Abstract
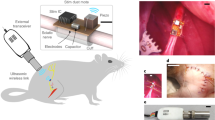
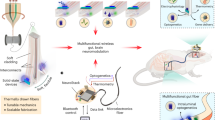
The fast-growing field of bioelectronic medicine aims to develop engineered systems that can relieve clinical conditions by stimulating the peripheral nervous system1,2,3,4,5. This type of technology relies largely on electrical stimulation to provide neuromodulation of organ function or pain. One example is sacral nerve stimulation to treat overactive bladder, urinary incontinence and interstitial cystitis (also known as bladder pain syndrome)4,6,7. Conventional, continuous stimulation protocols, however, can cause discomfort and pain, particularly when treating symptoms that can be intermittent (for example, sudden urinary urgency)8. Direct physical coupling of electrodes to the nerve can lead to injury and inflammation9,10,11. Furthermore, typical therapeutic stimulators target large nerve bundles that innervate multiple structures, resulting in a lack of organ specificity. Here we introduce a miniaturized bio-optoelectronic implant that avoids these limitations by using (1) an optical stimulation interface that exploits microscale inorganic light-emitting diodes to activate opsins; (2) a soft, high-precision biophysical sensor system that allows continuous measurements of organ function; and (3) a control module and data analytics approach that enables coordinated, closed-loop operation of the system to eliminate pathological behaviours as they occur in real-time. In the example reported here, a soft strain gauge yields real-time information on bladder function in a rat model. Data algorithms identify pathological behaviour, and automated, closed-loop optogenetic neuromodulation of bladder sensory afferents normalizes bladder function. This all-optical scheme for neuromodulation offers chronic stability and the potential to stimulate specific cell types.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are either provided in the source data or are available from the corresponding authors upon reasonable request. iOS code is available at https://github.com/noh21/bladder_cloc.
References
Birmingham, K. et al. Bioelectronic medicines: a research roadmap. Nat. Rev. Drug Discov. 13, 399–400 (2014).
Cameron, T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J. Neurosurg. 100, 254–267 (2004).
De Ferrari, G. M. et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 32, 847–855 (2011).
de Groat, W. C. & Tai, C. Impact of bioelectronic medicine on the neural regulation of pelvic visceral function. Bioelectron. Med. 2015, 25–36 (2015).
Famm, K., Litt, B., Tracey, K. J., Boyden, E. S. & Slaoui, M. Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161 (2013).
Janknegt, R. A. et al. Long-term effectiveness of sacral nerve stimulation for refractory urge incontinence. Eur. Urol. 39, 101–106 (2001).
Siegel, S. W. et al. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology 56, 87–91 (2000).
Kavvadias, T., Huebner, M., Brucker, S. Y. & Reisenauer, C. Management of device-related complications after sacral neuromodulation for lower urinary tract disorders in women: a single center experience. Arch. Gynecol. Obstet. 295, 951–957 (2017).
del Valle, J. & Navarro, X. Interfaces with the peripheral nerve for the control of neuroprostheses. Int. Rev. Neurobiol. 109, 63–83 (2013).
Rossini, P. M. et al. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin. Neurophysiol. 121, 777–783 (2010).
Yoshida, K., Farina, D., Akay, M. & Jensen, W. Multichannel intraneural and intramuscular techniques for multiunit recording and use in active prostheses. Proc. IEEE 98, 432–449 (2010).
Miftahof, R. & Nam, H. G. Biomechanics of the Human Urinary Bladder (Springer, 2013).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2012).
Zerboni, L. et al. Herpes simplex virus 1 tropism for human sensory ganglion neurons in the severe combined immunodeficiency mouse model of neuropathogenesis. J. Virol. 87, 2791–2802 (2013).
DeBerry, J. J. et al. Differential regulation of bladder pain and voiding function by sensory afferent populations revealed by selective optogenetic activation. Front. Integr. Nuerosci. 12, 5 (2018).
Beaudry, H., Daou, I., Ase, A. R., Ribeiro-da-Silva, A. & Séguéla, P. Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain 158, 2329–2339 (2017).
Boada, M. D. et al. Fast-conducting mechanoreceptors contribute to withdrawal behavior in normal and nerve injured rats. Pain 155, 2646–2655 (2014).
Iyer, S. M. et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol. 32, 274–278 (2014).
Iyer, S. M. et al. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci. Rep. 6, 30570 (2016).
Li, B. et al. A novel analgesic approach to optogenetically and specifically inhibit pain transmission using TRPV1 promoter. Brain Res. 1609, 12–20 (2015).
Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Towne, C., Montgomery, K. L., Iyer, S. M., Deisseroth, K. & Delp, S. L. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One 8, e72691 (2013).
ClinicalTrials.Gov. RST-001 phase I/II trial for advanced retinitis pigmentosa. https://ClinicalTrials.gov/show/NCT02556736 (2018).
ClinicalTrials.Gov. Dose-escalation study to evaluate the safety and tolerability of GS030 in subjects with retinitis pigmentosa. https://ClinicalTrials.gov/show/NCT03326336 (2018).
Gutierrez, C. A. & Meng, E. Low-cost carbon thick-film strain sensors for implantable applications. J. Micromech. Microeng. 20, 095028 (2010).
Lu, N., Lu, C., Yang, S. & Rogers, J. Highly sensitive skin-mountable strain gauges based entirely on elastomers. Adv. Funct. Mater. 22, 4044–4050 (2012).
Damaser, M. S. & Lehman, S. L. Does it matter, the shape of the bladder? Neurourol. Urodyn. 12, 277–280 (1993).
Korkmaz, I. & Rogg, B. A simple fluid-mechanical model for the prediction of the stress-strain relation of the male urinary bladder. J. Biomech. 40, 663–668 (2007).
Kelly, P. Mechanics Lecture Notes: An introduction to Solid Mechanics. http://homepages.engineering.auckland.ac.nz/~pkel015/SolidMechanicsBooks/index.html, 185–194 (2018).
Ren, Y., Qi, H., Chen, Q. & Ruan, L. Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int. J. Heat Mass Transfer 106, 212–221 (2017).
Samineni, V. K. et al. Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain 158, 2108–2116 (2017).
Bhattacharya, A. & Mahajan, R. L. Temperature dependence of thermal conductivity of biological tissues. Physiol. Meas. 24, 769–783 (2003).
Liu, J., Zhu, L. & Xu, L. X. Studies on the three-dimensional temperature transients in the canine prostate during transurethral microwave thermal therapy. J. Biomech. Eng. 122, 372–379 (2000).
Uvin, P. et al. The use of cystometry in small rodents: a study of bladder chemosensation. J. Vis. Exp. 66, e3869 (2012).
Andersson, K. E., Soler, R. & Füllhase, C. Rodent models for urodynamic investigation. Neurourol. Urodyn. 30, 636–646 (2011).
Samineni, V. K. et al. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Sci. Rep. 7, 15865 (2017).
Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Valtcheva, M. V. et al. Surgical extraction of human dorsal root ganglia from organ donors and preparation of primary sensory neuron cultures. Nat. Protocols 11, 1877–1888 (2016).
Lai, H. et al. Animal models of urologic chronic pelvic pain syndromes: findings from the multidisciplinary approach to the study of chronic pelvic pain research network. Urology 85, 1454–1465 (2015).
Parsons, B. A. & Drake, M. J. in Urinary Tract. Handbook of Experimental Pharmacology (eds Andersson, K. E. & Michel, M.) 15–43 (Springer, Berlin, 2011).
Crock, L. W. et al. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J. Neurosci. 32, 14217–14226 (2012).
Acknowledgements
We acknowledge the generosity of the donor families, as well as Mid-America Transplant for making the studies of human sensory neurons possible. J. Lemen provided instrumental help during human DRG surgical extractions. We thank J. Sinn-Hanlon for the illustrations, L. Strong for technical assistance with the CT imaging, S. Vogt for technical support and C. Morgan for early input on the project. This work was funded by an NIH Director’s Transformative Research Award TR01 NS081707 (R.W.G. and J.A.R.), an NIH SPARC Award via the NIBIB of the NIH U18 EB021793 (R.W.G. and J.A.R.), R01 NS42595 (R.W.G.), NRSA F32 DK115122 (A.D.M.), the McDonnell Center for Cellular and Molecular Neurobiology Postdoctoral Fellowship (A.D.M.), K01 DK115634 (V.K.S.), the Urology Care Foundation Research Scholars Program and Kailash Kedia Research Scholar Endowment (V.K.S.), NSF Grant 1635443 (Y.H.), the Ryan Fellowship and the Northwestern University International Institute for Nanotechnology (Y.X.), T32 DA007261 (L.A.M.), T32 DK108742 (K.W.M.), T32 GM 108539 (B.A.C.), Washington University BioSURF Fellowship (P.S.) DK082315 (H.H.L.) and K08 DK094964 (H.H.L.).
Reviewer information
Nature thanks T. Chai, C. Moritz, E. Roche and S. Zderic for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.D.M., S.M.W., K.N.N. and J.Y. contributed equally to the work; A.D.M., S.M.W., K.N.N., K.W.M., J.Y., S.-I.P., J.A.R. and R.W.G. conceived of and designed experiments and material devices; S.M.W., K.N.N., J.Y., K.E.C., B.H.K., S.M., D.H.K., Y.Y., H.J. and S.-I.P. designed and provided optoelectronic devices; S.M.W., K.N.N. and J.Y. conducted all bench top tests; S.M.W. provided device illustration; Y.X., J.Z., Y.L., and Y.H. contributed to mechanical and thermal simulations; A.D.M. performed all surgical implantations and viral injections; A.D.M., L.A.M., P.S. and Y.S. collected and analysed histological data; A.D.M. performed and analysed data from cytometry stain gauges and gait analysis; V.K.S. collected and analysed data from open field experiments; M.A.P. collected and analysed VMR data; L.A.M. and B.A.C. collected and analysed data from the electrophysiology experiments; A.D.M., S.M.W. and K.N.N. designed and tested the closed-loop algorithm; K.N.N. programed and tested the iOS software; H.H.L. provided guidance and resources for cystometric and metabolic studies; and A.D.M., S.M.W., K.N.N., J.A.R. and R.W.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.A.R. and R.W.G. are co-founders of Neurolux, a company that manufactures wireless optoelectronic devices. The device described here uses similar technology, but is distinct from the current Neurolux portfolio.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Demonstration of optoelectronic stimulation and sensing module features and implantation of the whole CLOC system.
a, Demonstration of strain gauge placement on a mimic bladder illustrating how the two silicone bands wrap around the bladder and how stretch is exerted on the strain gauge as the bladder expands. b, Detailed surgical procedure for implantation of the wireless closed-loop optogenetics-based system for peripheral neuromodulation. The strain gauge is placed on the bladder (1), and using curved forceps the lower band is pulled under the bladder and wrapped back on top of the optoelectronic stimulation and sensing module (2). Kwik-Sil is then applied to secure the lower band to the top of the optoelectronic stimulation and sensing module (3). The upper band is then wrapped around the largest part of the bladder dome and the buckle secured. Then a small suture is placed through the buckle into the bladder smooth muscle layer to secure the upper band to the bladder (4). The bladder is then placed back into the abdominal cavity (5) and muscle layers are closed with sutures (6). The WCP is inserted between the skin and the muscle layer (7) and the skin is closed with surgical staples (8, 9).
Extended Data Fig. 2 Computational and experimental studies of the bladder strain gauge and material properties of rat bladder.
a, Left, schematic illustration of a strain gauge, part of the optoelectronic stimulation and sensing module. Right, cross-sectional side view of the device components at the level of the dashed line shown on the left. b, Image of a rat bladder (left) and digitally manipulated version (right) to allow measurements of the volumetric changes in size during cystometry. c, Graph of changes in bladder size (red) and changes in strain gauge resistance (black). d, Fractional change in resistance of the strain gauge as a function of uniaxial tensile strain. e, Fractional change in resistance under 1,000 cycles of stretching to a maximum strain of 20%. f, Uniaxial strain–stress curve of carbon black (CB)–silicone composite and undoped neat silicone. g, Length and width of the bladder measured using Vernier calipers at different inner pressures. h, Representative optical image of a rat bladder at different inner pressures: 3.2 mm Hg (left) and 18.4 mm Hg (right) (ruler, 1 mm). i, Simulation results for the width and length of the bladder based on measured values of the inner pressure and the initial length and width.
Extended Data Fig. 3 Optical and thermal characteristics of the µ-ILEDs.
a, Schematic illustration of the µ-ILED portion of the optoelectronic stimulation and sensing module. b, Electrical and optical characteristics of a pair of µ-ILEDs. c, Measured power associated with penetration of light from a µ-ILED through the whole rat bladder (both layers) at fully inflated and empty states; additional calculations were performed on single bladder layer relaxed and manually stretched. Performed in three biologically independent samples, mean ± s.e.m. d, Results from in vivo testing of the temperature associated with operation of the µ-ILEDs for an hour. e, The material properties of the model. f, Simulated results for the temperature associated with operation of the µ-ILEDs with and without the stainless-steel substrate. g, Schematic illustration of optoelectronic stimulation and sensing module embedded in tissue with dimensions of 30 × 30 × 30 mm3 in the FEA model. h, The effect of number of elements in the FEA model on the temperature increments in vivo with power of 70 mW for steady-state analysis.
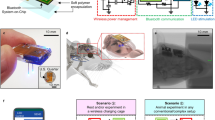
Extended Data Fig. 4 Layout and operation of the wireless control and power module.
a, Layout and component information for the WCP module. b, Normalized wireless power distribution, in dB, inside the rat cage (30 cm × 60 cm) at heights of 0 and 5 cm from the ground. c, Normalized wireless power received, in dB, at different out-of-plane orientation angles of the receiver antenna relative to the transmission antenna, for positions in a corner and at the centre of the cage. d, A plot of the supercapacitor voltage, which is proportional to the stored energy, as a function of time after deactivation of the transmission antenna while the system is otherwise fully operational.
Extended Data Fig. 5 Effect of optoelectronic stimulation and sensing module implantation or sham surgery on bladder cystometric properties, histology and animal health.
a, Representative traces of anaesthetized constant infusion (0.1 ml min–1) cystometric voiding from rats that had undergone sham surgery or CLOC device implantation. b, Quantification of peak pressure (PP), base pressure (BP), and threshold pressure (TP) indicates no significant differences between sham-operated and device-implanted groups. The data indicate no significant differences in intercontraction interval (ICI) or bladder compliance (Δvolume/Δpressure) between sham-operated and device-implanted animals (n = 6 rats per group). c, Representative H&E staining of bladder tissue in direct contact with the strain gauge and the full CLOC device in implanted rats, and the same tissue from sham-surgery rats (no overt histological differences were observed in these tissues (n = 3 for each group) in biologically independent samples with similar results). Scale bar, 100 µm. d, e, Examples (scale bar, 250 µm; d) and quantification (e) of bladder thickness, comparing device-implanted and sham-operated animals (n = 3 biologically independent tissues in each group). f, Sham-operated and device-implanted animals gained similar amounts of weight by post-operative day (POD) 7 (n = 5 biologically independent animals in each group). g, Animals from both groups ran similar distances in a novel arena (42 × 42 × 30 cm) (n = 5 biologically independent animals in each group). h, Implantation of the CLOC system did not significantly affect measurements of gait, including average run speed, run speed variation, step sequence regularity and steps per second (cadence) (n = 5 rats per group). All data represented as mean ± s.e.m analysed by two-way ANOVA with Sidak’s multiple comparisons test, unpaired t-test or Mann–Whitney test (details in Supplementary Table 1).
Extended Data Fig. 6 Bladder cystometric properties and markers of inflammation are not significantly altered by injection of HSV-eYFP compared to sham surgery.
a, Representative traces of constant infusion (0.1 ml min–1) anaesthetized (urethane) cystometric voiding from sham-injected and virus-injected (HSV-CMV-eYFP) rats. b, Quantification of peak pressure (PP), base pressure (BP), and threshold pressure (TP) indicated no significant differences between sham-injected and virus-injected groups. c, d, There were no significant differences in intercontraction interval (ICI) (c) or bladder compliance (Δvolume/Δpressure) (d) between sham-injected and virus-injected rats. e, f, No significant difference was observed in average number of mast cells (e) or degree of degranulation (f), indicating that no overt inflammatory response is detected in bladders injected with HSV-eYFP versus sham surgery 7 days after injection. n = 4 rats per group; all data mean ± s.e.m; analysed by two-way ANOVA with Sidak’s multiple comparisons test or unpaired t-test (details in Supplementary Table 1).
Extended Data Fig. 7 Activation of Arch in cultured bladder-projecting rat DRG neurons and human DRGs reduces neuronal excitability.
a, Example of a neuron transduced with HSV-Arch–eYFP (green) and identified as a neuron that projects to the bladder by labelling with DiI (red) following DiI injection into the bladder wall (7 days before). Scale bar, 20 μm; similar to results obtained from three independent experiments. b, Representative traces of DRG neuron firing properties in response to step current injection at 1–4× threshold with (green) and without (black) green light illumination (530 nm) (similar to results obtained from three independent cultures). c, d, Quantification of Arch-induced current amplitude in voltage clamp (c) and membrane hyperpolarization in current clamp (d) at peak and steady states. e, Quantification of action potential (AP) frequency with and without illumination, demonstrating light-dependent inhibition of AP firing. No light n = 13; 3.3 mW mm–2 n = 8; 10 mW mm–2 n = 5 different cells, from three biologically independent cultures; all data mean ± s.e.m.; one-way ANOVA with Dunnett’s multiple comparisons test; *P < 0.05 (further statistical details can be found in Supplementary Table 1). f, Example of a patched human DRG neuron transduced with HSV-Arch–eYFP. Scale bar, 20 μm. g, Example voltage clamp trace demonstrating the photocurrent elicited by green light (530 nm). h, Representative current clamp trace demonstrating the ability of Arch to inhibit neuronal firing in response to 3× threshold current injection. i, Current clamp traces with (green) and without (black) activation of Arch, showing a reduction in action potential firing in response to 1–4× threshold ramp currents. j, k, Quantification of light-induced current in voltage clamp (j) and hyperpolarization in current clamp (k) at peak and steady state (SS) in Arch-expressing human DRG neurons. n = 5 neurons; all data mean ± s.e.m. f–h, Repeated in 5 different cells from one culture, with similar biological results. Cartoon images in a and f are from Servier Medical Art by Servier (https://smart.servier.com/), and are covered by a Creative Commons 3.0 attribution license (https://creativecommons.org/licenses/by/3.0/). No changes were made to the original artwork.
Extended Data Fig. 8 Effect of Arch activation and CYP-induced bladder dysfunction on voiding properties.
a, b, Representative traces (a) and grouped data (b) that demonstrate a significant increase in cystometric inter-contraction interval (ICI) during green light illumination in HSV-Arch-injected animals compared to HSV-eYFP-injected controls, as defined by the strain gauge and pressure recordings. n = 4 rats per group; unpaired t-test *P < 0.05. c, Activation of Arch in bladder sensory afferents does not affect base, threshold or peak pressure. No changes were observed during bladder illumination in base pressure, threshold pressure or peak pressure in anaesthetized (urethane) cystometry (0.1 ml min–1) when comparing HSV-Arch–eYFP-injected rats to HSV-eYFP-injected rats. n = 4; unpaired t-test or Mann–Whitney test. d, Effect of Arch activation on normal voiding in awake, non-anaesthetized rats. Illumination of HSV-Arch-injected bladders (Arch denotes HSV-Arch–eYFP/LED-ON) did not significantly alter the number of voids or time to first void compared to HSV-eYFP (eYFP denotes HSV-eYFP/LED-ON) and HSV-injected/No LED groups in non-inflamed animals. n = 6; two-way ANOVA with Tukey’s multiple comparison test. e, Rats injected with CYP have significantly more voids and less time to first void during the 3 h after CYP injection as compared to baseline. n = 6; unpaired t-test or Mann–Whitney test, **P < 0.01. f, A single dose of CYP does not cause a significant increase in evoked VMR. n = 8 saline, n = 9 CYP; one-way ANOVA with Sidak’s multiple comparisons test. All data mean ± s.e.m (further statistical details can be found in Supplementary Table 1).
Extended Data Fig. 9 Automatic identification of voiding events from raw strain gauge data.
a, Diagram demonstrating the step-by-step process (top to bottom) for identifying voids from raw strain gauge data. b, Quantification of the number of missed voids, false voids, false voids per hour and per cent correct during 8 h of recording at baseline and 8 h after CYP injection using the void detection algorithm. n = 9 rats per group; mean ± s.e.m. c, Per cent change in void size after CYP administration (75 mg kg–1) (n = 11 biologically independent animals; mean ± s.e.m.). d, In vitro demonstration of the void volume threshold component of the closed-loop algorithm. With a threshold of 15 arbitrary units (AU) (20% fractional change of resistance), the closed-loop system did not activate when the void volumes were larger than 15 AU (right), while the closed-loop system was triggered and turned on the µ-ILEDs when void sizes were smaller than 15 AU (left). e, Demonstration of prolonged battery-free recording (repeated twice in independent animals with similar results).
Extended Data Fig. 10 Results from in vitro testing of the strain gauge.
a, Photograph of the in vitro setup, including a strain gauge on a mimic bladder (Ecoflex-0030) in saline solution (pH 7.4), a syringe pump (0.175 µl min–1) to control the size of the bladder, a function generator to supply power (7 Hz, 10 V) for external vibration, a pressure gauge and a digital multimeter to monitor pressure in the bladder. b, Change in resistance of the strain gauge on a mimic bladder during inflation at an inner pressure of 1 kPa, in otherwise ambient laboratory conditions. c, Similar changes during inflation with saline solution at 1 kPa. d, Change in resistance change of the strain gauge under the various conditions; vibration, stirring, and poking.
Supplementary information
Supplementary Table 1
This table contains a summary of all statistical parameters and results.
Rights and permissions
About this article
Cite this article
Mickle, A.D., Won, S.M., Noh, K.N. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019). https://doi.org/10.1038/s41586-018-0823-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0823-6
This article is cited by
-
Monolithic silicon for high spatiotemporal translational photostimulation
Nature (2024)
-
Squid-inspired and wirelessly controllable display for active camouflage in aquatic-environment
npj Flexible Electronics (2024)
-
Body-conformable light-emitting materials and devices
Nature Photonics (2024)
-
Intelligent block copolymer self-assembly towards IoT hardware components
Nature Reviews Electrical Engineering (2024)
-
Wearable and Implantable Light-Emitting Diodes and Their Biomedical Applications
Korean Journal of Chemical Engineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.