Abstract
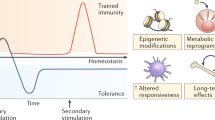
Intravesical BCG instillation is the gold-standard adjuvant immunotherapy for patients with high-risk non-muscle-invasive bladder cancer. However, the precise mechanism of action by which BCG asserts its beneficial effects is still unclear. BCG has been shown to induce a non-specific enhancement of the biological function in cells of the innate immune system, creating a de facto heterologous immunological memory that has been termed trained immunity. Trained immunity or innate immune memory enables innate immune cells to mount a more robust response to secondary non-related stimuli after being initially primed (or trained) by a challenge such as BCG. BCG-induced trained immunity is characterized by the metabolic rewiring of monocyte intracellular metabolism and epigenetic modifications, which subsequently lead to functional reprogramming effects, such as an increased production of cytokines, on restimulation. Results from BCG vaccination studies in humans show that trained immunity might at least partly account for the heterologous beneficial effects of BCG vaccination. Additionally, immunity might have a role in the effect of BCG immunotherapy for bladder cancer. Based on these indications, we propose that trained immunity could be one of the important mechanisms mediating BCG immunotherapy and could provide a basis for further improvements towards a personalized approach to BCG therapy in non-muscle-invasive bladder cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Power, N. E. & Izawa, J. Comparison of guidelines on non-muscle invasive bladder cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer 2, 27–36 (2016).
Babjuk, M. et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) — 2019 update. Eur. Urol. 76, 639–657 (2019).
Shelley, M. D. et al. A systematic review of intravesical bacillus Calmette–Guerin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 88, 209–216 (2001).
Shelley, M. D. et al. Intravesical bacillus Calmette–Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int. 93, 485–490 (2004).
Sylvester, R. J., van der Meijden, A. P., Witjes, J. A. & Kurth, K. Bacillus Calmette–Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J. Urol. 174, 86–91 (2005).
Sylvester, R. J., van der, M. A. & Lamm, D. L. Intravesical bacillus Calmette–Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol. 168, 1964–1970 (2002).
Han, R. F. & Pan, J. G. Can intravesical bacillus Calmette–Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 67, 1216–1223 (2006).
Cambier, S. et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette–Guerin. Eur. Urol. 69, 60–69 (2016).
Hemdan, T. et al. 5-Year outcome of a randomized prospective study comparing bacillus Calmette–Guerin with epirubicin and interferon-alpha2b in patients with T1 bladder cancer. J. Urol. 191, 1244–1249 (2014).
Redelman-Sidi, G., Glickman, M. S. & Bochner, B. H. The mechanism of action of BCG therapy for bladder cancer — a current perspective. Nat. Rev. Urol. 11, 153–162 (2014).
Pettenati, C. & Ingersoll, M. A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 15, 615–625 (2018).
Jallad, S., Goubet, S., Symes, A., Larner, T. & Thomas, P. Prognostic value of inflammation or granuloma after intravesical BCG in non-muscle-invasive bladder cancer. BJU Int. 113, E22–E27 (2014).
Pieras-Ayala, E. et al. Prognostic value of cystocopically pseudotumoral lesions (inflammation/granuloma) in primary stage T1 grade 3 bladder tumors treated with BCG. Int. Urol. Nephrol. 33, 469–472 (2001).
Bisiaux, A. et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus Calmette–Guerin therapy in patients with superficial bladder cancer. J. Urol. 181, 1571–1580 (2009).
Saint, F. et al. Urinary IL-2 assay for monitoring intravesical bacillus Calmette–Guerin response of superficial bladder cancer during induction course and maintenance therapy. Int. J. Cancer 107, 434–440 (2003).
Saint, F. et al. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guerin treatment for superficial bladder cancer. J. Urol. 167, 364–367 (2002).
Bekkering, S. et al. In vitro experimental model of trained innate immunity in human primary monocytes. Clin. Vaccine Immunol. 23, 926–933 (2016).
Cheng, S. C. et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
van der Heijden, C. et al. Epigenetics and trained immunity. Antioxid. Redox Signal. 29, 1023–1040 (2018).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016).
Arts, R. J. W. et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 17, 2562–2571 (2016).
Arts, R. J. et al. Long-term in vitro and in vivo effects of gamma-irradiated BCG on innate and adaptive immunity. J. Leukoc. Biol. 98, 995–1001 (2015).
Wang, X. et al. MLL1, a H3K4 methyltransferase, regulates the TNFalpha-stimulated activation of genes downstream of NF-kappaB. J. Cell Sci. 125, 4058–4066 (2012).
Austenaa, L. et al. The histone methyltransferase Wbp7 controls macrophage function through GPI glycolipid anchor synthesis. Immunity 36, 572–585 (2012).
Kimball, A. S. et al. The histone methyltransferase MLL1 directs macrophage-mediated inflammation in wound healing and is altered in a murine model of obesity and type 2 diabetes. Diabetes 66, 2459–2471 (2017).
Kleinnijenhuis, J. et al. Bacille Calmette–Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Arts, R. J. et al. Vitamin A induces inhibitory histone methylation modifications and down-regulates trained immunity in human monocytes. J. Leukoc. Biol. 98, 129–136 (2015).
Keating, S. T. & El-Osta, A. Epigenetics and metabolism. Circ. Res. 116, 715–736 (2015).
Arts, R. J. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23, 89–100 (2018).
Buffen, K. et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 10, e1004485 (2014).
Kleinnijenhuis, J. et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6, 152–158 (2014).
Xie, Z. & Klionsky, D. J. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 (2007).
Shibutani, S. T., Saitoh, T., Nowag, H., Munz, C. & Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 16, 1014–1024 (2015).
Jo, E. K. Autophagy as an innate defense against mycobacteria. Pathog. Dis. 67, 108–118 (2013).
Songane, M., Kleinnijenhuis, J., Netea, M. G. & van Crevel, R. The role of autophagy in host defence against Mycobacterium tuberculosis infection. Tuberculosis 92, 388–396 (2012).
Zullo, A. J. & Lee, S. Mycobacterial induction of autophagy varies by species and occurs independently of mammalian target of rapamycin inhibition. J. Biol. Chem. 287, 12668–12678 (2012).
Brooks, M. N. et al. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell. Microbiol. 13, 402–418 (2011).
Schenk, M. et al. Human NOD2 recognizes structurally unique muramyl dipeptides from Mycobacterium leprae. Infect. Immun. 84, 2429–2438 (2016).
Park, J.-H. et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178, 2380–2386 (2007).
Bourigault, M. L. et al. Relative contribution of IL-1alpha, IL-1beta and TNF to the host response to Mycobacterium tuberculosis and attenuated M. bovis BCG. Immun. Inflamm. Dis. 1, 47–62 (2013).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161 (2018).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190 (2018).
King, K. Y. & Goodell, M. A. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 11, 685–692 (2011).
Wen, A. Q. et al. Effects of haplotypes in the interleukin 1beta promoter on lipopolysaccharide-induced interleukin 1beta expression. Shock 26, 25–30 (2006).
Levine, M. I. & Sackett, M. F. Results of BCG immunization in New York City. Am. Rev. Tuberc. 53, 517–532 (1946).
Aronson, J. D. Protective vaccination against tuberculosis, with special reference to BCG vaccine. Minn. Med. 31, 1336 (1948).
Ferguson, R. G. & Simes, A. B. BCG vaccination of Indian infants in Saskatchewan. Tubercle 30, 5–11 (1949).
Rosenthal, S. R. et al. BCG vaccination in tuberculous households. Am. Rev. Respir. Dis. 84, 690–704 (1961).
Trunz, B. B., Fine, P. & Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367, 1173–1180 (2006).
Aaby, P. et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 204, 245–252 (2011).
Biering-Sorensen, S. et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette–Guerin vaccination at first health center contact. Pediatr. Infect. Dis. J. 31, 306–308 (2012).
Higgins, J. P. et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ 355, i5170 (2016).
Moorlag, S., Arts, R. J. W., van Crevel, R. & Netea, M. G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 25, 1473–1478 (2019).
Walk, J. et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 10, 874 (2019).
Kleinnijenhuis, J. et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 155, 213–219 (2014).
van ‘t Wout, J. W., Poell, R. & van Furth, R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 36, 713–719 (1992).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Conti, P. et al. Bacillus Calmette–Guerin potentiates monocyte responses to lipopolysaccharide-induced tumor necrosis factor and interleukin-1, but not interleukin-6 in bladder cancer patients. Cancer Immunol. Immunother. 38, 365–371 (1994).
Kim, C. I., Shin, J. S., Kim, H. I., Lee, J. M. & Kim, S. J. Production of tumor necrosis factor by intravesical administration of bacillus Calmette–Guerin in patients with superficial bladder cancer. Yonsei Med. J. 34, 356–364 (1993).
Calais da Silva, F. M. et al. Systemic humoral responses of non-muscle-invasive bladder cancer during BCG treatment: less is more. J. Cancer Metastasis Treat. 3, 116–126 (2017).
Adeli, K. et al. Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures survey. Clin. Chem. 61, 1075–1086 (2015).
Luo, Y., Chen, X. & O’Donnell, M. A. Mycobacterium bovis bacillus Calmette–Guerin (BCG) induces human CC- and CXC-chemokines in vitro and in vivo. Clin. Exp. Immunol. 147, 370–378 (2007).
Noguera-Ortega, E. et al. Intravesical Mycobacterium brumae triggers both local and systemic immunotherapeutic responses against bladder cancer in mice. Sci. Rep. 8, 15102 (2018).
Biot, C. et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci. Transl Med. 4, 137ra172 (2012).
Shintani, Y. et al. Intravesical instillation therapy with bacillus Calmette–Guerin for superficial bladder cancer: study of the mechanism of bacillus Calmette–Guerin immunotherapy. Int. J. Urol. 14, 140–146 (2007).
Taniguchi, K. et al. Systemic immune response after intravesical instillation of bacille Calmette–Guerin (BCG) for superficial bladder cancer. Clin. Exp. Immunol. 115, 131–135 (1999).
Kamat, A. M. et al. Cytokine panel for response to intravesical therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette–Guerin. Eur. Urol. 69, 197–200 (2016).
de Reijke, T. M., de Boer, E. C., Kurth, K. H. & Schamhart, D. H. Urinary cytokines during intravesical bacillus Calmette–Guerin therapy for superficial bladder cancer: processing, stability and prognostic value. J. Urol. 155, 477–482 (1996).
de Reijke, T. M., De Boer, E. C., Kurth, K. H. & Schamhart, D. H. Urinary interleukin-2 monitoring during prolonged bacillus Calmette–Guerin treatment: can it predict the optimal number of instillations? J. Urol. 161, 67–71 (1999).
Reale, M. et al. Production of MCP-1 and RANTES in bladder cancer patients after bacillus Calmette–Guerin immunotherapy. Cancer Immunol. Immunother. 51, 91–98 (2002).
Arends, T. J. H. et al. Urinary cytokines in patients treated with intravesical mitomycin-C with and without hyperthermia. World J. Urol. 33, 1411–1417 (2015).
Foster, S. L., Hargreaves, D. C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Toyonaga, K. et al. C-type lectin receptor DCAR recognizes mycobacterial phosphatidyl-inositol mannosides to promote a Th1 response during infection. Immunity 45, 1245–1257 (2016).
Torrelles, J. B., Azad, A. K. & Schlesinger, L. S. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J. Immunol. 177, 1805–1816 (2006).
Yonekawa, A. et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 41, 402–413 (2014).
Ishikawa, E., Mori, D. & Yamasaki, S. Recognition of mycobacterial lipids by immune receptors. Trends Immunol. 38, 66–76 (2017).
Jones, B. W., Heldwein, K. A., Means, T. K., Saukkonen, J. J. & Fenton, M. J. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann. Rheumatic Dis. 60, iii6–iii12 (2001).
Jones, B. W. et al. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 69, 1036–1044 (2001).
Yan, R. & Liu, Z. LRRK2 enhances Nod1/2-mediated inflammatory cytokine production by promoting Rip2 phosphorylation. Protein Cell 8, 55–66 (2017).
de Jong, E., Lim, A., Waterer, G. & Price, P. Monocyte-derived macrophages do not explain susceptibility to pulmonary non-tuberculous mycobacterial disease. Clin. Transl Immunol. 1, e2 (2012).
Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198 (2004).
Lee, K. W. et al. Immunostimulatory oligodeoxynucleotide isolated from genome wide screening of Mycobacterium bovis chromosomal DNA. Mol. Immunol. 43, 2107–2118 (2006).
Thomas-White, K. et al. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 9, 1557 (2018).
Meshkibaf, S., Fritz, J., Gottschalk, M. & Kim, S. O. Preferential production of G-CSF by a protein-like Lactobacillus rhamnosus GR-1 secretory factor through activating TLR2-dependent signaling events without activation of JNKs. BMC Microbiol. 15, 238 (2015).
Uehori, J. et al. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette–Guerin peptidoglycan. Infect. Immun. 71, 4238–4249 (2003).
Tsuji, S. et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette–Guerin: involvement of Toll-like receptors. Infect. Immun. 68, 6883–6890 (2000).
Astarie-Dequeker, C. et al. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 5, e1000289 (2009).
Oldenburg, R. et al. Mycobacterial phenolic glycolipids selectively disable TRIF-dependent TLR4 signaling in macrophages. Front. Immunol. 9, 2 (2018).
Arbues, A. et al. Trisaccharides of phenolic glycolipids confer advantages to pathogenic mycobacteria through manipulation of host–cell pattern-recognition receptors. ACS Chem. Biol. 11, 2865–2875 (2016).
Kohka Takahashi, H. et al. Role of cell–cell interactions in high mobility group Box 1 cytokine activity in human peripheral blood mononuclear cells and mouse splenocytes. Eur. J. Pharmacol. 701, 194–202 (2013).
Andersson, U. et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 192, 565–570 (2000).
Aneja, R. K. et al. Preconditioning with high mobility group Box 1 (HMGB1) induces lipopolysaccharide (LPS) tolerance. J. Leukoc. Biol. 84, 1326–1334 (2008).
Zhang, G. et al. HMGB1 release by urothelial carcinoma cells in response to bacillus Calmette–Guerin functions as a paracrine factor to potentiate the direct cellular effects of bacillus Calmette–Guerin. J. Urol. 190, 1076–1082 (2013).
Zhang, G., Chen, F., Cao, Y. & See, W. A. Contributors to HMGB1 release by urothelial carcinoma cells in response to bacillus Calmette–Guerin. J. Urol. 190, 1398–1403 (2013).
Shah, G. et al. The dose–response relationship of bacillus Calmette–Guerin and urothelial carcinoma cell biology. J. Urol. 195, 1903–1910 (2016).
See, W. A. et al. Bacille Calmette–Guerin induces caspase-independent cell death in urothelial carcinoma cells together with release of the necrosis-associated chemokine high molecular group Box protein 1. BJU Int. 103, 1714–1720 (2009).
Chen, B. et al. S100A9 induced inflammatory responses are mediated by distinct damage associated molecular patterns (DAMP) receptors in vitro and in vivo. PLoS One 10, e0115828 (2015).
Tolson, J. P. et al. Differential detection of S100A8 in transitional cell carcinoma of the bladder by pair wise tissue proteomic and immunohistochemical analysis. Proteomics 6, 697–708 (2006).
Afonina, I. S. et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1alpha. Mol. Cell 44, 265–278 (2011).
Di Paolo, N. C. & Shayakhmetov, D. M. Interleukin 1alpha and the inflammatory process. Nat. Immunol. 17, 906–913 (2016).
Grimm, S. et al. Malignancy of bladder cancer cells is enhanced by tumor-associated fibroblasts through a multifaceted cytokine–chemokine loop. Exp. Cell Res. 335, 1–11 (2015).
Garg, M. et al. Heat-shock protein 70-2 (HSP70-2) expression in bladder urothelial carcinoma is associated with tumour progression and promotes migration and invasion. Eur. J. Cancer 46, 207–215 (2010).
Behnsawy, H. M., Miyake, H., Kusuda, Y. & Fujisawa, M. Small interfering RNA targeting heat shock protein 70 enhances chemosensitivity in human bladder cancer cells. Urologic Oncol. 31, 843–848 (2013).
Vabulas, R. M. et al. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 277, 15107–15112 (2002).
Fang, H. et al. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 286, 30393–30400 (2011).
Lee, K. H., Jeong, J. & Yoo, C. G. Positive feedback regulation of heat shock protein 70 (Hsp70) is mediated through Toll-like receptor 4-PI3K/Akt-glycogen synthase kinase-3beta pathway. Exp. Cell Res. 319, 88–95 (2013).
Yang Han, Z. & Oppenheim, J. J. Alarmins and immunity. Immunol. Rev. 280, 41–56 (2017).
Daniels, M. J. et al. Contemporary oncologic outcomes of second induction course BCG in patients with nonmuscle invasive bladder cancer. Urologic Oncol. 38, 5 (2020).
Grimm, M. O. et al. Treatment of high-grade non-muscle-invasive bladder carcinoma by standard number and dose of BCG instillations versus reduced number and standard dose of BCG instillations: results of the European Association of Urology Research Foundation randomised phase III clinical trial ‘NIMBUS’. Eur. Urol. https://doi.org/10.1016/j.eururo.2020.04.066 (2020).
Oddens, J. et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette–Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur. Urol. 63, 462–472 (2013).
Kamat, A. M. et al. Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat. Rev. Urol. 12, 225–235 (2015).
D’Andrea, D. et al. Comparative effectiveness of intravesical BCG-Tice and BCG-Moreau in patients with non-muscle-invasive bladder cancer. Clin. Genitourin. Cancer 18, 20–25 (2020).
Niwa, N. et al. Does switching the bacillus Calmette–Guerin strain affect clinical outcome in patients with recurrent non-muscle-invasive bladder cancer after initial bacillus Calmette–Guerin therapy? Urologic Oncol. 36, 306 (2018).
Krajewski, W. et al. Are there differences in toxicity and efficacy between various bacillus Calmette–Guerin strains in bladder cancer patients? Analysis of 844 patients. Urol. Int. 101, 277–284 (2018).
Niwa, N. et al. Purified protein derivative skin test prior to bacillus Calmette–Guerin therapy may have therapeutic impact in patients with nonmuscle invasive bladder cancer. J. Urol. 199, 1446–1451 (2018).
Hofbauer, S. L. et al. The Moreau strain of bacillus Calmette–Guerin (BCG) for high-risk non-muscle invasive bladder cancer: an alternative during worldwide BCG shortage? Urol. Int. 96, 46–50 (2016).
Gan, C., Mostafid, H., Khan, M. S. & Lewis, D. J. BCG immunotherapy for bladder cancer — the effects of substrain differences. Nat. Rev. Urol. 10, 580–588 (2013).
Boehm, B. E. et al. Efficacy of bacillus Calmette–Guerin strains for treatment of nonmuscle invasive bladder cancer: a systematic review and network meta-analysis. J. Urol. 198, 503–510 (2017).
Witjes, J. A. et al. The efficacy of BCG TICE and BCG Connaught in a cohort of 2,099 patients with T1G3 non-muscle-invasive bladder cancer. Urol. Oncol. 34, 484 (2016).
Secanella-Fandos, S., Noguera-Ortega, E., Olivares, F., Luquin, M. & Julian, E. Killed but metabolically active Mycobacterium bovis bacillus Calmette–Guerin retains the antitumor ability of live bacillus Calmette–Guerin. J. Urol. 191, 1422–1428 (2014).
Liu, Y., Lu, J., Huang, Y. & Ma, L. Clinical spectrum of complications induced by intravesical immunotherapy of bacillus Calmette–Guerin for bladder cancer. J. Oncol. 2019, 6230409 (2019).
Nakagawa, T. et al. Reiter’s syndrome following intravesical bacillus Calmette–Guerin therapy for bladder carcinoma: a report of five cases. Int. Cancer Conf. J. 7, 148–151 (2018).
Arts, R. J. W., Joosten, L. A. B. & Netea, M. G. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front. Immunol. 9, 298 (2018).
Cabău, G., Crișan, T. O., Klück, V., Popp, R. A. & Joosten, L. A. B. Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 294, 92–105 (2020).
Arbues, A., Lugo-Villarino, G., Neyrolles, O., Guilhot, C. & Astarie-Dequeker, C. Playing hide-and-seek with host macrophages through the use of mycobacterial cell envelope phthiocerol dimycocerosates and phenolic glycolipids. Front. Cell. Infect. Microbiol. 4, 173 (2014).
Cambier, C. J. et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505, 218–222 (2014).
Kleinnijenhuis, J., Oosting, M., Joosten, L. A., Netea, M. G. & Van Crevel, R. Innate immune recognition of Mycobacterium tuberculosis. Clin. Dev. Immunol. 2011, 405310 (2011).
Chen, J. M., Islam, S. T., Ren, H. & Liu, J. Differential productions of lipid virulence factors among BCG vaccine strains and implications on BCG safety. Vaccine 25, 8114–8122 (2007).
Dinarello, C. A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nature reviews. Rheumatology 15, 612–632 (2019).
Netea, M. G. & Joosten, L. A. B. Trained immunity and local innate immune memory in the lung. Cell 175, 1463–1465 (2018).
Yao, Y. et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175, 1634–1650 (2018).
Krajewski, W. et al. Does Mantoux test results predict BCG immunotherapy efficiency and severe toxicity in non-muscle invasive bladder cancer. Urol. J. 16, 458–462 (2019).
Svatek, R. S., Tangen, C., Delacroix, S., Lowrance, W. & Lerner, S. P. Background and update for S1602 ‘A phase III randomized trial to evaluate the influence of BCG strain differences and T cell priming with intradermal BCG before intravesical therapy for BCG-naive high-grade non-muscle-invasive bladder cancer’. Eur. Urol. Focus. 4, 522–524 (2018).
Novakovic, B. et al. Beta-glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167, 1354–1368 (2016).
Netea, M. G., Joosten, L. A. B. & van der Meer, J. W. M. Hypothesis: stimulation of trained immunity as adjunctive immunotherapy in cancer. J. Leukoc. Biol. 102, 1323–1332 (2017).
Mulder, W. J. M., Ochando, J., Joosten, L. A. B., Fayad, Z. A. & Netea, M. G. Therapeutic targeting of trained immunity. Nat. Rev. Drug Discov. 18, 553–566 (2019).
Larsen, E. S., Joensen, U. N., Poulsen, A. M., Goletti, D. & Johansen, I. S. Bacillus Calmette–Guerin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. Apmis 128, 92–103 (2020).
van der Wel, N. et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129, 1287–1298 (2007).
Shah, G. et al. Loss of bacillus Calmette–Guerin viability adversely affects the direct response of urothelial carcinoma cells to bacillus Calmette–Guerin exposure. J. Urol. 191, 823–829 (2014).
Noguera-Ortega, E. et al. Nonpathogenic Mycobacterium brumae inhibits bladder cancer growth in vitro, ex vivo, and in vivo. Eur. Urol. Focus 2, 67–76 (2016).
Packiam, V. T., Pearce, S. M. & Steinberg, G. D. The role of mycobacterial cell wall nucleic acid complex in the treatment of bacillus Calmette–Guerin failures for non-muscle-invasive bladder cancer. Ther. Adv. Urol. 8, 29–37 (2016).
Morales, A. et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette–Guerin. J. Urol. 193, 1135–1143 (2015).
Acknowledgements
M.G.N. was supported by a Netherlands Organization for Scientific Research Spinoza Grant (NWO SPI 94-212). L.A.B.J. was supported by a Competitiveness Operational Programme grant of the Romanian Ministry of European Funds (P_37_762, MySMIS 103587). S.H.V. is partly supported by a grant from the Netherlands Organization for Scientific Research (NWO Vidi 91717334).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to discussion of content and reviewed and edited the manuscript before submission. S.H.V., J.H.v.P. and S.T.K. researched data for the article. S.H.V., J.H.v.P., S.T.K. and L.A.B.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.A.B.J. and M.G.N. declare that they are scientific founders of Trained Therapeutics Discovery and are the owners of two patents related to trained immunity. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks G. Redelman-Sidi, E. Julián and T. Ratliff for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Puffelen, J.H., Keating, S.T., Oosterwijk, E. et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat Rev Urol 17, 513–525 (2020). https://doi.org/10.1038/s41585-020-0346-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-0346-4
This article is cited by
-
Immunologic Aspects of Endometriosis
Current Obstetrics and Gynecology Reports (2024)
-
The androgen receptor in bladder cancer
Nature Reviews Urology (2023)
-
Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis
Nature Immunology (2023)
-
The dynamic roles of the bladder tumour microenvironment
Nature Reviews Urology (2022)
-
Immune evasion and provocation by Mycobacterium tuberculosis
Nature Reviews Microbiology (2022)