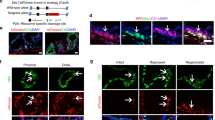
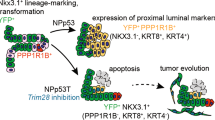
Abstract
Stem and progenitor cells of the adult prostate epithelium have historically been believed to reside mainly or exclusively within the basal cell compartment and to possess basal-like phenotypic characteristics. Within the past decade, evidence of the existence of luminal epithelial cells exhibiting stem/progenitor properties has been obtained by lineage tracing and by functional characterization of sorted luminal-like cells. In 2020, the boom of single-cell transcriptomics led to increasingly exhaustive profiling of putative mouse luminal progenitor cells and, importantly, to the identification of cognate cells in the human prostate. The enrichment of luminal progenitor cells in genetically modified mouse models of prostate inflammation, benign prostate hypertrophy and prostate cancer, and the intrinsic castration tolerance of these cells, suggest their potential role in prostate pathogenesis and in resistance to androgen deprivation therapy. This Review bridges different approaches that have been used in the field to characterize luminal progenitor cells, including the unification of multiple identifiers employed to define these cells (names and markers). It also provides an overview of the intrinsic functional properties of luminal progenitor cells, and addresses their relevance in mouse and human prostate pathophysiology.
Key points
-
LSCmed defines a prototypic population of epithelial cells discovered in 2014 in the mouse prostate that exhibit luminal and stem-like features and were therefore called luminal progenitor cells. Their signature includes Krt4 and Psca.
-
Phenotypically similar cells have been identified in the human prostate. In both species, these cells reside mainly, but not only, in proximal regions of the gland (peri-urethral area and/or central transition zones).
-
In the adult mouse prostate in situ, luminal progenitor cells are unipotent stem-like cells that contribute to post-castration prostatic tissue regeneration in cooperation with alternative cell-driven mechanisms.
-
Luminal progenitor cells are amplified in various pathological states (such as inflammation, benign prostatic hyperplasia and cancer). The underlying molecular and cellular mechanisms are virtually unknown but might involve complex circuitry with inflammatory and stromal cells.
-
Luminal progenitor cells are more castration-tolerant than differentiated luminal cells. Thus, they could contribute to resistance to androgen-targeting therapeutic strategies (5α-reductase inhibitors and androgen deprivation therapy) and promote disease progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Devlin, C. M., Simms, M. S. & Maitland, N. J. Benign prostatic hyperplasia — what do we know? BJU Int. 127, 389–399 (2021).
Strand, D. W., Costa, D. N., Francis, F., Ricke, W. A. & Roehrborn, C. G. Targeting phenotypic heterogeneity in benign prostatic hyperplasia. Differentiation 96, 49–61 (2017).
Lee, S. W. H., Chan, E. M. C. & Lai, Y. K. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Sci. Rep. 7, 7984 (2017).
Bell, K. J., Del Mar, C., Wright, G., Dickinson, J. & Glasziou, P. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int. J. Cancer 137, 1749–1757 (2015).
Gandaglia, G. et al. Epidemiology and prevention of prostate cancer. Eur. Urol. Oncol. 4, 877–892 (2021).
Neal, D. E. et al. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the ProtecT randomised controlled trial according to treatment received. Eur. Urol. 77, 320–330 (2020).
Cunha, G. R., Hayward, S. W. & Wang, Y. Z. Role of stroma in carcinogenesis of the prostate. Differentiation 70, 473–485 (2002).
Hayward, S. W., Rosen, M. A. & Cunha, G. R. Stromal-epithelial interactions in the normal and neoplastic prostate. Br. J. Urol. 79 (Suppl. 2), 18–26 (1997).
Cunha, G. R., Donjacour, A. A. & Sugimura, Y. Stromal-epithelial interactions and heterogeneity of proliferative activity within the prostate. Biochem. Cell Biol. 64, 608–614 (1986).
Abate-Shen, C. & Shen, M. M. Molecular genetics of prostate cancer. Genes Dev. 14, 2410–2434 (2000).
Zhang, D., Zhao, S., Li, X., Kirk, J. S. & Tang, D. G. Prostate luminal progenitor cells in development and cancer. Trends Cancer 4, 769–783 (2018).
Li, J. J. & Shen, M. M. Prostate stem cells and cancer stem cells. Cold Spring Harb. Perspect. Med. 9, a030395 (2019).
Rycaj, K. & Tang, D. G. Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res. 75, 4003–4011 (2015).
Ittmann, M. Anatomy and histology of the human and murine prostate. Cold Spring Harb. Perspect. Med. 8, a030346 (2018).
Roy-Burman, P., Wu, H., Powell, W. C., Hagenkord, J. & Cohen, M. B. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr. Relat. Cancer 11, 225–254 (2004).
Shappell, S. B. et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 64, 2270–2305 (2004).
Bruchovsky, N., Lesser, B., Van Doorn, E. & Craven, S. Hormonal effects on cell proliferation in rat prostate. Vitam. Horm. 33, 61–102 (1975).
English, H. F., Santen, R. J. & Isaacs, J. T. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11, 229–242 (1987).
Wang, Y., Hayward, S., Cao, M., Thayer, K. & Cunha, G. Cell differentiation lineage in the prostate. Differentiation 68, 270–279 (2001).
Taylor, R. A., Toivanen, R. & Risbridger, G. P. Stem cells in prostate cancer: treating the root of the problem. Endocr. Relat. Cancer 17, R273–R285 (2010).
Goldstein, A. S., Stoyanova, T. & Witte, O. N. Primitive origins of prostate cancer: in vivo evidence for prostate-regenerating cells and prostate cancer-initiating cells. Mol. Oncol. 4, 385–396 (2010).
Lawson, D. A., Xin, L., Lukacs, R. U., Cheng, D. & Witte, O. N. Isolation and functional characterization of murine prostate stem cells. Proc. Natl Acad. Sci. USA 104, 181–186 (2007).
Evans, G. S. & Chandler, J. A. Cell proliferation studies in rat prostate. I. The proliferative role of basal and secretory epithelial cells during normal growth. Prostate 10, 163–178 (1987).
Evans, G. S. & Chandler, J. A. Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11, 339–351 (1987).
Wang, X. et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 (2009).
Choi, N., Zhang, B., Zhang, L., Ittmann, M. & Xin, L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21, 253–265 (2012).
Liu, J. et al. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol. Endocrinol. 25, 1849–1857 (2011).
Lawson, D. A. et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc. Natl Acad. Sci. USA 107, 2610–2615 (2010).
Sackmann-Sala, L. et al. Prolactin-induced prostate tumorigenesis links sustained stat5 signaling with the amplification of basal/stem cells and emergence of putative luminal progenitors. Am. J. Pathol. 184, 3105–3119 (2014).
Kwon, O. J., Zhang, L. & Xin, L. Stem cell antigen-1 identifies a distinct androgen-independent murine prostatic luminal cell lineage with bipotent potential. Stem Cell 34, 191–202 (2016).
Sackmann Sala, L. et al. A rare castration-resistant progenitor cell population is highly enriched in Pten-null prostate tumors. J. Pathol. 243, 54–64 (2017).
Guo, W. et al. Single-cell transcriptomics identifies a distinct luminal progenitor cell type in distal prostate invagination tips. Nat. Genet. 52, 908–918 (2020).
Karthaus, W. R. et al. Regenerative potential of prostate luminal cells revealed by single-cell analysis. Science 368, 497–505 (2020).
Crowley, L. et al. A single-cell atlas of the mouse and human prostate reveals heterogeneity and conservation of epithelial progenitors. eLife https://doi.org/10.7554/eLife.59465 (2020).
Mevel, R. et al. RUNX1 marks a luminal castration-resistant lineage established at the onset of prostate development. eLife 9, e59465. (2020).
Joseph, D. B. et al. Urethral luminal epithelia are castration-insensitive cells of the proximal prostate. Prostate 80, 872–884 (2020).
Joseph, D. B., Turco, A. E., Vezina, C. M. & Strand, D. W. Progenitors in prostate development and disease. Dev. Biol. 473, 50–58 (2021).
Freeland, J., Crowell, P. D., Giafaglione, J. M., Boutros, P. C. & Goldstein, A. S. Aging of the progenitor cells that initiate prostate cancer. Cancer Lett. 515, 28–35 (2021).
Lukacs, R. U., Goldstein, A. S., Lawson, D. A., Cheng, D. & Witte, O. N. Isolation, cultivation and characterization of adult murine prostate stem cells. Nat. Protoc. 5, 702–713 (2010).
Mulholland, D. J. et al. Lin−Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 69, 8555–8562 (2009).
Wang, Z. A. et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat. Cell Biol. 15, 274–283 (2013).
Goffin, V., Hoang, D. T., Bogorad, R. L. & Nevalainen, M. T. Prolactin regulation of the prostate gland: a female player in a male game. Nat. Rev. Urol. 8, 597–607 (2011).
Crowell, P. D. et al. Expansion of luminal progenitor cells in the aging mouse and human prostate. Cell Rep. 28, 1499–1510.e6 (2019).
Kwon, O. J. et al. The Sca-1+ and Sca-1− mouse prostatic luminal cell lineages are independently sustained. Stem Cell 38, 1479–1491 (2020).
Tsujimura, A. et al. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J. Cell Biol. 157, 1257–1265 (2002).
Lawson, D. A. & Witte, O. N. Stem cells in prostate cancer initiation and progression. J. Clin. Invest. 117, 2044–2050 (2007).
Burger, P. E. et al. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc. Natl Acad. Sci. USA 102, 7180–7185 (2005).
Goldstein, A. S. et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl Acad. Sci. USA 105, 20882–20887 (2008).
Shen, M. M. & Abate-Shen, C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000 (2010).
Puig Lombardi, E., Baures, M., Dariane, C., Guidotti, J.-E. & Goffin, V. In silico computed clusters of prostate luminal progenitors match FACS-Enriched LSCmed cells. Preprint at bioRxiv https://doi.org/10.1101/2021.06.16.448624 (2021).
Henry, G. H. et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 25, 3530–3542.e5 (2018).
Hu, W. Y. et al. Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem Cell Res. 23, 1–12 (2017).
Liu, X. et al. Low CD38 identifies progenitor-like inflammation-associated luminal cells that can initiate human prostate cancer and predict poor outcome. Cell Rep. 17, 2596–2606 (2016).
Zhang, D. et al. Histone 2B-GFP label-retaining prostate luminal cells possess progenitor cell properties and are intrinsically resistant to castration. Stem Cell Rep. 10, 228–242 (2018).
Kwon, O. J., Zhang, L., Jia, D. & Xin, L. Sox2 is necessary for androgen ablation-induced neuroendocrine differentiation from Pten null Sca-1+ prostate luminal cells. Oncogene 40, 203–214 (2021).
Sugimura, Y., Cunha, G. R., Donjacour, A. A., Bigsby, R. M. & Brody, J. R. Whole-mount autoradiography study of DNA synthetic activity during postnatal development and androgen-induced regeneration in the mouse prostate. Biol. Reprod. 34, 985–995 (1986).
Yoo, Y. A. et al. Bmi1 marks distinct castration-resistant luminal progenitor cells competent for prostate regeneration and tumour initiation. Nat. Commun. 7, 12943 (2016).
Yoo, Y. A. et al. The role of castration-resistant Bmi1+Sox2+ cells in driving recurrence in prostate cancer. J. Natl Cancer Inst. 111, 311–321 (2019).
Henry, G. H. & Strand, D. W. Strand Lab analysis of single-cell RNA sequencing. Zenodo https://doi.org/10.5281/zenodo.3687064 (2020).
Leong, K. G., Wang, B. E., Johnson, L. & Gao, W. Q. Generation of a prostate from a single adult stem cell. Nature 456, 804–808 (2008).
Richardson, G. D. et al. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 117, 3539–3545 (2004).
Agarwal, S. et al. Identification of different classes of luminal progenitor cells within prostate tumors. Cell Rep. 13, 2147–2158 (2015).
Wang, L. et al. Expansion of prostate epithelial progenitor cells after inflammation of the mouse prostate. Am. J. Physiol. Renal Physiol. 308, F1421–F1430 (2015).
Wang, B. E. et al. Castration-resistant Lgr5+ cells are long-lived stem cells required for prostatic regeneration. Stem Cell Rep. 4, 768–779 (2015).
Barros-Silva, J. D. et al. Single-cell analysis identifies LY6D as a marker linking castration-resistant prostate luminal cells to prostate progenitors and cancer. Cell Rep. 25, 3504–3518.e6 (2018).
McAuley, E. et al. Sox2 expression marks castration-resistant progenitor cells in the adult murine prostate. Stem Cell 37, 690–700 (2019).
Korsten, H., Ziel-van der Made, A., Ma, X., van der Kwast, T. & Trapman, J. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS ONE 4, e5662 (2009).
Xin, L., Lawson, D. A. & Witte, O. N. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc. Natl Acad. Sci. USA 102, 6942–6947 (2005).
Wei, X. et al. Spatially restricted stromal Wnt signaling restrains prostate epithelial progenitor growth through direct and indirect mechanisms. Cell Stem Cell 24, 753–768.e6 (2019).
Ousset, M. et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol. 14, 1131–1138 (2012).
Wang, J. et al. Symmetrical and asymmetrical division analysis provides evidence for a hierarchy of prostate epithelial cell lineages. Nat. Commun. 5, 4758 (2014).
Wuidart, A. et al. Quantitative lineage tracing strategies to resolve multipotency in tissue-specific stem cells. Genes Dev. 30, 1261–1277 (2016).
Tika, E., Ousset, M., Dannau, A. & Blanpain, C. Spatiotemporal regulation of multipotency during prostate development. Development 146, dev180224 (2019).
Shibata, M., Epsi, N. J., Xuan, S., Mitrofanova, A. & Shen, M. M. Bipotent progenitors do not require androgen receptor for luminal specification during prostate organogenesis. Stem Cell Rep. 15, 1026–1036 (2020).
Ceder, J. A., Aalders, T. W. & Schalken, J. A. Label retention and stem cell marker expression in the developing and adult prostate identifies basal and luminal epithelial stem cell subpopulations. Stem Cell Res. Ther. 8, 95 (2017).
Moad, M. et al. Multipotent basal stem cells, maintained in localized proximal niches, support directed long-ranging epithelial flows in human prostates. Cell Rep. 20, 1609–1622 (2017).
Treutlein, B. et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509, 371–375 (2014).
Rawlins, E. L. et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525–534 (2009).
Karthaus, W. R. et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175 (2014).
Chua, C. W. et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 16, 951–954 (2014).
Pignon, J. C. et al. Cell kinetic studies fail to identify sequentially proliferating progenitors as the major source of epithelial renewal in the adult murine prostate. PLoS ONE 10, e0128489 (2015).
Shivdasani, R. A., Clevers, H. & de Sauvage, F. J. Tissue regeneration: reserve or reverse? Science 371, 784–786 (2021).
Brennen, W. N. & Isaacs, J. T. Mesenchymal stem cells and the embryonic reawakening theory of BPH. Nat. Rev. Urol. 15, 703–715 (2018).
Claus, S., Wrenger, M., Senge, T. & Schulze, H. Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol. Res. 21, 305–308 (1993).
Izumi, K., Li, L. & Chang, C. Androgen receptor and immune inflammation in benign prostatic hyperplasia and prostate cancer. Clin. Investig. 4, 935–950 (2014).
Roehrborn, C. G. et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur. Urol. 57, 123–131 (2010).
McConnell, J. D. et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 349, 2387–2398 (2003).
Cornu, J. N. et al. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur. Urol. 67, 1066–1096 (2015).
Kindblom, J. et al. Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology 144, 2269–2278 (2003).
Nevalainen, M. T. et al. Prolactin and prolactin receptors are expressed and functioning in human prostate. J. Clin. Invest. 99, 618–627 (1997).
Pigat, N. et al. Combined Sabal and Urtica extracts (WS® 1541) exert anti-proliferative and anti-inflammatory effects in a mouse model of benign prostate hyperplasia. Front. Pharmacol. 10, 311 (2019).
Bernichtein, S. et al. Anti-inflammatory properties of Lipidosterolic extract of Serenoa repens (Permixon®) in a mouse model of prostate hyperplasia. Prostate 75, 706–722 (2015).
Lai, K. P. et al. Targeting stromal androgen receptor suppresses prolactin-driven benign prostatic hyperplasia (BPH). Mol. Endocrinol. 27, 1617–1631 (2013).
Isaacs, J. T. & Coffey, D. S. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 2, 33–50 (1989).
Kwon, O. J., Zhang, L., Ittmann, M. M. & Xin, L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl Acad. Sci. USA 111, E592–E600 (2014).
Schalken, J. A. Inflammation in the pathophysiology of benign prostatic hypertrophy. Eur. Urol. Suppl. 14, e1455–e1458 (2015).
McNeal, J. E. Origin and evolution of benign prostatic enlargement. Invest. Urol. 15, 340–345 (1978).
De Nunzio, C., Presicce, F. & Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 13, 613–626 (2016).
Bushman, W. A. & Jerde, T. J. The role of prostate inflammation and fibrosis in lower urinary tract symptoms. Am. J. Physiol. Renal Physiol. 311, F817–F821 (2016).
Wang, H. H. et al. Characterization of autoimmune inflammation induced prostate stem cell expansion. Prostate 75, 1620–1631 (2015).
Zhang, B. et al. Non-cell-autonomous regulation of prostate epithelial homeostasis by androgen receptor. Mol. Cell 63, 976–989 (2016).
Yu, Y. et al. Mesenchymal stem cells recruited by castration-induced inflammation activation accelerate prostate cancer hormone resistance via chemokine ligand 5 secretion. Stem Cell Res. Ther. 9, 242 (2018).
Shi, X., Gipp, J., Dries, M. & Bushman, W. Prostate progenitor cells proliferate in response to castration. Stem Cell Res. 13, 154–163 (2014).
Spehar, K., Pan, A. & Beerman, I. Restoring aged stem cell functionality: current progress and future directions. Stem Cell 38, 1060–1077 (2020).
Stoyanova, T. et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc. Natl Acad. Sci. USA 110, 20111–20116 (2013).
Goldstein, A. S. et al. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nat. Protoc. 6, 656–667 (2011).
Wang, Z. A., Toivanen, R., Bergren, S. K., Chambon, P. & Shen, M. M. Luminal cells are favored as the cell of origin for prostate cancer. Cell Rep. 8, 1339–1346 (2014).
Goldstein, A. S., Huang, J., Guo, C., Garraway, I. P. & Witte, O. N. Identification of a cell of origin for human prostate cancer. Science 329, 568–571 (2010).
Lu, T. L. et al. Conditionally ablated Pten in prostate basal cells promotes basal-to-luminal differentiation and causes invasive prostate cancer in mice. Am. J. Pathol. 182, 975–991 (2013).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003).
Parisotto, M. et al. PTEN deletion in luminal cells of mature prostate induces replication stress and senescence in vivo. J. Exp. Med. 215, 1749–1763 (2018).
Ratnacaram, C. K. et al. Temporally controlled ablation of PTEN in adult mouse prostate epithelium generates a model of invasive prostatic adenocarcinoma. Proc. Natl Acad. Sci. USA 105, 2521–2526 (2008).
Mulholland, D. J. et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 19, 792–804 (2011).
Zhang, J. et al. Proteomic and transcriptomic profiling of Pten gene-knockout mouse model of prostate cancer. Prostate 80, 588–605 (2020).
Abu El Maaty, M. A. et al. Single-cell analyses unravel cell type-specific responses to a vitamin D analog in prostatic precancerous lesions. Sci. Adv. 7, eabg5982 (2021).
Humphrey, P. A. Diagnosis of adenocarcinoma in prostate needle biopsy tissue. J. Clin. Pathol. 60, 35–42 (2007).
Shah, R. B., Zhou, M., LeBlanc, M., Snyder, M. & Rubin, M. A. Comparison of the basal cell-specific markers, 34βE12 and p63, in the diagnosis of prostate cancer. Am. J. Surg. Pathol. 26, 1161–1168 (2002).
Toivanen, R. et al. A preclinical xenograft model identifies castration-tolerant cancer-repopulating cells in localized prostate tumors. Sci. Transl. Med. 5, 187ra171 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Fizazi, K. et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 377, 352–360 (2017).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Fizazi, K. et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N. Engl. J. Med. 383, 1040–1049 (2020).
Smith, M. R. et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 378, 1408–1418 (2018).
Davies, A. H., Beltran, H. & Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 15, 271–286 (2018).
Mu, P. et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 (2017).
Ku, S. Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017).
Kwon, O. J. et al. De novo induction of lineage plasticity from human prostate luminal epithelial cells by activated AKT1 and c-Myc. Oncogene 39, 7142–7151 (2020).
Hsu, E. C. et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc. Natl Acad. Sci. USA 117, 2032–2042 (2020).
Mottahedeh, J. et al. CD38 is methylated in prostate cancer and regulates extracellular NAD+. Cancer Metab. 6, 13 (2018).
Chung, J. S. et al. Circulating tumor cell-based molecular classifier for predicting resistance to abiraterone and enzalutamide in metastatic castration-resistant prostate cancer. Neoplasia 21, 802–809 (2019).
Blanpain, C. & Simons, B. D. Unravelling stem cell dynamics by lineage tracing. Nat. Rev. Mol. Cell Biol. 14, 489–502 (2013).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Acknowledgements
V.G. and J.-E.G. are grateful to L. Sackmann Sala for her pioneering work that led to the discovery of LSCmed cells. The authors thank the following sources of financial support: Ligue contre le cancer (RS16/75-18, RS17/75-1, RS18/75-48, RS19/75-63, RS20/75-93 and RS21 /75-35), FONCER contre le cancer, Association pour la recherche sur les tumeurs de la prostate (ARTP), Cancéropôle Ile-de-France and Institut National du Cancer (INCa_6672), Inserm and the Université de Paris. M.B. is supported by a fellowship from the Ministry of Research, C.D. by research/mobility fellowships from Inserm, the Association Française d’Urologie and Assistance Publique Hôpitaux de Paris (APHP), and E.T. by a Fonds de la Recherche Scientific (FNRS) fellowship. C.B. is an investigator of WELBIO.
Author information
Authors and Affiliations
Contributions
V.G., M.B., C.D., E.P.L. and J.-E.G. researched data for the article. V.G., M.B., C.D., E.T., E.P.L., C.B. and J.-E.G. wrote the article. V.G., M.B., C.D., N.B.D., C.B. and J.-E.G. reviewed/edited the manuscript before submission. All authors made a substantial contribution to discussion of content.
Corresponding author
Ethics declarations
Competing interests
C.D. is a consultant for Janssen, Astellas and Bayer. C.B. is a consultant for Genentech, Nestlé and Chromacure. N.B.D. is a consultant for Koelis and Affluent Medical. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
Most references were retrieved from our personal collection of references, and some were suggested by the peer reviewers. We also searched for original and review articles in PubMed using the following search terms, alone or in combination: prostate, progenitor, stem cells, luminal cells, neuroendocrine, CARN, CARB, Sox2, organoid, lineage-tracing, castration, treatment resistance, recurrence, 5α-reductase, cancer, benign prostate hyperplasia, inflammation, development, single cell, RNA-seq, and biomarkers. All papers identified were full text papers in English. We also searched the reference lists of identified articles for further relevant papers.
Rights and permissions
About this article
Cite this article
Baures, M., Dariane, C., Tika, E. et al. Prostate luminal progenitor cells: from mouse to human, from health to disease. Nat Rev Urol 19, 201–218 (2022). https://doi.org/10.1038/s41585-021-00561-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00561-2