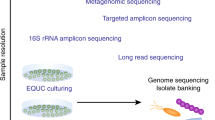
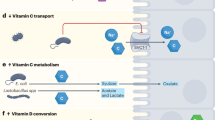
Abstract
Kidney stone disease affects ~10% of the global population and the incidence continues to rise owing to the associated global increase in the incidence of medical conditions associated with kidney stone disease including, for example, those comprising the metabolic syndrome. Considering that the intestinal microbiome has a substantial influence on host metabolism, that evidence has suggested that the intestinal microbiome might have a role in maintaining oxalate homeostasis and kidney stone disease is unsurprising. In addition, the discovery that urine is not sterile but, like other sites of the human body, harbours commensal bacterial species that collectively form a urinary microbiome, is an additional factor that might influence the induction of crystal formation and stone growth directly in the kidney. Collectively, the microbiomes of the host could influence kidney stone disease at multiple levels, including intestinal oxalate absorption and direct crystal formation in the kidneys.
Key points
-
Composition of the intestinal microbiome influences host metabolism and overall health.
-
Patients with recurrent kidney stone disease have a disrupted intestinal microbiome composition, including reduced overall abundance of butyrate-producing species.
-
Butyrate is a key short-chain fatty acid responsible for overall intestinal epithelial integrity and health, a major determinant affecting the absorption of oxalate and other ions relevant to kidney stone disease.
-
Long-term history of antibiotic use is associated with an antibiotics-driven shift in intestinal microbiome composition and increased risk of kidney stone disease.
-
Members of the microbiome have a role in different processes of stone formation, including intestinal oxalate absorption and crystal formation.
-
Urine also has a native urinary microbiome, the composition of which is better able to differentiate between stone and non-stone formers than that of the intestine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Malla, M. A. et al. Exploring the human microbiome: the potential future role of next-generation sequencing in disease diagnosis and treatment. Front. Immunol. 9, 2868 (2018).
NIH Human Microbiome Portfolio Analysis Team. A review of 10 years of human microbiome research activities at the US National Institutes of Health, fiscal years 2007–2016. Microbiome 7, 31 (2019).
Clarke, G. et al. Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 28, 1221–1238 (2014).
Oliphant, K. & Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91 (2019).
Fulde, M. & Hornef, M. W. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol. Rev. 260, 21–34 (2014).
Kamada, N., Chen, G. Y., Inohara, N. & Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 14, 685–690 (2013).
Ijssennagger, N. et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl Acad. Sci. USA 112, 10038–10043 (2015).
Reinhardt, C. et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 483, 627–631 (2012).
Neuman, H., Debelius, J. W., Knight, R. & Koren, O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 39, 509–521 (2015).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Canfora, E. E., Jocken, J. W. & Blaak, E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591 (2015).
Tuddenham, S. & Sears, C. L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 28, 464–470 (2015).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016).
Durack, J. & Lynch, S. V. The gut microbiome: relationships with disease and opportunities for therapy. J. Exp. Med. 216, 20–40 (2019).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Schirmer, M. et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat. Microbiol. 3, 337–346 (2018).
Gevers, D. et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392 (2014).
Fujimura, K. E. & Lynch, S. V. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe 17, 592–602 (2015).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Larsen, N. et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5, e9085 (2010).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Marin, I. A. et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 7, 43859 (2017).
Kang, D. W. et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5, 10 (2017).
Clapp, M. et al. Gut microbiota’s effect on mental health: the gut-brain axis. Clin. Pract. 7, 987 (2017).
Wilkins, L. J., Monga, M. & Miller, A. W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 9, 1–10 (2019).
Moe, O. W. Kidney stones: pathophysiology and medical management. Lancet 367, 333–344 (2006).
Robertson, W. G., Peacock, M., Marshall, R. W., Marshall, D. H. & Nordin, B. C. Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N. Engl. J. Med. 294, 249–252 (1976).
Kaufman, D. W. et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J. Am. Soc. Nephrol. 19, 1197–1203 (2008).
Sidhu, H. et al. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet 352, 1026–1029 (1998).
Kumar, R. et al. Role of Oxalobacter formigenes in calcium oxalate stone disease: a study from North India. Eur. Urol. 41, 318–322 (2002).
Duncan, S. H. et al. Oxalobacter formigenes and its potential role in human health. Appl. Environ. Microbiol. 68, 3841–3847 (2002).
Allison, M. J., Dawson, K. A., Mayberry, W. R. & Foss, J. G. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch. Microbiol. 141, 1–7 (1985).
Miller, A. W. & Dearing, D. The metabolic and ecological interactions of oxalate-degrading bacteria in the mammalian gut. Pathogens 2, 636–652 (2013).
Campieri, C. et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 60, 1097–1105 (2001).
Turroni, S. et al. Oxalate consumption by lactobacilli: evaluation of oxalyl-CoA decarboxylase and formyl-CoA transferase activity in Lactobacillus acidophilus. J. Appl. Microbiol. 103, 1600–1609 (2007).
Turroni, S. et al. Oxalate-degrading activity in Bifidobacterium animalis subsp. lactis: impact of acidic conditions on the transcriptional levels of the oxalyl coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes. Appl. Environ. Microbiol. 76, 5609–5620 (2010).
Batagello, C. A., Monga, M. & Miller, A. W. Calcium oxalate urolithiasis: a case of missing microbes? J. Endourol. 32, 995–1005 (2018).
Hoppe, B. et al. A randomised phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr. Nephrol. 32, 781–790 (2017).
Hoppe, B. et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol. Dial. Transplant. 26, 3609–3615 (2011).
Hoppe, B. et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 70, 1305–1311 (2006).
Milliner, D., Hoppe, B. & Groothoff, J. A randomised phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46, 313–323 (2018).
Jairath, A. et al. Oxalobacter formigenes: opening the door to probiotic therapy for the treatment of hyperoxaluria. Scand. J. Urol. 49, 334–337 (2015).
Lieske, J. C., Goldfarb, D. S., De Simone, C. & Regnier, C. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 68, 1244–1249 (2005).
Lieske, J. C. et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 78, 1178–1185 (2010).
Ferraz, R. R. N. et al. Effects of Lactobacillus casei and Bifidobacterium breve on urinary oxalate excretion in nephrolithiasis patients. Urol. Res. 37, 95–100 (2009).
Okombo, J. & Liebman, M. Probiotic-induced reduction of gastrointestinal oxalate absorption in healthy subjects. Urol. Res. 38, 169–178 (2010).
Al-Wahsh, I., Wu, Y. & Liebman, M. Acute probiotic ingestion reduces gastrointestinal oxalate absorption in healthy subjects. Urol. Res. 40, 191–196 (2012).
Siener, R. et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 83, 1144–1149 (2013).
Goldfarb, D. S., Modersitzki, F. & Asplin, J. R. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2, 745–749 (2007).
Hoppe, B. & Martin-Higueras, C. Improving treatment options for primary hyperoxaluria. Drugs https://doi.org/10.1007/s40265-022-01735-x (2022).
Stern, J. M. et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44, 399–407 (2016).
Tang, R. et al. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis https://doi.org/10.1007/s00240-018-1037-y (2018).
Suryavanshi, M. V. et al. Hyperoxaluria leads to dysbiosis and drives selective enrichment of oxalate metabolizing bacterial species in recurrent kidney stone endures. Sci. Rep. 6, 34712 (2016).
Suryavanshi, M. V., Bhute, S. S., Gune, R. P. & Shouche, Y. S. Functional eubacteria species along with trans-domain gut inhabitants favour dysgenic diversity in oxalate stone disease. Sci. Rep. 8, 1–11 (2018).
Ticinesi, A. et al. Understanding the gut–kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106 (2018).
Miller, A. W., Choy, D., Penniston, K. L. & Lange, D. Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 96, 180–188 (2019).
Zampini, A., Nguyen, A. H., Rose, E., Monga, M. & Miller, A. W. Defining dysbiosis in patients with urolithiasis. Sci. Rep. 9, 5425 (2019).
Miller, A. W., Orr, T., Dearing, D. & Monga, M. Loss of function dysbiosis associated with antibiotics and high fat, high sugar diet. ISME J. 13, 1379–1390 (2019).
Miller, A. W., Oakeson, K. F., Dale, C. & Dearing, M. D. Microbial community transplant results in increased and long-term oxalate degradation. Microb. Ecol. 72, 470–478 (2016).
Miller, A. W., Dale, C. & Dearing, M. D. The induction of oxalate metabolism in vivo is more effective with functional microbial communities than with functional microbial species. mSystems 2, e00088-17 (2017).
Stern, J. M. et al. Fecal transplant modifies urine chemistry risk factors for urinary stone disease. Physiol. Rep. 7, e14012 (2019).
Wolfe, A. J. et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol. 50, 1376–1383 (2012).
Xie, J. et al. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol. 20, 41 (2020).
Schwaderer, A. L. & Wolfe, A. J. The association between bacteria and urinary stones. Ann. Transl. Med. 5, 32 (2017).
Dornbier, R. A. et al. The microbiome of calcium-based urinary stones. Urolithiasis 48, 191–199 (2019).
Barr-Beare, E. et al. The interaction between Enterobacteriaceae and calcium oxalate deposits. PLoS ONE 10, e0139575 (2015).
Tavichakorntrakool, R. et al. Extensive characterizations of bacteria isolated from catheterized urine and stone matrices in patients with nephrolithiasis. Nephrol. Dial. Transplant. 27, 4125–4130 (2012).
Hobbs, T., Schultz, L. N., Lauchnor, E. G., Gerlach, R. & Lange, D. Evaluation of biofilm induced urinary infection stone formation in a novel laboratory model system. J. Urol. 199, 178–185 (2018).
Chutipongtanate, S., Sutthimethakorn, S., Chiangjong, W. & Thongboonkerd, V. Bacteria can promote calcium oxalate crystal growth and aggregation. J. Biol. Inorg. Chem. 18, 299–308 (2013).
Kanlaya, R., Naruepantawart, O. & Thongboonkerd, V. Flagellum is responsible for promoting effects of viable Escherichia coli on calcium oxalate crystallization, crystal growth, and crystal aggregation. Front. Microbiol. 10, 2507 (2019).
Sivaguru, M. et al. Geobiology reveals how human kidney stones dissolve in vivo. Sci. Rep. 8, 1–9 (2018).
Sivaguru, M. et al. Human kidney stones: a natural record of universal biomineralization. Nat. Rev. Urol. 18, 404–432 (2021).
Saw, J. et al. In vivo entombment of bacteria and fungi during calcium oxalate, brushite, and struvite urolithiasis. Kidney360 2, 298–311 (2021).
Flannigan, R., Choy, W. H., Chew, B. & Lange, D. Renal struvite stones-pathogenesis, microbiology, and management strategies. Nat. Rev. Urol. 11, 333–341 (2014).
Jansma, J. & El Aidy, S. Understanding the host-microbe interactions using metabolic modeling. Microbiome 9, 16 (2021).
Donia, M. S. & Fischbach, M. A. Human microbiota. Small molecules from the human microbiota. Science 349, 1254766 (2015).
Silva, Y. P., Bernardi, A. & Frozza, R. L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 11, 25 (2020).
van der Hee, B. & Wells, J. M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 29, 700–712 (2021).
Al-Harbi, N. O. et al. Short chain fatty acid, acetate ameliorates sepsis-induced acute kidney injury by inhibition of NADPH oxidase signaling in T cells. Int. Immunopharmacol. 58, 24–31 (2018).
Dawson, P. A. et al. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J. Clin. Invest. 120, 706–712 (2010).
Heilberg, I. P. & Goldfarb, D. S. Optimum nutrition for kidney stone disease. Adv. Chronic Kidney Dis. 20, 165–174 (2013).
Mitchell, T. et al. Dietary oxalate and kidney stone formation. Am. J. Physiol. Renal Physiol. 316, F409–F413 (2019).
Rowland, I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24 (2018).
Duan, X. et al. 1 H NMR-based metabolomic study of metabolic profiling for the urine of kidney stone patients. Urolithiasis 48, 27–35 (2020).
Pallister, T. et al. Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci. Rep. 7, 1–9 (2017).
Wang, X. et al. Identification of urine biomarkers for calcium-oxalate urolithiasis in adults based on UPLC-Q-TOF/MS. J. Chromatogr. B 1124, 290–297 (2019).
Iwanaga, T. & Kishimoto, A. Cellular distributions of monocarboxylate transporters: a review. Biomed. Res. 36, 279–301 (2015).
Schonfeld, P. & Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J. Lipid Res. 57, 943–954 (2016).
Suzuki, T., Yoshida, S. & Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 100, 297–305 (2008).
Macfarlane, G. T. & Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 95, 50–60 (2012).
Matey-Hernandez, M. L. et al. Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol. Genomics 50, 117–126 (2018).
den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 (2013).
Druart, C. et al. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS ONE 9, e87560 (2014).
Lehnen, T. E., da Silva, M. R., Camacho, A., Marcadenti, A. & Lehnen, A. M. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J. Int. Soc. Sports Nutr. 12, 36 (2015).
Andrade-Oliveira, V. et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 26, 1877–1888 (2015).
Machado, R. A. et al. Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol. Dial. Transpl. 27, 3136–3140 (2012).
Marzocco, S. et al. Supplementation of short-chain fatty acid, sodium propionate, in patients on maintenance hemodialysis: beneficial effects on inflammatory parameters and gut-derived uremic toxins, a pilot study (PLAN Study). J. Clin. Med. https://doi.org/10.3390/jcm7100315 (2018).
Yang, J. et al. Effects of prebiotic fiber xylooligosaccharide in adenine-induced nephropathy in mice. Mol. Nutr. Food Res. https://doi.org/10.1002/mnfr.201800014 (2018).
Vaziri, N. D. et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 9, e114881 (2014).
Wong, J. et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 39, 230–237 (2014).
Huang, W., Zhou, L., Guo, H., Xu, Y. & Xu, Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 68, 20–30 (2017).
Li, L. Z., Tao, S. B., Ma, L. & Fu, P. Roles of short-chain fatty acids in kidney diseases. Chin. Med. J. 132, 1228–1232 (2019).
Khan, S. R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J. Urol. 189, 803–811 (2013).
Khan, S. R., Canales, B. K. & Dominguez-Gutierrez, P. R. Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat. Rev. Nephrol. 17, 417–433 (2021).
Feng, W. et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell Physiol. Biochem. 47, 1617–1629 (2018).
Wang, H. B., Wang, P. Y., Wang, X., Wan, Y. L. & Liu, Y. C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 57, 3126–3135 (2012).
Alrefai, W. A. et al. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am. J. Physiol. Gastrointest. Liver Physiol. 293, G923–G934 (2007).
Raheja, G. et al. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G395–G401 (2010).
Peng, L., Li, Z. R., Green, R. S., Holzman, I. R. & Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139, 1619–1625 (2009).
Krishnan, S., Rajendran, V. M. & Binder, H. J. Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am. J. Physiol. Cell Physiol. 285, C1246–C1254 (2003).
Musch, M. W., Bookstein, C., Xie, Y., Sellin, J. H. & Chang, E. B. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G687–G693 (2001).
Kiela, P. R., Hines, E. R., Collins, J. F. & Ghishan, F. K. Regulation of the rat NHE3 gene promoter by sodium butyrate. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G947–G956 (2001).
Farre, R., Fiorani, M., Abdu Rahiman, S. & Matteoli, G. Intestinal permeability, inflammation and the role of nutrients. Nutrients https://doi.org/10.3390/nu12041185 (2020).
Freel, R. W., Whittamore, J. M. & Hatch, M. Transcellular oxalate and Cl- absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G520–G527 (2013).
Hesse, A., Schneeberger, W., Engfeld, S., Von Unruh, G. E. & Sauerbruch, T. Intestinal hyperabsorption of oxalate in calcium oxalate stone formers: application of a new test with [13C2]oxalate. J. Am. Soc. Nephrol. 10, S329–S333 (1999).
Whittamore, J. M. & Hatch, M. The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 45, 89–108 (2017).
Liu, Y. et al. The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. FASEB J. 34, 11200–11214 (2020).
Denburg, M. R. et al. Perturbations of the gut microbiome and metabolome in children with calcium oxalate kidney stone disease. J. Am. Soc. Nephrol. 31, 1358–1369 (2020).
Chen, Y., Michalak, M. & Agellon, L. B. Importance of nutrients and nutrient metabolism on human health. Yale J. Biol. Med. 91, 95–103 (2018).
Remer, T. Influence of nutrition on acid-base balance-metabolic aspects. Eur. J. Nutr. 40, 214–220 (2001).
Orzechowski, A., Ostaszewski, P., Jank, M. & Berwid, S. J. Bioactive substances of plant origin in food-impact on genomics. Reprod. Nutr. Dev. 42, 461–477 (2002).
McRorie, J. W. Jr & McKeown, N. M. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 117, 251–264 (2017).
Schmidt, T. S. B., Raes, J. & Bork, P. The human gut microbiome: from association to modulation. Cell 172, 1198–1215 (2018).
Schroeder, B. O. & Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089 (2016).
Suryavanshi, M. V., Bhute, S. S., Gune, R. P. & Shouche, Y. S. Functional eubacteria species along with trans-domain gut inhabitants favour dysgenic diversity in oxalate stone disease. Sci. Rep. 8, 16598 (2018).
Miller, A. W., Dale, C. & Dearing, M. D. Microbiota diversification and crash induced by dietary oxalate in the mammalian herbivore Neotoma albigula. mSphere https://doi.org/10.1128/mSphere.00428-17 (2017).
Seck, E. H. et al. Salt in stools is associated with obesity, gut halophilic microbiota and Akkermansia muciniphila depletion in humans. Int. J. Obes. 43, 862–871 (2019).
Bernardino, M. & Parmar, M. S. Oxalate nephropathy from cashew nut intake. CMAJ 189, E405–E408 (2017).
Murphy, E. A., Velazquez, K. T. & Herbert, K. M. Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 18, 515–520 (2015).
Abate, N., Chandalia, M., Cabo-Chan, A. V. Jr., Moe, O. W. & Sakhaee, K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 65, 386–392 (2004).
Ticinesi, A. et al. Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106 (2018).
Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2018. CDC https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2018.html (2018).
Shallcross, L., Beckley, N., Rait, G., Hayward, A. & Petersen, I. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J. Antimicrob. Chemother. 72, 1818–1824 (2017).
Modi, S. R., Collins, J. J. & Relman, D. A. Antibiotics and the gut microbiota. J. Clin. Invest. 124, 4212–4218 (2014).
Ferrer, M., Méndez-García, C., Rojo, D., Barbas, C. & Moya, A. Antibiotic use and microbiome function. Biochem. Pharmacol. 134, 114–126 (2017).
Neuman, H., Forsythe, P., Uzan, A., Avni, O. & Koren, O. Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol. Rev. 42, 489–499 (2018).
Ianiro, G., Tilg, H. & Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 65, 1906–1915 (2016).
Risnes, K. R., Belanger, K., Murk, W. & Bracken, M. B. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am. J. Epidemiol. 173, 310–318 (2011).
Kronman, M. P., Zaoutis, T. E., Haynes, K., Feng, R. & Coffin, S. E. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 130, e794–e803 (2012).
Ferraro, P. M., Curhan, G. C., Gambaro, G. & Taylor, E. N. Antibiotic use and risk of incident kidney stones in female nurses. Am. J. Kidney Dis. 74, 736–741 (2019).
Tasian, G. E. et al. Oral antibiotic exposure and kidney stone disease. J. Am. Soc. Nephrol. 29, 1731–1740 (2018).
Kelly, J. P., Curhan, G. C., Cave, D. R., Anderson, T. E. & Kaufman, D. W. Factors related to colonization with Oxalobacter formigenes in US adults. J. Endourol. 25, 673–679 (2011).
Lange, J. N. et al. Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology 79, 1286–1289 (2012).
Pietzke, M., Meiser, J. & Vazquez, A. Formate metabolism in health and disease. Mol. Metab. 33, 23–37 (2020).
Gamage, K. N. et al. The role of fluid intake in the prevention of kidney stone disease: a systematic review over the last two decades. Turk. J. Urol. 46, S92–S103 (2020).
Bowyer, R. C. E. et al. Associations between UK tap water and gut microbiota composition suggest the gut microbiome as a potential mediator of health differences linked to water quality. Sci. Total. Environ. 739, 139697 (2020).
Vandeputte, D. et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62 (2016).
Falony, G. et al. Population-level analysis of gut microbiome variation. Science 352, 560–564 (2016).
Zhao, Y. & Yu, Y. B. Intestinal microbiota and chronic constipation. Springerplus 5, 1130 (2016).
Gerritsen, J., Smidt, H., Rijkers, G. T. & de Vos, W. M. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 6, 209–240 (2011).
Kirgizov, I. V., Sukhorukov, A. M., Dudarev, V. A. & Istomin, A. A. Hemostasis in children with dysbacteriosis in chronic constipation. Clin. Appl. Thromb. Hemost. 7, 335–338 (2001).
Khalif, I. L., Quigley, E. M., Konovitch, E. A. & Maximova, I. D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 37, 838–849 (2005).
Alford, A., Furrow, E., Borofsky, M. & Lulich, J. Animal models of naturally occurring stone disease. Nat. Rev. Urol. 17, 691–705 (2020).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Sekirov, I. & Finlay, B. B. The role of the intestinal microbiota in enteric infection. J. Physiol. 587, 4159–4167 (2009).
Sekirov, I., Russell, S. L., Antunes, L. C. & Finlay, B. B. Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010).
Mukherjee, S., Joardar, N., Sengupta, S. & Sinha Babu, S. P. Gut microbes as future therapeutics in treating inflammatory and infectious diseases: lessons from recent findings. J. Nutr. Biochem. 61, 111–128 (2018).
Hatch, M. Gut microbiota and oxalate homeostasis. Ann. Transl. Med. 5, 36 (2017).
Sanchez-Tapia, M. et al. Consumption of cooked black beans stimulates a cluster of some clostridia class bacteria decreasing inflammatory response and improving insulin sensitivity. Nutrients https://doi.org/10.3390/nu12041182 (2020).
Scholz-Ahrens, K. E. & Schrezenmeir, J. Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J. Nutr. 137, 2513S–2523S (2007).
Xu, X. et al. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. 5, 17046 (2017).
Kiousi, D. E. et al. Probiotics in extraintestinal diseases: current trends and new directions. Nutrients https://doi.org/10.3390/nu11040788 (2019).
Weaver, C. M. & Legette, L. L. Equol, via dietary sources or intestinal production, may ameliorate estrogen deficiency-induced bone loss. J. Nutr. 140, 1377S–1379S (2010).
Blaut, M. & Clavel, T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J. Nutr. 137, 751S–755S (2007).
Guo, S. et al. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J. Immunol. 195, 4999–5010 (2015).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. A.W.M., K.L.P., G.T. and D.L. contributed substantially to discussion of the content. All authors wrote the article. A.W.M., K.L.P., G.T. and D.L. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks John Lieske and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miller, A.W., Penniston, K.L., Fitzpatrick, K. et al. Mechanisms of the intestinal and urinary microbiome in kidney stone disease. Nat Rev Urol 19, 695–707 (2022). https://doi.org/10.1038/s41585-022-00647-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00647-5
This article is cited by
-
Identification of biological components for sialolith formation organized in circular multi-layers
Scientific Reports (2023)