D.P., H.P.M., P.G., E.N., A.M.L.L., S.G.E., L.W., M.P., I.H.G., H.P.M. and A.J.I. were involved in the critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Blood cell populations over time.
3TC, lamivudine; CsA, cyclosporine-A; DTG, dolutegravir; RPV, rilpivirine; WBC, white blood cells.
Extended Data Fig. 2 Susceptibility of CD4 T cells from the index patient to CCR5-tropic and CXCR4-tropic HIV-1.
a, Experimental flow for measurement of infection by intracellular p24 Gag staining. Control cells were from a healthy HIV−CCR5 donor. b, Flow cytometry analysis of PBMCs following three days of stimulation exhibiting the expression pattern of CCR5 receptor within CD3 CD4 T cells in both healthy donor (control) and index patient. c. Culture supernatants from CD4 T cells infected with CCR5- and CXCR4-tropic viruses were collected on days three and seven to measure infectivity on HeLa TZM-bl reporter cells. Infectivity is measured as a reduction in the gene expression of the Tat-induced firefly luciferase reporter in TZM-bl cells. data are mean ±s.e.m. n = 2: one donor and one index patient. Experiments were repeated three times with similar results.
Extended Data Fig. 3 CD8 T cell responses and CD4 T cell responses to HIV.
Representative fluorescence-activated cell sorting plots showing the percentage of virus-specific CD8 T cells (top) and CD4 T cells (bottom) identified by intracellular staining for IFNγ, following stimulation with HIV Pol, Env and Nef peptide pools after HSCT at days 96 and 819.
Supplementary information
Rights and permissions
About this article
Cite this article
Gupta, R.K., Abdul-Jawad, S., McCoy, L.E. et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568, 244–248 (2019). https://doi.org/10.1038/s41586-019-1027-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1027-4
This article is cited by
-
The chemokine receptor CCR5: multi-faceted hook for HIV-1
Retrovirology (2024)
-
Immune targeting of HIV-1 reservoir cells: a path to elimination strategies and cure
Nature Reviews Microbiology (2024)
-
Ripretinib inhibits HIV-1 transcription through modulation of PI3K-AKT-mTOR
Acta Pharmacologica Sinica (2024)
-
HIV-1 mRNA knockdown with CRISPR/CAS9 enhances neurocognitive function
Journal of NeuroVirology (2024)
-
In-house ELISA protocols for capsid p24 detection of diverse HIV isolates
Virology Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.