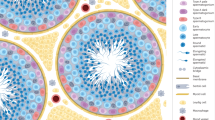
Abstract
Male factor infertility affects 50% of infertile couples worldwide; the most severe form, non-obstructive azoospermia (NOA), affects 10–15% of infertile males. Treatment for individuals with NOA is limited to microsurgical sperm extraction paired with in vitro fertilization intracytoplasmic sperm injection. Unfortunately, spermatozoa are only retrieved in ~50% of patients, resulting in live birth rates of 21–46%. Regenerative therapies could provide a solution; however, understanding the cell-type-specific mechanisms of cellular dysfunction is a fundamental necessity to develop precision medicine strategies that could overcome these abnormalities and promote regeneration of spermatogenesis. A number of mechanisms of cellular dysfunction have been elucidated in NOA testicular cells. These mechanisms include abnormalities in both somatic cells and germ cells in NOA testes, such as somatic cell immaturity, aberrant growth factor signalling, increased inflammation, increased apoptosis and abnormal extracellular matrix regulation. Future cell-type-specific investigations in identifying modulators of cellular transcription and translation will be key to understanding upstream dysregulation, and these studies will require development of in vitro models to functionally interrogate spermatogenic niche dysfunction in both somatic and germ cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vahidi, S. et al. Success rate and art outcome of microsurgical sperm extraction in non obstructive azoospermia: a retrospective study. Int. J. Reprod. Biomed. 19, 781–788 (2021).
Schlegel, P. N. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum. Reprod. 14, 131–135 (1999).
Punjani, N., Flannigan, R., Kang, C., Khani, F. & Schlegel, P. N. Quantifying heterogeneity of testicular histopathology in men with nonobstructive azoospermia. J. Urol. 206, 1268–1275 (2021).
Chiba, K., Enatsu, N. & Fujisawa, M. Management of non-obstructive azoospermia. Reprod. Med. Biol. 15, 165–173 (2016).
O’Donnell, L. et al. Endocrinology of the Male Reproductive System and Spermatogenesis. Endotext [internet] https://www.ncbi.nlm.nih.gov/books/NBK279031/ (updated 11 Jan 2017).
Sohni, A. et al. The neonatal and adult human testis defined at the single-cell level. Cell Rep. 26, 1501–1517.e4 (2019).
Guo, J. et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 28, 764–778.e4 (2021).
Hajkova, P. et al. Epigenetic reprogramming in mouse primordial germ cells. Mechanisms Dev. 117, 15–23 (2002).
Nikolic, A., Volarevic, V., Armstrong, L., Lako, M. & Stojkovic, M. Primordial germ cells: current knowledge and perspectives. Stem Cell Int. 2016, 1741072 (2016).
Guo, J. et al. The dynamic transcriptional cell atlas of testis development during human puberty. Cell Stem Cell 26, 262–273.e4 (2020).
Loveland, K. L. & Hedger, M. P. in Sertoli Cell Biology (eds Skinner, M. & Griswold, M.) 201–232 (Elsevier, 2015).
Le Magueresse-Battistoni, B. Serine proteases and serine protease inhibitors in testicular physiology: the plasminogen activation system. Reproduction 134, 721–729 (2007).
Charron, M. & Wright, W. W. in: Skinner, M. & Griswold, M. (eds) Sertoli Cell Biology. 121–152 (Elsevier, 2005).
França, L. R., Hess, R. A., Dufour, J. M., Hofmann, M. C. & Griswold, M. D. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology 4, 189–212 (2016).
Griswold, M. D. Protein secretions of Sertoli cells. Int. Rev. Cytol. 110, 133–156 (1988).
Peng, Y. J. et al. Sertoli cells are the source of stem cell factor for spermatogenesis. Development 150, dev200706 (2023).
Washburn, R. L., Hibler, T., Kaur, G. & Dufour, J. M. Sertoli cell immune regulation: a double-edged sword. Front. Immunol. 13, 913502 (2022).
Hofmann, M.-C. & McBeath, E. Sertoli cell-germ cell interactions within the niche: paracrine and juxtacrine molecular communications. Front. Endocrinol. 13, 897062 (2022).
Nakanishi, Y. & Shiratsuchi, A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biol. Pharm. Bull. 27, 13–16 (2004).
Jensen, C. F. et al. Sertoli and germ cells within atrophic seminiferous tubules of men with non-obstructive azoospermia. Front. Endocrinol. 13, 825904 (2022).
Aumüller, G., Schulze, C. & Viebahn, C. Intermediate filaments in Sertoli cells. Microsc. Res. Tech. 20, 50–72 (1992).
Steger, K. Reversion of the differentiated phenotype and maturation block in Sertoli cells in pathological human testis. Hum. Reprod. 14, 136–143 (1999).
Zhao, L. Y. et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat. Commun. 11, 5683 (2020).
Boukari, K. et al. Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-mullerian hormone repression during early human testis development. J. Clin. Endocrinol. Metab. 94, 1818–1825 (2009).
Yao, C. et al. MiRNA-133B promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget 7, 2201–2219 (2016).
Yang, C. et al. Mir-202-3p regulates Sertoli cell proliferation, synthesis function, and apoptosis by targeting LRP6 and cyclin D1 of wnt/β-catenin signaling. Mol. Ther. Nucleic Acids 14, 1–19 (2019).
Dabaja, A. A. et al. Possible germ cell-Sertoli cell interactions are critical for establishing appropriate expression levels for the Sertoli cell-specific microRNA, mir-202-5p, in human testis. Basic. Clin. Androl. 25, 2 (2015).
Lian, J. et al. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod. Biol. Endocrinol. 7, 13 (2009).
Mak, I. W. Y., Singh, S., Turcotte, R. & Ghert, M. The epigenetic regulation of SOX9 by mir-145 in human chondrosarcoma. J. Cell. Biochem. 116, 37–44 (2014).
Pan, S., Bearelly, P. & Oates, R. D. Fertility in men with Klinefelter syndrome and Y chromosome microdeletions: an update. Curr. Opin. Endocr. Metab. Res. 6, 21–28 (2019).
Mahyari, E. et al. Comparative single-cell analysis of biopsies clarifies pathogenic mechanisms in Klinefelter syndrome. Am. J. Hum. Gen. 108, 1924–1945 (2021).
Wang, M. et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 23, 599–614.e4 (2018).
Alfano, M. et al. Aging, inflammation and DNA damage in the somatic testicular niche with idiopathic germ cell aplasia. Nat. Commun. 12, 5205 (2021).
Abraham, R. T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes. Dev. 15, 2177–2196 (2001).
Nie, X. et al. Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev. Cell 57, 1160–1176.e5 (2022).
Zhang, X. et al. Comprehensive analysis of the association between human non-obstructive azoospermia and plasticisers via single-cell and traditional RNA sequencing methods. Exposure Health 14, 829–842 (2022).
Chen, S. et al. Human obstructive (postvasectomy) and nonobstructive azoospermia — insights from scRNA-seq and transcriptome analysis. Genes. Dis. 9, 766–776 (2022).
Mruk, D. D. & Cheng, C. Y. The mammalian blood-testis barrier: its biology and regulation. Endocr. Rev. 36, 564–591 (2015).
Aydin, S. et al. Evaluation of blood–testis barrier integrity in terms of adhesion molecules in nonobstructive azoospermia. Andrologia 52, e13636 (2020).
Chiba, K., Yamaguchi, K., Ando, M., Miyake, H. & Fujisawa, M. Expression pattern of testicular claudin-11 in infertile men. Urology 80, 1161.e13-7 (2012).
Zhu, R. et al. The alteration of Rhoa geranylgeranylation and Ras farnesylation breaks the integrity of the blood–testis barrier and results in hypospermatogenesis. Cell Death Dis. 10, 450 (2019).
Sun, H. et al. Presence of metastasis-associated protein 1 in Sertoli cells is required for proper contact between Sertoli cells and adjacent germ cells. Urology 81, 66–73 (2013).
Sen, N., Gui, B. & Kumar, R. Physiological functions of MTA family of proteins. Cancer Metastasis Rev. 33, 869–877 (2014).
Xiang, W. et al. CR16 forms a complex with N-WASP in human testes. Cell Tissue Res. 344, 519–526 (2011).
Fargeas, C. A. et al. Identification of novel prominin-1/CD133 splice variants with alternative C-termini and their expression in epididymis and testis. J. Cell Sci. 117, 4301–4311 (2004).
Yukselten, Y., Aydos, O. S., Sunguroglu, A. & Aydos, K. Investigation of CD133 and CD24 as candidate azoospermia markers and their relationship with spermatogenesis defects. Gene 706, 211–221 (2019).
Wu, X. et al. Role of laminin and collagen chains in human spermatogenesis — insights from studies in rodents and scRNA-seq transcriptome profiling. Semin. Cell Dev. Biol. 121, 125–132 (2022).
Aydos, O. S., Yukselten, Y., Ozkavukcu, S., Sunguroglu, A. & Aydos, K. ADAMTS1 and ADAMTS5 metalloproteases produced by Sertoli cells: a potential diagnostic marker in azoospermia. Syst. Biol. Reprod. Med. 65, 29–38 (2018).
Longin, J. & Le Magueresse-Battistoni, B. Evidence that MMP-2 and Timp-2 are at play in the FSH-induced changes in Sertoli cells. Mol. Cell. Endocrinol. 189, 25–35 (2002).
Robinson, L. L. L., Sznajder, N. A., Riley, S. C. & Anderson, R. A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human fetal testis and ovary. Mol. Hum. Reprod. 7, 641–648 (2001).
Walczak-Jędrzejowska, R. et al. Expression of G-protein-coupled estrogen receptor (GPER) in whole testicular tissue and laser-capture microdissected testicular compartments of men with normal and aberrant spermatogenesis. Biology 11, 373 (2022).
Zhao, Y., Wu, T.-Y., Zhao, M.-F. & Li, C.-J. The balance of protein farnesylation and geranylgeranylation during the progression of nonalcoholic fatty liver disease. J. Biol. Chem. 295, 5152–5162 (2020).
Wang, M. & Casey, P. J. Protein prenylation: unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol. 17, 110–122 (2016).
Wang, X.-X. et al. Altered protein prenylation in Sertoli cells is associated with adult infertility resulting from childhood mumps infection. J. Exp. Med. 210, 1559–1574 (2013).
Azizi, H., Hashemi Karoii, D. & Skutella, T. Whole exome sequencing and in silico analysis of human Sertoli in patients with non-obstructive azoospermia. Int. J. Mol. Sci. 23, 12570 (2022).
Ma, M. et al. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype. Hum. Reprod. 28, 1863–1873 (2013).
Ohta, H., Yomogida, K., Dohmae, K. & Nishimune, Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development 127, 2125–2131 (2000).
Meng, X. et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493 (2000).
He, Z., Kokkinaki, M. & Dym, M. Signaling molecules and pathways regulating the fate of spermatogonial stem cells. Microsc. Res. Tech. 72, 586–595 (2009).
Chui, K. et al. Characterization and functionality of proliferative human Sertoli cells. Cell Transpl. 20, 619–635 (2011).
Fujita, K. et al. Expression of inhibin α, glial cell line-derived neurotrophic factor and stem cell factor in Sertoli cell-only syndrome: relation to successful sperm retrieval by microdissection testicular sperm extraction. Hum. Reprod. 20, 2289–2294 (2005).
Hai, Y. et al. BMP4 promotes human Sertoli cell proliferation via Smad1/5 and ID2/3 pathway and its abnormality is associated with azoospermia. Discov. Med. 19 105, 311–325 (2015).
Tian, R. et al. Fibroblast growth factor-5 promotes spermatogonial stem cell proliferation via ERK and Akt activation. Stem Cell Res. Ther. 10, 40 (2019).
Kato, Y., Shiraishi, K. & Matsuyama, H. Expression of testicular androgen receptor in non-obstructive azoospermia and its change after hormonal therapy. Andrology 2, 734–740 (2014).
Kuyucu, Y. et al. Immunohistochemical examination of androgen receptor and estrogen receptor alpha expressions in obstructive and non-obstructive azoospermia. Syst. Biol. Reprod. Med. 67, 463–470 (2021).
Lan, K.-C. et al. Up-regulation of SOX9 in Sertoli cells from testiculopathic patients accounts for increasing Anti-Mullerian hormone expression via impaired androgen receptor signaling. PLoS One 8, e76303 (2013).
Almeida, C. et al. Caspase signalling pathways in human spermatogenesis. J. Assist. Reprod. Genet. 30, 487–495 (2013).
Kim, S.-K., Yoon, Y.-D., Park, Y.-S., Seo, J. T. & Kim, J.-H. Involvement of the FAS–FAS ligand system and active caspase-3 in abnormal apoptosis in human testes with maturation arrest and Sertoli cell-only syndrome. Fertil. Steril. 87, 547–553 (2007).
Sinha Hikim, A. P. et al. Deciphering the pathways of germ cell apoptosis in the testis. J. Steroid Biochem. Mol. Biol. 85, 175–182 (2003).
Vogl, A. W., Vaid, K. S. & Guttman, J. A. The Sertoli cell cytoskeleton. Adv. Exp. Med. Biol. 636, 186–211 (2009).
Wu, X. et al. Defects of microtubule cytoskeletal organization in NOA Human testes. Reprod. Biol. Endocrinol. 20, 154 (2022).
Nogales, E. Structural insights into microtubule function. Annu. Rev. Biochem. 69, 277–302 (2000).
Tang, E. I., Mok, K.-W., Lee, W. M. & Cheng, C. Y. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats: an in vitro study. Endocrinology 156, 680–693 (2014).
Wen, Q. et al. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am. J. Physiol. Endocrinol. Metab. 315, E924–E948 (2018).
Wu, S. et al. KIF15 supports spermatogenesis via its effects on Sertoli cell microtubule, actin, vimentin, and septin cytoskeletons. Endocrinology 162, bqab010 (2021).
Voutilainen, R. Differentiation of the fetal gonad. Horm. Res. 38, 66–71 (1992).
Carreau S. in: Payne, A. H. & Hardy, M. P. (eds) The Leydig Cell in Health and Disease. 189-195 (Contemporary Endocrinology, 2007).
Sokwala, S. in: Rehman, R. & Sheikh, A. (eds) Subfertility: Recent Advances for Management and Prevention. 39-64 (Elsevier, 2021).
Walker, W. H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 1, 116–120 (2011).
Walker, W. H. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 365, 1557–1569 (2010).
Rettew, J. A., Huet-Hudson, Y. M. & Marriott, I. Testosterone reduces macrophage expression in the mouse of Toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 78, 432–437 (2008).
Griffeth, R. J., Bianda, V. & Nef, S. The emerging role of insulin-like growth factors in testis development and function. Basic Clin. Androl. 24, 12 (2014).
Jaworowski, A., Wilson, N. J., Christy, E., Byrne, R. & Hamilton, J. A. Roles of the mitogen-activated protein kinase family in macrophage responses to colony stimulating factor-1 addition and withdrawal. J. Biol. Chem. 274, 15127–15133 (1999).
Mohamad, N. V., Soelaiman, I.-N. & Chin, K.-Y. A concise review of testosterone and bone health. Clin. Interv. Aging 11, 1317–1324 (2016).
Bachman, E. et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A. Biol. Sci. Med. Sci. 69, 725–735 (2013).
Mahran, A. M., Elgamal, D. A., Ghafeer, H. H., Abdel-Maksoud, S. A. & Farrag, A. A. Histological alterations in Leydig cells and macrophages in azoospermic men. Andrologia 49, https://doi.org/10.1111/and.12714 (2016).
Hauptman, D. et al. Leydig cells in patients with non-obstructive azoospermia: do they really proliferate? Life 11, 1266 (2021).
Holm, M., Rajpert-De Meyts, E., Andersson, A.-M. & Skakkebaek, N. E. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. J. Pathol. 199, 378–386 (2003).
Adamczewska, D., Słowikowska-Hilczer, J. & Walczak-Jędrzejowska, R. The fate of Leydig cells in men with spermatogenic failure. Life 12, 570 (2022).
Tang, X.-J. et al. Single-cell transcriptomics-based study of transcriptional regulatory features in the non-obstructive azoospermia testis. Front. Genet 13, 875762 (2022).
Ghaffari Novin, M., Mirfakhraie, R. & Nazarian, H. Aberrant Wnt/β-catenin signaling pathway in testis of azoospermic men. Adv. Pharm. Bull. 5, 373–377 (2015).
Goluža, T. et al. Macrophages and Leydig cells in testicular biopsies of azoospermic men. Biomed. Res. Int. 2014, 828697 (2014).
Shiraishi, K., Oka, S. & Matsuyama, H. Testicular testosterone and estradiol concentrations and aromatase expression in men with nonobstructive azoospermia. J. Clin. Endocrinol. Metab. 106, 1803–1815 (2020).
Lardone, M. C. et al. Leydig cell dysfunction is associated with post-transcriptional deregulation of CYP17A1 in men with Sertoli cell-only syndrome. Mol. Hum. Reprod. 24, 203–210 (2018).
Sato, Y. et al. A change in the steroid metabolic pathway in human testes showing deteriorated spermatogenesis. Reprod. Biol. 20, 210–219 (2020).
Sato, Y., Nozawa, S., Yoshiike, M., Otoi, T. & Iwamoto, T. Glycoconjugates recognized by peanut agglutinin lectin in the inner acellular layer of the lamina propria of seminiferous tubules in human testes showing impaired spermatogenesis. Hum. Reprod. 27, 659–668 (2012).
Yanaihara, T. & Troen, P. Studies of the human testis. I. Biosynthetic pathways for androgen formation in human testicular tissue in vitro. J. Clin. Endocrinol. Metab. 34, 783–792 (1972).
Bar-Shira Maymon, B. et al. Detection of calretinin expression in abnormal immature Sertoli cells in non-obstructive azoospermia. Acta Histochem. 107, 105–112 (2005).
Spiess, A.-N. et al. Cross-platform gene expression signature of human spermatogenic failure reveals inflammatory-like response. Hum. Reprod. 22, 2936–2946 (2007).
Wren, A. M. et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141, 4325–4328 (2000).
Ishikawa, T., Fujioka, H., Ishimura, T., Takenaka, A. & Fujisawa, M. Ghrelin expression in human testis and serum testosterone level. J. Androl. 28, 320–324 (2006).
Ozkanli, S. et al. The ghrelin and orexin activity in testicular tissues of patients with idiopathic non-obstructive azoospermia. Kaohsiung J. Med. Sci. 34, 564–568 (2018).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Domke, L. M. & Franke, W. W. The cell–cell junctions of mammalian testes: II. the lamellar smooth muscle monolayer cells of the peritubular wall are laterally connected by vertical adherens junctions — a novel architectonic cell–cell junction system. Cell Tissue Res. 375, 451–482 (2018).
Flenkenthaler, F. et al. Secretome analysis of testicular peritubular cells: a window into the human testicular microenvironment and the spermatogonial stem cell niche in man. J. Proteome Res. 13, 1259–1269 (2014).
Spinnler, K., Kohn, F. M., Schwarzer, U. & Mayerhofer, A. Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum. Reprod. 25, 2181–2187 (2010).
Hedger, M. P. & Winnall, W. R. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol. Cell. Endocrinol. 359, 30–42 (2012).
Mayerhofer, A. Human testicular peritubular cells: more than meets the eye. Reproduction 145, R107–R116 (2013).
Di Persio, S. et al. Single-cell RNA-seq unravels alterations of the human spermatogonial stem cell compartment in patients with impaired spermatogenesis. Cell Rep. Med. 2, 100395 (2021).
Pop, O. T. et al. Histological and ultrastructural analysis of the seminiferous tubule wall in ageing testis. Rom. J. Morphol. Embryol. 52, 241–248 (2011).
Sato, Y., Nozawa, S. & Iwamoto, T. Study of spermatogenesis and thickening of lamina propria in the human seminiferous tubules. Fertil. Steril. 90, 1310–1312 (2008).
Lan, K.-C. et al. Expression of androgen receptor co-regulators in the testes of men with azoospermia. Fertil. Steril. 89, 1397–1405 (2008).
Kikuchi, H. et al. Ara54 is involved in transcriptional regulation of the cyclin D1 gene in human cancer cells. Carcinogenesis 28, 1752–1758 (2007).
Hasirci, E. et al. Distribution and number of Cajal-like cells in testis tissue with azoospermia. Kaohsiung J. Med. Sci. 33, 181–186 (2017).
Briggs Boedtkjer, D. et al. Identification of interstitial Cajal-like cells in the human thoracic duct. Cell Tissues Organs 197, 145–158 (2012).
Mossadegh-Keller, N. & Sieweke, M. H. Testicular macrophages: guardians of fertility. Cell. Immunol. 330, 120–125 (2018).
Mossadegh-Keller, N. et al. Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 214, 2829–2841 (2017).
Chen, J.-J., Lukyanenko, Y. & Hutson, J. C. 25-hydroxycholesterol is produced by testicular macrophages during the early postnatal period and influences differentiation of Leydig cells in vitro. Biol. Reprod. 66, 1336–1341 (2002).
Nes, W. D. et al. Identification of the lipophilic factor produced by macrophages that stimulates steroidogenesis. Endocrinology 141, 953–958 (2000).
Hales, D. B. Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol. 57, 3–18 (2002).
Kostić, T., Andrić, S., Kovačević, R. & Marić, D. The involvement of nitric oxide in stress-impaired testicular steroidogenesis. Eur. J. Pharmacol. 346, 267–273 (1998).
DeFalco, T. et al. Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep. 12, 1107–1119 (2015).
Bhushan, S. et al. Immune cell subtypes and their function in the testis. Front. Immunol. 11, 583304 (2020).
Gong, J., Zeng, Q., Yu, D. & Duan, Y.-G. T lymphocytes and testicular immunity: a new insight into immune regulation in testes. Int. J. Mol. Sci. 22, 57 (2020).
Ruiz-Argüelles, A. in: Anaya JM et al. (eds) Autoimmunity: From Bench to Bedside. 113–123 (El Rosario University Press, 2013).
Krystel-Whittemore, M., Dileepan, K. N. & Wood, J. G. Mast cell: a multi-functional master cell. Front. Immunol. 6, 620 (2016).
Payne, V. & Kam, P. C. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia 59, 695–703 (2004).
Haidl, G. et al. The role of mast cells in male infertility. Expert. Rev. Clin. Immunol. 7, 627–634 (2011).
Abdel-Hamid, A. A., Atef, H., Zalata, K. R. & Abdel-Latif, A. Correlation between testicular mast cell count and spermatogenic epithelium in non-obstructive azoospermia. Int. J. Exp. Pathol. 99, 22–28 (2018).
Klein, B. et al. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum. Reprod. 31, 2192–2202 (2016).
O’Bryan, M. K. & Hedger, M. P. Inflammatory networks in the control of spermatogenesis. Adv. Exp. Med. Biol. 636, 92–114 (2009).
Loveland, K. L. et al. Cytokines in male fertility and reproductive pathologies: immunoregulation and beyond. Front. Endocrinol. 8, 307 (2017).
Bott, R. C., McFee, R. M., Clopton, D. T., Toombs, C. & Cupp, A. S. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the RAT1. Biol. Reprod. 75, 56–67 (2006).
Ergün, S., Stingl, J. & Holstein, A. F. Microvasculature of the human testis in correlation to Leydig cells and seminiferous tubules. Andrologia 26, 255–262 (2009).
Bhang, D. H. et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat. Commun. 9, 4379 (2018).
De Rooij, D. G. & Russell, L. D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 21, 776–798 (2013).
Guo, J. et al. The adult human testis transcriptional cell atlas. Cell Res. 28, 1141–1157 (2018).
Schwarzacher, T. Meiosis, recombination and chromosomes: a review of gene isolation and fluorescent in situ hybridization data in plants. J. Exp. Bot. 54, 11–23 (2003).
Cheng, C. Y. & Mruk, D. D. The blood-testis barrier and its implications for male contraception. Pharm. Rev. 64, 16–64 (2011).
Neto, F. T., Bach, P. V., Najari, B. B., Li, P. S. & Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 59, 10–26 (2016).
Yoshida, K. et al. Mapping of histone-binding sites in histone replacement-completed spermatozoa. Nat. Commun. 9, 3885 (2018).
Zhou, Y. et al. An epididymis-specific secretory protein HongrES1 critically regulates sperm capacitation and male fertility. PLoS One 3, e4106 (2008).
Du, L., Chen, W., Li, C., Cui, Y. & He, Z. RNF144B stimulates the proliferation and inhibits the apoptosis of human spermatogonial stem cells via the FCER2/NOTCH2/HES1 pathway and its abnormality is associated with azoospermia. J. Cell. Physiol. 237, 3565–3577 (2022).
He, H. et al. The novel key genes of non-obstructive azoospermia affect spermatogenesis: transcriptomic analysis based on RNA-seq and scRNA-seq data. Front. Genet. 12, 608629 (2021).
Yu, D., Lim, J., Wang, X., Liang, F. & Xiao, G. Enhanced construction of gene regulatory networks using hub gene information. BMC Bioinforma. 18, 186 (2017).
Emiralioğlu, N. et al. Genotype and phenotype evaluation of patients with primary ciliary dyskinesia: first results from Turkey. Pediatr. Pulmonol. 55, 383–393 (2019).
Wang, X. et al. Testis-specific serine/threonine protein kinase 4 (TSSK4) phosphorylates ODF2 at ser-76. Sci. Rep. 6, 22861 (2016).
Wang, X.-L., Wei, Y.-H., Fu, G.-L. & Yu, L. Testis specific serine/threonine protein kinase 4 (TSSK4) leads to cell apoptosis relying on its kinase activity. J. Huazhong Univ. Sci. Tech. Med. Sci. 35, 235–240 (2015).
Prabhu, S. M. et al. Expression of c-kit receptor mRNA and protein in the developing, adult and irradiated rodent testis. Reproduction 131, 489–499 (2006).
Mithraprabhu, S. & Loveland, K. L. Control of KIT signalling in male germ cells: what can we learn from other systems? Reproduction 138, 743–757 (2009).
Fu, H. et al. PAK1 promotes the proliferation and inhibits apoptosis of human spermatogonial stem cells via PDK1/KDR/Znf367 and ERK1/2 and Akt pathways. Mol. Ther. Nucleic Acids 12, 769–786 (2018).
Wang, M. et al. Deciphering the autophagy regulatory network via single-cell transcriptome analysis reveals a requirement for autophagy homeostasis in spermatogenesis. Theranostics 11, 5010–5027 (2021).
Sahani, M. H., Itakura, E. & Mizushima, N. Expression of the autophagy substrate SQSTM1/P62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 10, 431–441 (2014).
Peeters, J. G. C. et al. Transcriptional and epigenetic profiling of nutrient-deprived cells to identify novel regulators of autophagy. Autophagy 15, 98–112 (2018).
Liu, C. et al. The interaction between cancer stem cell marker CD133 and SRC protein promotes focal adhesion kinase (FAK) phosphorylation and cell migration. J. Biol. Chem. 291, 15540–15550 (2016).
Wyrwoll, M. J. et al. Analysis of copy number variation in men with non‐obstructive azoospermia. Andrology 10, 1593–1604 (2022).
Aoki, N. & Matsui, Y. Comprehensive analysis of mouse cancer/testis antigen functions in cancer cells and roles of TEKT5 in cancer cells and testicular germ cells. Mol. Cell. Biol. 39, e00154-19 (2019).
Rastgar Rezaei, Y. et al. MicroRNAs in the pathogenesis of non-obstructive azoospermia: the underlying mechanisms and therapeutic potentials. Syst. Biol. Reprod. Med. 67, 337–353 (2021).
Zhou, F. et al. miRNA-122-5p stimulates the proliferation and DNA synthesis and inhibits the early apoptosis of human spermatogonial stem cells by targeting CBL and competing with lncRNA Casc7. Aging 12, 25528–25546 (2020).
Jaruzelska, J. et al. Conservation of a Pumilio-Nanos complex from Drosophila germ plasm to human germ cells. Dev. Genes. Evol. 213, 120–126 (2003).
Kusz-Zamelczyk, K. et al. Mutations of NANOS1, a human homologue of the Drosophila morphogen, are associated with a lack of germ cells in testes or severe oligo-astheno-teratozoospermia. J. Med. Genet. 50, 187–193 (2013).
Huang, Y., Bai, J. Y. & Ren, H. T. PiRNAs biogenesis and its functions. Bioorg. Khim. 40, 320–326 (2014).
Nagirnaja, L. et al. Variant pnldc1, defective piRNA processing, and azoospermia. N. Engl. J. Med. 385, 707–719 (2021).
Nishimura, T. et al. PNLDC1, mouse pre‐piRNA trimmer, is required for meiotic and post‐meiotic male germ cell development. EMBO Rep. 19, e44957 (2018).
Nagirnaja, L. et al. Diverse monogenic subforms of human spermatogenic failure. Nat. Commun. 13, 7953 (2022).
Shoji, M. et al. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev. Cell 17, 775–787 (2009).
Arafat, M. et al. Mutation in TDRD9 causes non-obstructive azoospermia in infertile men. J. Med. Genet. 54, 633–639 (2017).
Kamaliyan, Z., Pouriamanesh, S., Amin-Beidokhti, M., Rezagholizadeh, A. & Mirfakhraie, R. HIWI2 rs508485 polymorphism is associated with non-obstructive azoospermia in Iranian patients. Rep. Biochem. Mol. Biol. 5, 108–111 (2017).
Gu, A. et al. Genetic variants in Piwi-interacting RNA pathway genes confer susceptibility to spermatogenic failure in a Chinese population. Hum. Reprod. 25, 2955–2961 (2010).
Kamaliyan, Z., Pouriamanesh, S., Soosanabadi, M., Gholami, M. & Mirfakhraie, R. Investigation of piwi-interacting RNA pathway genes role in idiopathic non-obstructive azoospermia. Sci. Rep. 8, 142 (2018).
Francavilla, S. Ultrastructural analysis of chromatin defects in testicular spermatids in azoospermic men submitted to Tese-ICSI. Hum. Reprod. 16, 1440–1448 (2001).
Adiga, S. K. et al. Reduced expression of DNMT3B in the germ cells of patients with bilateral spermatogenic arrest does not lead to changes in the global methylation status. Mol. Hum. Reprod. 17, 545–549 (2011).
Laurentino, S. et al. High-resolution analysis of germ cells from men with sex chromosomal aneuploidies reveals normal transcriptome but impaired imprinting. Clin. Epigenetics 11, 127 (2019).
Salido, E. C., Yen, P. H., Mohandas, T. K. & Shapiro, L. J. Expression of the X-inactivation-associated gene XIST during spermatogenesis. Nat. Genet. 2, 196–199 (1992).
Uysal, F., Akkoyunlu, G. & Ozturk, S. Decreased expression of DNA methyltransferases in the testes of patients with non-obstructive azoospermia leads to changes in global DNA methylation levels. Reprod. Fertil. Dev. 31, 1386 (2019).
Cui, X. et al. DNA methylation in spermatogenesis and male infertility. Exp. Ther. Med. 12, 1973–1979 (2016).
Han, F. et al. Epigenetic inactivation of SOX30 is associated with male infertility and offers a therapy target for non-obstructive azoospermia. Mol. Ther. Nucleic Acids 19, 72–83 (2020).
Han, F. et al. Epigenetic regulation of Sox30 is associated with testis development in mice. PLoS One 9, e97203 (2014).
Khazamipour, N., Noruzinia, M., Fatehmanesh, P., Keyhanee, M. & Pujol, P. MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: the role of epigenetics in male infertility. Hum. Reprod. 24, 2361–2364 (2009).
Uhlen, M. et al. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 28, 1248–1250 (2010).
Heyn, H. et al. Epigenetic disruption of the PIWI pathway in human spermatogenic disorders. PLoS One 7, e47892 (2012).
Bak, C. W., Yoon, T.-K. & Choi, Y. Functions of PIWI proteins in spermatogenesis. Clin. Exp. Reprod. Med. 38, 61 (2011).
Hadziselimovic, F., Hadziselimovic, N. O., Demougin, P., Krey, G. & Oakeley, E. Piwi-pathway alteration induces LINE-1 transposon derepression and infertility development in cryptorchidism. Sex. Dev. 9, 98–104 (2015).
Di Persio, S. et al. Whole-genome methylation analysis of testicular germ cells from cryptozoospermic men points to recurrent and functionally relevant DNA methylation changes. Clin. Epigenet. 13, 160 (2021).
Niu, Y. et al. N6-methyl-adenosine (M6A) in RNA: an old modification with a novel epigenetic function. Genomics Proteom. Bioinforma. 11, 8–17 (2013).
Cai, Z., Niu, Y. & Li, H. RNA N6-methyladenosine modification, spermatogenesis, and human male infertility. Mol. Hum. Reprod. 27, gaab020 (2021).
Ozturk, S. & Uysal, F. Potential roles of the poly(A)-binding proteins in translational regulation during spermatogenesis. J. Reprod. Dev. t 64, 289–296 (2018).
Ozturk, S. et al. The poly(A)-binding protein genes, EPAB, PABPC1, and PABPC3 are differentially expressed in infertile men with non-obstructive azoospermia. J. Assist. Reprod. Genet. 33, 335–348 (2016).
Hammoud, S., Emery, B. R., Dunn, D., Weiss, R. B. & Carrell, D. T. Sequence alterations in the YBX2 gene are associated with male factor infertility. Fertil. Steril. 91, 1090–1095 (2009).
Yang, J. et al. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc. Natl Acad. Sci. USA 102, 5755–5760 (2005).
Yang, J., Morales, C. R., Medvedev, S., Schultz, R. M. & Hecht, N. B. In the absence of the mouse DNA/RNA-binding protein MSY2, messenger RNA instability leads to spermatogenic arrest. Biol. Reprod. 76, 48–54 (2007).
McLaughlin, E. A., Sutherland, J. M., Siddall, N. A. & Hime, G. R. RNA binding proteins in spermatogenesis: an in depth focus on the Musashi family. Asian J. Androl. 17, 529 (2015).
Tesarik, J. et al. Differentiation of spermatogenic cells during in-vitro culture of testicular biopsy samples from patients with obstructive azoospermia: effect of recombinant follicle stimulating hormone. Hum. Reprod. 13, 2772–2781 (1998).
Tesarik, J., Guido, M., Mendoza, C. & Greco, E. Human spermatogenesis in vitro: respective effects of follicle-stimulating hormone and testosterone on meiosis, spermiogenesis, and Sertoli cell apoptosis. J. Clin. Endocrinol. Metab. 83, 4467–4473 (1998).
Tesarik, J., Bahceci, M., Özcan, C., Greco, E. & Mendoza, C. Restoration of fertility by in-vitro spermatogenesis. Lancet 353, 555–556 (1999).
Tanaka, A. et al. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil. Steril. 79, 795–801 (2003).
Cremades, N. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum. Reprod. 14, 1287–1293 (1999).
Lee, J.-H. et al. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 87, 824–833 (2007).
Skylar-Scott, M. A. et al. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 6, 449–462 (2022).
Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019).
Ji, S. & Guvendiren, M. Complex 3D bioprinting methods. APL Bioeng. 5, 011508 (2021).
Kang, H.-W. et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotech. 34, 312–319 (2016).
Robinson, M., Bedford, E., Witherspoon, L., Willerth, S. M. & Flannigan, R. Using clinically derived human tissue to 3-dimensionally bioprint personalized testicular tubules for in vitro culturing: first report. F. S Sci. 3, 130–139 (2022).
Robinson, M., Sparanese, S., Witherspoon, L. & Flannigan, R. Human in vitro spermatogenesis as a regenerative therapy — where do we stand? Nat. Rev. Urol. 20, 461–479 (2023).
Schiebinger, G. et al. Optimal-transport analysis of single-cell gene expression identifies developmental trajectories in reprogramming. Cell 176, 928–943.e22 (2019).
Cinà, D. P., Phillips, D. & Flannigan, R. CRISPR/Cas9 in male factor infertility. Curr. Tissue Microenviron. Rep. 1, 89–97 (2020).
Krausz, C. & Cioppi, F. Genetic factors of non-obstructive azoospermia: consequences on patients’ and offspring health. J. Clin. Med. 10, 4009 (2021).
Zhang, L. et al. c-kit and its related genes in spermatogonial differentiation. Spermatogenesis 1, 186–194 (2011).
Li, X., Sun, T., Wang, X., Tang, J. & Liu, Y. Restore natural fertility of KITW/Kitwv mouse with nonobstructive azoospermia through gene editing on SSCs mediated by CRISPR-Cas9. Stem Cell Res. Ther. 10, 271 (2019).
Gondo, Y. Trends in large-scale mouse mutagenesis: from genetics to functional genomics. Nat. Rev. Genet. 9, 803–810 (2008).
Yazawa, T. et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology 147, 4104–4111 (2006).
Crawford, P. A., Sadovsky, Y. & Milbrandt, J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol. Cell. Biol. 17, 3997–4006 (1997).
Jadhav, U. & Jameson, J. L. Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage. Endocrinology 152, 2870–2882 (2011).
Sonoyama, T. et al. Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells. Endocrinology 153, 4336–4345 (2012).
Yang, Y. et al. Directed mouse embryonic stem cells into Leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cell Dev. 24, 459–470 (2015).
Shin, E.-Y., Park, S., Choi, W. Y. & Lee, D. R. Rapid differentiation of human embryonic stem cells into testosterone-producing Leydig cell-like cells in vitro. Tissue Eng. Regen. Med. 18, 651–662 (2021).
Robinson, M. et al. Differentiation of peritubular myoid‐like cells from human induced pluripotent stem cells. Adv. Biol. 7, e2200322 (2023). 2200322.
Rodríguez Gutiérrez, D., Eid, W. & Biason-Lauber, A. A human gonadal cell model from induced pluripotent stem cells. Front. Genet. 9, 498 (2018).
Riggs, J. W. et al. Induced pluripotency and oncogenic transformation are related processes. Stem Cell Dev. 22, 37–50 (2013).
Maan, Z., Masri, N. Z. & Willerth, S. M. Smart bioinks for the printing of human tissue models. Biomolecules 12, 141 (2022).
Ramsoomair, C. K., Alver, C. G., Flannigan, R., Ramasamy, R. & Agarwal, A. Spermatogonial stem cells and in vitro spermatogenesis: how far are we from a human testis on a chip? Eur. Urol. Focus. 9, 46–48 (2023).
Yuan, Y. et al. In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res. 30, 244–255 (2020).
Loai, S. et al. Clinical perspectives on 3D bioprinting paradigms for regenerative medicine. Regen. Med. Front. https://doi.org/10.20900/rmf20190004 (2019).
Acknowledgements
The authors thank the American Urologic Association Rising Star Award, Michael Smith Health Research Foundation Health Professional Investigator and Vancouver Coastal Health Research Institute for support related to R.F.’s work in this field.
Author information
Authors and Affiliations
Contributions
A.P., S.S. and R.F. researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Ranjith Ramasamy, Elizabeth Bromfield and Kenneth Aston for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Piechka, A., Sparanese, S., Witherspoon, L. et al. Molecular mechanisms of cellular dysfunction in testes from men with non-obstructive azoospermia. Nat Rev Urol 21, 67–90 (2024). https://doi.org/10.1038/s41585-023-00837-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-023-00837-9