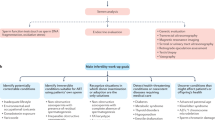
Abstract
Infertility affects one in six couples worldwide, and fertility continues to deteriorate globally, partly owing to a decline in semen quality. Sperm analysis has a central role in diagnosing and treating male factor infertility. Many emerging techniques, such as digital holography, super-resolution microscopy and next-generation sequencing, have been developed that enable improved analysis of sperm motility, morphology and genetics to help overcome limitations in accuracy and consistency, and improve sperm selection for infertility treatment. These techniques have also improved our understanding of fundamental sperm physiology by enabling discoveries in sperm behaviour and molecular structures. Further progress in sperm analysis and integrating these techniques into laboratories and clinics requires multidisciplinary collaboration, which will increase discovery and improve clinical outcomes.
Key points
-
Technical advances have improved the accuracy, speed and efficiency in the analysis of sperm motility, morphology and genetics.
-
Advances in sperm analysis improve sperm selection for infertility treatment by measuring sperm behaviour and biomarkers of fertility potential.
-
Discoveries in sperm locomotion and behaviour improve understanding of sperm motion and guidance within the female reproductive tract.
-
Analyses of sperm structure and molecules such as CatSper channels and ubiquitin increase understanding of sperm physiology and contribute to the identification of biomarkers of fertility potential.
-
Further progress in sperm analysis promises non-invasive testing for sperm selection, increased efficiency and accuracy via automation and artificial intelligence, and improved access to point-of-care assays.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
10 August 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41585-021-00512-x
References
Vos, T. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016).
Agarwal, A. et al. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 13, 37 (2015).
Skakkebaek, N. E. et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol. Rev. 96, 55–97 (2016).
Vollset, S. E. et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet 396, 1285–1306 (2020).
Carlsen, E. et al. Evidence for decreasing quality of semen during past 50 years. BMJ 305, 609–613 (1992).
Levine, H. et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update 23, 646–659 (2017).
Sripada, S. et al. Trends in Semen Parameters in the Northeast of Scotland. J. Androl. 28, 313–319 (2007).
Centola, G. M. et al. Decline in sperm count and motility in young adult men from 2003 to 2013: observations from a U . S . sperm bank. Andrology 4, 270–276 (2016).
Virtanen, H. E. & Toppari, J. Semen quality in the 21 st century. Nat. Rev. Urol. 14, 120–130 (2017).
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. https://www.who.int/docs/default-source/reproductive-health/srhr-documents/infertility/examination-and-processing-of-human-semen-5ed-eng.pdf?sfvrsn=5227886e_2 (2010).
David, S. et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 345, 1388–1393 (2001).
Donnelly, E. T. et al. In vitro fertilization and pregnancy rates: the influence of sperm motility and morphology on IVF outcome. Fertil. Steril. 70, 305–314 (1998).
Bartoov, B. et al. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J. Androl. 23, 1–8 (2002).
De Vos, A. et al. Influence of individual sperm morphology on fertilization, embryo morphology, and pregnancy outcome of intracytoplasmic sperm injection. Fertil. Steril. 79, 42–48 (2003).
Evenson, D. P. The Sperm Chromatin Structure Assay (SCSA) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 169, 56–75 (2016).
Ramasamy, R., Besada, S. & Lamb, D. J. Fluorescent in situ hybridization of human sperm: Diagnostics, indications, and therapeutic implications. Fertil. Steril. 102, 1534–1539 (2014).
Zini, A., San Gabriel, M. & Baazeem, A. Antioxidants and sperm DNA damage: a clinical perspective. J. Assist. Reprod. Genet. 26, 427–432 (2009).
Baldi, E., & Muratori, M. Genetic Damage in Human Spermatozoa (Springer, 2014).
Zidi-Jrah, I. et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil. Steril. 105, 58–64 (2016).
Esteves, S. C., Roque, M., Bradley, C. K. & Garrido, N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil. Steril. 108, 456–467 (2017).
Nosrati, R. et al. Microfluidics for sperm analysis and selection. Nat. Rev. Urol. 14, 707 (2017).
Esteves, S. C., Roque, M., Bedoschi, G., Haahr, T. & Humaidan, P. Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat. Rev. Urol. 15, 1–28 (2018).
Adamson, G. D. et al. International committee for monitoring assisted reproductive technology: world report on assisted reproductive technology, 2011. Fertil. Steril. 110, 1067–1080 (2018).
Sunderam, S. et al. Assisted reproductive technology surveillance — United States, 2012. Morbidity Mortal. Wkly. Report Surveill. Summaries 64, 1–29 (2015).
Wyns, C. et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum. Reprod. Open 3, hoaa032 (2020).
Gnoth, C. et al. Final ART success rates: a 10 years survey. Hum. Reprod. 26, 2239–2246 (2011).
Em, S. et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod. Biomed. Online 27, 325–337 (2013).
Eamer, L. et al. Turning the corner in fertility: high DNA integrity of boundary-following sperm. Lab. Chip 16, 2418–2422 (2016).
Parmegiani, L. et al. “Physiologic ICSI”: Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil. Steril. 93, 598–604 (2010).
Hoogendijk, C. F., Ph, D., Kruger, T. F., Bouic, P. J. D. & Ph, D. A novel approach for the selection of human sperm using annexin V-binding and flow cytometry. Fertil. Steril. 91, 1285–1292 (2009).
Miller, D. et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two-group, randomised trial. Lancet 393, 416–422 (2019).
Sakkas, D., Ramalingam, M., Garrido, N. & Barratt, C. L. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum. Reprod. Update 21, 711–726 (2015).
Amann, R. P. & Katz, D. F. Andrology lab corner: reflections on CASA after 25 years. J. Androl. 25, 317–325 (2004).
Daloglu, M. U. et al. Label-free 3D computational imaging of spermatozoon locomotion, head spin and flagellum beating over a large volume. Light. Sci. Appl. 7, 17111–17121 (2018).
Dai, C. et al. Automated non-invasive measurement of single sperm’s motility and morphology. IEEE Trans. Med. Imaging 37, 2257–2265 (2018).
Sánchez, V. et al. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 98, 1124–1129 (2012).
Nazarenko, R. V., Irzhak, A. V., Pomerantsev, A. L. & Rodionova, O. Y. Confocal Raman spectroscopy and multivariate data analysis for evaluation of spermatozoa with normal and abnormal morphology. A feasibility study. Chemom. Intell. Lab. Syst. 182, 172–179 (2018).
Sequencing, W. et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science 338, 1627–1631 (2012).
Tran, Q. T. et al. Chromosomal scan of single sperm cells by combining fluorescence-activated cell sorting and next-generation sequencing. J. Assist. Reprod. Genet. 36, 91–97 (2019).
Nosrati, R. et al. Rapid selection of sperm with high DNA integrity. Lab. Chip 14, 1142 (2014).
Wagenaar, B. D., Dekker, S., Olthuis, W., Berg, A. V. D. & Segerink, L. I. Towards microfluidic sperm refinement: continuous flow label-free analysis and sorting of sperm cells. Lab. Chip 16, 528–530 (2015).
Zaferani, M., Cheong, S. H. & Abbaspourrad, A. Rheotaxis-based separation of sperm with progressive motility using a microfluidic corral system. Proc. Natl Acad. Sci. USA 115, 8272–8277 (2018).
Bucar, S. et al. DNA fragmentation in human sperm after magnetic-activated cell sorting. J. Assist. Reprod. Genet. 32, 147–154 (2015).
Su, T.-W., Xue, L. & Ozcan, A. High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories. Proc. Natl Acad. Sci. USA 109, 16018–16022 (2012).
Su, T. W. et al. Sperm trajectories form chiral ribbons. Sci. Rep. 3, 1–8 (2013).
Nosrati, R., Driouchi, A., Yip, C. M. & Sinton, D. Two-dimensional slither swimming of sperm within a micrometre of a surface. Nat. Commun. 6, 8703 (2015).
Denissenko, P., Kantsler, V., Smith, D. J. & Kirkman-Brown, J. Human spermatozoa migration in microchannels reveals boundary-following navigation. Proc. Natl Acad. Sci. USA 109, 8007–8010 (2012).
Chung, J. J. et al. Structurally distinct Ca2+ signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 157, 808–822 (2014).
Frolikova, M., Sebkova, N., Ded, L. & Dvorakova-Hortova, K. Characterization of CD46 and β1 integrin dynamics during sperm acrosome reaction. Sci. Rep. 6, 1–15 (2016).
Sutovsky, P., Terada, Y. & Schatten, G. Ubiquitin-based sperm assay for the diagnosis of male factor infertility. Hum. Reprod. 16, 250–258 (2001).
Schiza, C. G., Jarvi, K., Diamandis, E. P. & Drabovich, A. P. An emerging role of TEX101 protein as a male infertility biomarker. J. Int. Fed. Clin. Chem. Lab. Med. 25, 9–26 (2014).
Huszar, G. Biochemical markers of sperm function: male fertility and sperm selection for ICSI. Reprod. Biomed. Online 7, 462–468 (2003).
Louis, G. M. B. et al. Semen quality and time to pregnancy: the longitudinal investigation of fertility and the environment study. Fertil. Steril. 101, 453–462 (2014).
Zinaman, M. J., Brown, C. C., Selevan, S. G. & Clegg, E. D. Semen quality and human fertility: a prospective study with healthy couples. J. Androl. 21, 145–153 (2000).
Bonde, J. P. E. et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 352, 1172–1177 (1998).
Bungum, M. et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum. Reprod. 22, 174–179 (2007).
Duran, E. H., Morshedi, M., Taylor, S. & Oehninger, S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum. Reprod. 17, 3122–3128 (2002).
Marques, C. J. et al. Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol. Hum. Reprod. 14, 67–73 (2008).
Jenkins, T. G. et al. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst. Biol. Reprod. Med. 63, 69–76 (2017).
Turner, R. M. Moving to the beat: a review of mammalian sperm motility regulation. Reprod. Fertil. Dev. 18, 25–38 (2005).
Gaffney, E. et al. Mammalian sperm motility: observation and theory. Annu. Rev. Fluid Mech. 43, 501–528 (2011).
Lindemann, C. B. & Lesich, K. A. Functional anatomy of the mammalian sperm flagellum. Cytoskeleton 73, 652–669 (2016).
Piomboni, P., Focarelli, R., Stendardi, A., Ferramosca, A. & Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 35, 109–124 (2012).
Vernon, G. G. & Woolley, D. M. Basal sliding and the mechanics of oscillation in a mammalian sperm flagellum. Biophys. J. 87, 3934–3944 (2004).
Guzick, D. S. et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 345, 1388–1393 (2001).
Lu, J. C., Huang, Y. F. & Lu, N. Q. Computer‐aided sperm analysis: past, present and future. Andrologia 46, 329–338 (2014).
Amann, R. P. & Waberski, D. Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology 81, 5–17 (2014).
Chenouard, N. et al. Objective comparison of particle tracking methods. Nat. Methods 11, 281–289 (2014).
Urbano, L., Masson, P., VerMilyea, M. & Kam, M. Automatic tracking and motility analysis of human sperm in time-lapse images. IEEE Trans. Med. Imaging 36, 792–801 (2016).
Tomlinson, M. J. et al. Validation of a novel computer-assisted sperm analysis (CASA) system using multitarget-tracking algorithms. Fertil. Steril. 93, 1911–1920 (2010).
Agarwal, A., Henkel, R., Huang, C. C. & Lee, M. S. Automation of human semen analysis using a novel artificial intelligence optical microscopic technology. Andrologia 51, 13440 (2019).
Menkveld, R. et al. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: an effort towards standardization of in-vivo thresholds. Hum. Reprod. 16, 1165–1171 (2001).
Bijar, A., Benavent, A. P., Mikaeili, M. & Khayati, R. Fully automatic identification and discrimination of sperm’s parts in microscopic images of stained human semen smear. J. Biomed. Sci. Eng. 5, 384–395 (2012).
Maree, L., Du Plessis, S. S., Menkveld, R. & Van der Horst, G. Morphometric dimensions of the human sperm head depend on the staining method used. Hum. Reprod. 25, 1369–1382 (2010).
Perdrix, A. & Rives, N. Motile sperm organelle morphology examination (MSOME) and sperm head vacuoles: state of the art in 2013. Hum. Reprod. Update 19, 527–541 (2013).
Berkovitz, A. et al. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum. Reprod. 20, 185–190 (2005).
Setti, A. S., Braga, D. P., Iaconelli, A., Aoki, T. & Borges, E. Twelve years of MSOME and IMSI: a review. Reprod. Biomed. Online 27, 338–352 (2013).
Hammoud, I. et al. Selection of normal spermatozoa with a vacuole-free head (×6300) improves selection of spermatozoa with intact DNA in patients with high sperm DNA fragmentation rates. Andrologia 45, 163–170 (2013).
Balaban, B. et al. Clinical outcome of intracytoplasmic injection of spermatozoa morphologically selected under high magnification: a prospective randomized study. Reprod. Biomed. Online 22, 472–476 (2011).
De Vos, A. et al. Does intracytoplasmic morphologically selected sperm injection improve embryo development? A randomized sibling-oocyte study. Hum. Reprod. 28, 617–626 (2013).
Ebner, T., Shebl, O., Oppelt, P. & Mayer, R. B. Some reflections on intracytoplasmic morphologically selected sperm injection. Int. J. Fertil. Steril. 8, 105 (2014).
Rougier, N. et al. Changes in DNA fragmentation during sperm preparation for intracytoplasmic sperm injection over time. Fertil. Steril. 100, 69–74 (2013).
Vingris, L. et al. Sperm morphological normality under high magnification predicts laboratory and clinical outcomes in couples undergoing ICSI. Hum. Fertil. 18, 81–86 (2015).
Dai, C. et al. Automated motility and morphology measurement of live spermatozoa. Andrology https://doi.org/10.1111/andr.13002 (2021).
Yin, Z., Kanade, T. & Chen, M. Understanding the phase contrast optics to restore artifact-free microscopy images for segmentation. Med. Image Anal. 16, 1047–1062 (2012).
Obara, B., Roberts, M. A., Armitage, J. P. & Grau, V. Bacterial cell identification in differential interference contrast microscopy images. BMC Bioinform. 14, 1–3 (2013).
Shaked, N. T. Label-free quantitative imaging of sperm for in-vitro fertilization using interferometric phase microscopy. JFIV Reprod. Med. Genet. 4, 190 (2016).
Coppola, G. et al. Digital holographic microscopy for the evaluation of human sperm structure. Zygote 22, 446–454 (2014).
Haifler, M. et al. Interferometric phase microscopy for label-free morphological evaluation of sperm cells. Fertil. Steril. 104, 43–47 (2015).
Almeling, R. Selling genes, selling gender: Egg agencies, sperm banks, and the medical market in genetic material. Am. Sociol. Rev. 72, 319–340 (2007).
Bisht, S., Faiq, M., Tolahunase, M. & Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 14, 470–85 (2017).
Tang, S. et al. Biallelic mutations in CFAP43 and CFAP44 cause male infertility with multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 100, 854–864 (2017).
Firat-karalar, E. N., Sante, J., Elliott, S. & Stearns, T. Proteomic analysis of mammalian sperm cells identifies new components of the centrosome. J. Cell Sci. 3, 4128–4133 (2014).
Coutton, C. et al. Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am. J. Hum. Genet. 104, 331–340 (2019).
Zhu, F. et al. Biallelic SUN5 mutations cause autosomal-recessive acephalic spermatozoa syndrome. Am. J. Hum. Genet. 99, 942–949 (2016).
Zhu, F. et al. Mutations in PMFBP1 cause acephalic spermatozoa syndrome. Am. J. Hum. Genet. 103, 188–199 (2018).
Krausz, C. & Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 15, 1–16 (2018).
Lewis, S. E. & Simon, L. Clinical implications of sperm DNA damage. Hum. Fertil. 13, 201–207 (2010).
Evenson, D. P. Evaluation of sperm chromatin structure and DNA strand breaks is an important part of clinical male fertility assessment. Transl. Androl. Urol. 6, 2–7 (2017).
Evenson, D. & Wixon, R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod. Biomed. Online 12, 466–472 (2006).
Shamsi, M. B., Imam, S. N. & Dada, R. Sperm DNA integrity assays: Diagnostic and prognostic challenges and implications in management of infertility. J. Assist. Reprod. Genet. 28, 1073–1085 (2011).
Mishra, S., Kumar, R., Malhotra, N., Singh, N. & Dada, R. Mild oxidative stress is beneficial for sperm telomere length maintenance. World J. Methodol. 6, 163 (2016).
Tremellen, K. Oxidative stress and male infertility — a clinical perspective. Hum. Reprod. Update 14, 243–258 (2008).
Lewis, S. E. & Aitken, R. J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 322, 33–41 (2005).
Kumar, S. B., Chawla, B., Bisht, S., Yadav, R. K. & Dada, R. Tobacco use increases oxidative DNA damage in sperm-possible etiology of childhood cancer. Asian Pac. J. Cancer Prev. 16, 6967–6972 (2015).
Vorilhon, S. et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum. Reprod. 33, 553–562 (2018).
Muratori, M. et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol. Med. 21, 109–122 (2015).
Ni, K., Spiess, A. N., Schuppe, H. C. & Steger, K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: a systematic review and meta‐analysis. Andrology 4, 789–799 (2006).
Sakkas, D., Seli, E., Bizzaro, D., Tarozzi, N. & Manicardi, G. C. Abnormal spermatozoa in the ejaculate: abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online 7, 428–432 (2003).
Virro, M. R., Larson-cook, K. L. & Evenson, D. P. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil. Steril. 81, 1289–1295 (2004).
Henkel, R., Hoogendijk, C. F., Bouic, P. J. D. & Kruger, T. F. TUNEL assay and SCSA determine different aspects of sperm DNA damage. Andrologia 42, 305–313 (2010).
Sergerie, M., Laforest, G., Bujan, L., Bissonnette, F. & Bleau, G. Sperm DNA fragmentation: threshold value in male fertility. Hum. Reprod. 20, 3446–3451 (2005).
Ribeiro, S. et al. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology 5, 477–485 (2017).
Mitchell, L. A., Iuliis, G. N. D. & Aitken, R. J. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int. J. Androl. 34, 2–13 (2010).
Muratori, M., Forti, G. & Baldi, E. Comparing flow cytometry and fluorescence microscopy for analyzing human sperm DNA fragmentation by TUNEL labeling. Cytom. A 73, 785–787 (2008).
Cho, C. L. & Agarwal, A. Role of sperm DNA fragmentation in male factor infertility: a systematic review. Arab. J. Urol. 16, 21–34 (2018).
Muriel, L. et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J. Androl. 24, 59–66 (2003).
Morris, I. D., Ilott, S., Dixon, L. & Brison, D. R. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum. Reprod. 17, 990–998 (2002).
Afanasieva, K. & Sivolob, A. Biophysical chemistry physical principles and new applications of comet assay. Biophys. Chem. 238, 1–7 (2018).
Collins, A. R. et al. The comet assay: topical issues. Mutagenesis 23, 143–151 (2008).
Olive, P. L. & Banáth, J. P. The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 1, 23 (2006).
Simon, L. et al. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil. Steril. 95, 652–657 (2011).
Ribas-Maynou, J. et al. Double stranded sperm DNA breaks measured by comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS ONE 7, 44679 (2012).
Sanchez, V. et al. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 98, 1124–9 (2012).
Angelis, A. De et al. Combined Raman spectroscopy and digital holographic microscopy for sperm cell quality analysis. J. Spectrosc. 2017, 1–15 (2017).
Boydston-white, S., Mattha, C., Romeo, M. & Diem, M. A. X. Raman and infrared microspectral imaging of mitotic cells. Appl. Spectrosc. 60, 1–8 (2006).
Costa, R. Da, Amaral, S., Redmann, K., Kliesch, S. & Id, S. S. Spectral features of nuclear DNA in human sperm assessed by raman microspectroscopy: effects of UV-irradiation and hydration. PLoS ONE 13, 1–15 (2018).
Wang, Y. et al. Prediction of DNA integrity from morphological parameters using a single-sperm DNA fragmentation index assay. Adv. Sci. 6, 1900712 (2019).
Barnea, I. et al. Stain-free interferometric phase microscopy correlation with DNA fragmentation stain in human spermatozoa. J. Biophotonics 11, 1–10 (2018).
McCallum, C. et al. Deep learning-based selection of human sperm with high DNA integrity. Commun. Biol. 2, 1–100 (2019).
Calogero, A. E. et al. Sperm aneuploidy in infertile men. Reprod. Biomed. Online 6, 310–317 (2003).
Shi, Q. & Martin, R. H. Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet. Cell Genet. 90, 219–226 (2000).
Hassold, T. & Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291 (2001).
Muriel, L. et al. Increased aneuploidy rate in sperm with fragmented DNA as determined by the sperm chromatin dispersion (SCD) test and FISH analysis. J. Androl. 28, 38–49 (2007).
Huber, D., Voithenberg, L. V. V. & Kaigala, G. V. Fluorescence in situ hybridization (FISH): History, limitations and what to expect from micro-scale FISH? Micro Nano Eng. 1, 15–24 (2018).
Song, S. H. et al. Genome-wide screening of severe male factor infertile patients using BAC-array comparative genomic hybridization (CGH). Gene 506, 248–252 (2012).
Karampetsou, E., Morrogh, D. & Chitty, L. Microarray technology for the diagnosis of fetal chromosomal aberrations: which platform should we use? J. Clin. Med. 3, 663–678 (2014).
Rubio, C. et al. Use of array comparative genomic hybridization (array-CGH) for embryo assessment: clinical results. Fertil. Steril. 99, 1044–1048 (2013).
Patassini, C. et al. Molecular karyotyping of human single sperm by array- comparative genomic hybridization. PLoS ONE 8, 60922 (2013).
Xi, R., Kim, T. & Park, P. J. Detecting structural variations in the human genome using next generation sequencing. Brief. Funct. Genomics 9, 405–415 (2011).
Koboldt, D. C., Steinberg, K. M., Larson, D. E., Wilson, R. K. & Mardis, E. R. The next-generation sequencing revolution and its impact on genomics. Cell 155, 27–38 (2013).
Cheung, S., Parrella, A., Rosenwaks, Z. & Palermo, G. D. Genetic and epigenetic profiling of the infertile male. PLoS ONE 14, e0214275 (2019).
Kazda, A. et al. Chromosome end protection by blunt-ended telomeres. Genes Dev. 26, 1703–1713 (2012).
Nandakumar, J. & Cech, T. R. Finding the end: recruitment of telomerase to telomeres. Nat. Rev. Mol. Cell Biol. 14, 69–82 (2013).
Thilagavathi, J. et al. Analysis of sperm telomere length in men with idiopathic infertility. Arch. Gynecol. Obstet. 287, 803–807 (2013).
Yang, Q. et al. Sperm telomere length is positively associated with the quality of early embryonic development. Hum. Reprod. 30, 1876–1881 (2015).
Boniewska-Bernacka, E., Pańczyszyn, A. & Cybulska, N. Telomeres as a molecular marker of male infertility. Hum. Fertil. 22, 78–87 (2019).
Lafuente, R. et al. Sperm telomere length in motile sperm selection techniques: a qFISH approach. Andrologia 50, e12840 (2018).
Turner, S. & Hartshorne, G. M. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol. Hum. Reprod. 19, 510–518 (2013).
Cariati, F. et al. Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality. Reprod. Biomed. Online 33, 404–411 (2016).
Zhao, F., Yang, Q., Shi, S., Luo, X. & Sun, Y. Semen preparation methods and sperm telomere length: density gradient centrifugation versus the swim up procedure. Sci. Rep. 6, 39051 (2016).
Rocca, M. S., Foresta, C. & Ferlin, A. Telomere length: lights and shadows on their role in human reproduction. Biol. Reprod. 100, 305–317 (2019).
Jenkins, T. G. & Carrell, D. T. The sperm epigenome and potential implications for the developing embryo. Reproduction 143, 727 (2012).
Hammoud, S. S. et al. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum. Reprod. 26, 2558–2569 (2011).
Denomme, M. M., McCallie, B. R., Parks, J. C., Schoolcraft, W. B. & Katz-Jaffe, M. G. Alterations in the sperm histone-retained epigenome are associated with unexplained male factor infertility and poor blastocyst development in donor oocyte IVF cycles. Hum. Reprod. 32, 2443–2455 (2017).
Aston, K. I. et al. Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil. Steril. 104, 1388–1397 (2015).
Schrott, R. et al. Sperm DNA methylation altered by tHc and nicotine: vulnerability of neurodevelopmental genes with bivalent chromatin. Sci. Rep. 10, 1–12 (2020).
Maamar, M. B. et al. Developmental origins of transgenerational sperm DNA methylation epimutations following ancestral DDT exposure. Dev. Biol. 445, 280–293 (2019).
Jenkins, T. G. et al. Intra-sample heterogeneity of sperm DNA methylation. Mol. Hum. Reprod. 21, 313–319 (2015).
Keravnou, A. et al. Whole-genome fetal and maternal DNA methylation analysis using MeDIP-NGS for the identification of differentially methylated regions. Genet. Res. 98, 1–9 (2016).
Luján, S. et al. Sperm DNA methylation epimutation biomarkers for male infertility and FSH therapeutic responsiveness. Sci. Rep. 9, 1–12 (2019).
Oostlander, A. E., Meijer, G. A. & Ylstra, B. Microarray‐based comparative genomic hybridization and its applications in human genetics. Clin. Genet. 66, 488–495 (2004).
Houshdaran, S. et al. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS ONE 2, e1289 (2007).
Aston, K. I. et al. Genome-wide sperm deoxyribonucleic acid methylation is altered in some men with abnormal chromatin packaging or poor in vitro fertilization embryogenesis. Fertil. Steril. 97, 285–292 (2012).
Brykczynska, U. et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 17, 679 (2010).
Steilmann, C. et al. Presence of histone H3 acetylated at lysine 9 in male germ cells and its distribution pattern in the genome of human spermatozoa. Reprod. Fertil. Dev. 23, 997–1011 (2011).
Hammoud, S. S. et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature 460, 473–478 (2009).
Boissonnas, C., Ph, D. & Jouannet, P. Epigenetic disorders and male subfertility. Fertil. Steril. 99, 624–631 (2013).
Hamatani, T. Human spermatozoal RNAs. Fertil. Steril. 97, 275–281 (2012).
Pelloni, M. et al. Molecular study of human sperm RNA: ropporin and CABYR in asthenozoospermia. J. Endocrinol. Invest. 41, 781–787 (2018).
Gòdia, M. et al. A RNA-Seq analysis to describe the boar sperm transcriptome and its seasonal changes. Front. Genet. 10,, 299 (2019).
Wein, S. et al. A computational platform for high-throughput analysis of RNA sequences and modifications by mass spectrometry. Nat. Commun. 11, 1–12 (2020).
Kukurba, K. R. & Montgomery, S. B. RNA sequencing and analysis. Cold Spring Harb. Protoc. 2015, 951–969 (2015).
Aoki, V. W., Liu, L. & Carrell, D. T. A novel mechanism of protamine expression deregulation highlighted by abnormal protamine transcript retention in infertile human males with sperm protamine deficiency. Mol. Hum. Reprod. 12, 41–50 (2006).
Ostermeier, G. C., Miller, D., Huntriss, J. D., Diamond, M. P. & Krawetz, S. A. Delivering spermatozoan RNA to the oocyte. Nature 429, 154 (2004).
Rassoulzadegan, M. et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 441, 469–474 (2006).
Sone, Y. et al. Nuclear translocation of phospholipase C-zeta, an egg-activating factor, during early embryonic development. Biochem. Biophys. Res. Commun. 330, 690–694 (2005).
Fu, Y., Dominissini, D., Rechavi, G. & He, C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat. Rev. Genet. 15, 293 (2014).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Zini, A., Finelli, A., Phang, D. & Jarvi, K. Influence of semen processing technique on human sperm DNA integrity. Urology 56, 1081–1084 (2000).
Xue, X. et al. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J. Assist. Reprod. Genet. 31, 1161–1166 (2014).
Henkel, R. R. & Schill, W. B. Sperm preparation for ART. Reprod. Biol. Endocrinol. 1, 108 (2003).
Zaferani, M., Palermo, G. D. & Abbaspourrad, A. Strictures of a microchannel impose fierce competition to select for highly motile sperm. Sci. Adv. 5, 2111 (2019).
Li, K. et al. Novel distance — progesterone — combined selection approach improves human sperm quality. J. Transl. Med. 16, 1–10 (2018).
Tasoglu, S. et al. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small 9, 3374–3384 (2013).
Nosrati, R., Graham, P. J., Liu, Q. & Sinton, D. Predominance of sperm motion in corners. Sci. Rep. 6, 1–9 (2016).
Zaferani, M., Hon, S. & Abbaspourrad, A. Rheotaxis-based separation of sperm with progressive motility using a microfluidic corral system. Proc. Natl Acad. Sci. USA 115, 8272–8277 (2018).
Kaupp, U. B., Kashikar, N. D. & Weyand, I. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 70, 93–117 (2008).
Huszar, G. et al. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil. Steril. 79, 1616–1624 (2003).
Simopoulou, M. et al. Improving ICSI: a review from the spermatozoon perspective. Syst. Biol. Reprod. Med. 62, 359–371 (2016).
Liu, T. et al. Detection of apoptosis based on the interaction between annexin V and phosphatidylserine. Anal. Chem. 81, 2410–2413 (2009).
Gil, M., Sar-Shalom, V., Sivira, Y. M., Carreras, R. & Checa, M. A. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: a systematic review and meta-analysis. J. Assist. Reprod. Genet. 30, 479–485 (2013).
Galatioto, G. P. et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J. Urol. 26, 97–102 (2008).
Jun, L. et al. Quantitative analysis of locomotive behavior of human sperm head and tail. Biomed. Eng. IEEE Trans. 60, 390–396 (2013).
Zhang, Z. et al. An automated system for investigating sperm orientation in fluid flow. IEEE Int. Conf. Robot. Autom. https://doi.org/10.1109/ICRA.2016.7487551 (2016).
Hernandez-Herrera, P., Montoya, F., Rendón-Mancha, J. M., Darszon, A. & Corkidi, G. 3-D human sperm flagellum tracing in low SNR fluorescence images. IEEE Trans. Med. Imaging 37, 2236–2247 (2018).
Saggiorato, G. et al. Human sperm steer with second harmonics of the flagellar beat. Nat. Commun. 8, 1–9 (2017).
Friedrich, B. M., Riedel-Kruse, I. H., Howard, J. & Julicher, F. High-precision tracking of sperm swimming fine structure provides strong test of resistive force theory. J. Exp. Biol. 213, 1226–1234 (2010).
Gade, H., Gaffney, E. A. & Smith, D. J. Nonlinear instability in flagellar dynamics: a novel modulation mechanism in sperm migration? J. R. Soc. Interface 7, 1689–1697 (2010).
Zhang, Z. et al. Human sperm rheotaxis: a passive physical process. Sci. Rep. 6, 23553 (2016).
Bukatin, A., Kukhtevich, I., Stoop, N., Dunkel, J. & Kantsler, V. Bimodal rheotactic behavior reflects flagellar beat asymmetry in human sperm cells. Proc. Natl Acad. Sci. USA 112, 15904–15909 (2015).
Pérez-Cerezales, S. et al. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 5, 16146 (2015).
Di Caprio, G. et al. 4D tracking of clinical seminal samples for quantitative characterization of motility parameters. Biomed. Opt. Express 5, 690–700 (2014).
Di Caprio, G. et al. Holographic imaging of unlabelled sperm cells for semen analysis: a review. J. Biophotonics 8, 779–789 (2015).
Suarez, S. S. & Pacey, A. A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 12, 23–37 (2006).
Gadêlha, H., Hernández-Herrera, P., Montoya, F., Darszon, A. & Corkidi, G. Human sperm uses asymmetric and anisotropic flagellar controls to regulate swimming symmetry and cell steering. Sci. Adv. 6, eaba5168 (2020).
Elgeti, J., Kaupp, U. B. & Gompper, G. Hydrodynamics of sperm cells near surfaces. Biophys J 99, 1018–1026 (2010).
Zhang, X. et al. Lensless imaging for simultaneous microfluidic sperm monitoring and sorting. Lab. Chip 11, 2535 (2011).
Bazylewski, P. & Ezugwu, S. A review of three-dimensional scanning near-field optical microscopy (3D-SNOM) and its applications in nanoscale light management. Appl. Sci. 7, 973 (2017).
Andolfi, L. et al. The application of scanning near field optical imaging to the study of human sperm morphology. J. Nanobiotechnol. 13, 2 (2015).
Chemes, H. E. & Rawe, V. Y. Sperm pathology: a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum. Reprod. Update 9, 405–428 (2003).
Xu, J., Tehrani, K. F., Kner, P., States, U. & Avenue, C. Multicolor 3D super-resolution imaging by quantum dot stochastic optical reconstruction microscopy. ACS Nano 9, 2917–2925 (2015).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–814 (2008).
Chung, J. J. et al. CatSperζ regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. eLife 6, e23082 (2007).
Strünker, T. et al. The CatSper channel mediates progesterone-induced Ca 2+ influx in human sperm. Nature 471, 382–386 (2011).
Gervasi, G., Xu, X., Carbajal-Gonzalez, B., Buffone, M. G. & Visconti, P. E. The actin cytoskeleton of the mouse sperm flagellum is organized in a helical structure. J. Cell Sci. 131, 1–9 (2018).
Dunleavy, J. E., O’Bryan, M. K., Stanton, P. G. & O’Donnell, L. The cytoskeleton in spermatogenesis. Reproduction 157, 53–72 (2019).
Paës, G., Habrant, A. & Terryn, C. Fluorescent nano-probes to image plant cell walls by super-resolution STED microscopy. Plants 7, 1–9 (2018).
Aminski, C. L. F. K. Frontiers in structured illumination microscopy. Optica 3, 667–677 (2016).
Gustafsson, M. G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Dan, D. et al. DMD-based LED-illumination super-resolution and optical sectioning microscopy. Sci. Rep. 3, 1–7 (2013).
Chang, B. J., Chou, L. J., Chang, Y. C. & Chiang, S. Y. Isotropic image in structured illumination microscopy patterned with a spatial light modulator. Opt. Express 17, 14710–14721 (2009).
Calvi, A. et al. SUN4 is essential for nuclear remodeling during mammalian spermiogenesis. Dev. Biol. 407, 321–330 (2015).
Yeh, C. H. et al. SEPT12/SPAG4/LAMINB1 complexes are required for maintaining the integrity of the nuclear envelope in postmeiotic male germ cells. PLoS ONE 10, e0120722 (2015).
Miller, M. R. et al. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352, 555–9 (2016).
Baker, M. A., Hetherington, L., Ecroyd, H., Roman, S. D. & Aitken, R. J. Analysis of the mechanism by which calcium negatively regulates the tyrosine phosphorylation cascade associated with sperm capacitation. J. Cell Sci. 117, 211–222 (2004).
Asquith, K. L., Baleato, R. M., McLaughlin, E. A., Nixon, B. & Aitken, R. J. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J. Cell Sci. 117, 3645–3657 (2004).
Awad, H., Khamis, M. M. & El-Aneed, A. Mass spectrometry, review of the basics: ionization. Appl. Spectrosc. Rev. 50, 158–175 (2015).
Platt, M. D., Salicioni, A. M., Hunt, D. F. & Visconti, P. E. Use of differential isotopic labeling and mass spectrometry to analyze capacitation-associated changes in the phosphorylation status of mouse sperm proteins. J. Proteome Res. 8, 1431–1440 (2009).
Ardito, F., Giuliani, M., Perrone, D., Troiano, G. & Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int. J. Mol. Med. 40, 271–280 (2017).
Castillo, J. et al. Proteomic changes in human sperm during sequential in vitro capacitation and acrosome reaction. Front. Cell Dev. Biol. 7, 295 (2019).
Drabovich, A. P. et al. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci. Transl. Med. 5, 212ra160 (2013).
Zerbinati, C. et al. Redox Biology Mass spectrometry pro fi ling of oxysterols in human sperm identi fi es 25- hydroxycholesterol as a marker of sperm function. Redox Biol. 11, 111–117 (2017).
Hafiz, P. et al. Predicting implantation outcome of in vitro fertilization and intracytoplasmic sperm injection using data mining techniques. Int. J. Fertil. Steril. 11, 184–190 (2017).
Comhaire, F., Messiaen, A. & Decleer, W. A mathematical model predicting the individual outcome of IVF through sperm-analysis: the role of the HaloSpermG2 DNA fragmentation test. Med. Hypotheses 117, 50–53 (2018).
Farias-hesson, E. et al. Semi-automated library preparation for high-throughput DNA sequencing platforms. J. Biomed. Biotechnol. 2010, 1–8 (2010).
Malm, J. et al. Semi-automated biobank sample processing with a 384 high density sample tube robot used in cancer and cardiovascular studies. Clin. Transl. Med. 4, 27 (2015).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. J. 42, 60–88 (2017).
Hicks, S. A. et al. Machine learning-based analysis of sperm videos and participant data for male fertility prediction. Sci. Rep. 9, 1–10 (2019).
Sobieranski, A. C. et al. Portable lensless wide-field microscopy imaging platform based on digital inline holography and multi-frame pixel super-resolution. Light. Sci. Appl. 4, 346 (2015).
Kanakasabapathy, M. K. et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci. Transl. Med. 9, 7863 (2017).
Carrilho, E., Martinez, A. W. & Whitesides, G. M. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 81, 7091–7095 (2009).
Matsuura, K. et al. Paper-based diagnostic devices for evaluating the quality of human sperm. Microfluid. Nanofluidics 16, 857–867 (2014).
Nosrati, R., Gong, M. M., Gabriel, C. S., Zini, A. & Sinton, D. Paper-based sperm DNA integrity analysis. Anal. Methods 8, 6260–6264 (2016).
Ribas-Maynou, J. et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 1, 715–722 (2013).
Eisenbach, M. & Giojalas, L. Sperm guidance in mammals — an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 7, 276–285 (2006).
Vanderzwalmen, P. et al. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod. Biomed. Online 17, 617–627 (2008).
Pandiyan, N. et al. in Male Infertility A Clinical Approach (Springer, 2016).
Rougier, N. et al. Changes in DNA fragmentation during sperm preparation for intracytoplasmic sperm injection over time. Fertil. Steril. 100, 69–74 (2013).
Fink, M. & Taylor, M. A. in A Clinician’ s Guide to Diagnosis Sperm DNA and Chromatin Damage (Springer, 2018).
Palermo, G. D., Colombero, L. T., Hariprashad, J. J., Schlegel, P. N. & Rosenwaks, Z. Chromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum. Reprod. 17, 570–575 (2002).
Lockwood, W. W., Chari, R., Chi, B. & Lam, W. L. Recent advances in array comparative genomic hybridization technologies and their applications in human genetics. Eur. J. Hum. Genet. 14, 139–148 (2006).
Author information
Authors and Affiliations
Contributions
Y.S., C.D., Z.Z. and K.J. contributed substantially to discussion of the content. C.D, Z.Z., G.S., L.-T.C. and Z.H. wrote the article. Y.S., S.M., C.L. and K.J. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks R. Dada, N. Garrido and R. Henkel for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, C., Zhang, Z., Shan, G. et al. Advances in sperm analysis: techniques, discoveries and applications. Nat Rev Urol 18, 447–467 (2021). https://doi.org/10.1038/s41585-021-00472-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00472-2
This article is cited by
-
Chromatin condensation but not DNA integrity of pig sperm is greater in the sperm-rich fraction
Journal of Animal Science and Biotechnology (2023)
-
How to select ICSI-viable sperm from the most challenging samples
Nature Reviews Urology (2022)
-
Evolution of the World Health Organization semen analysis manual: where are we?
Nature Reviews Urology (2022)