Abstract
Ever-present in our environments, light entrains circadian rhythms over long timescales, influencing daily activity patterns, health and performance. Increasing evidence indicates that light also acts independently of the circadian system to directly impact physiology and behaviour, including cognition. Exposure to light stimulates brain areas involved in cognition and appears to improve a broad range of cognitive functions. However, the extent of these effects and their mechanisms are unknown. Intrinsically photosensitive retinal ganglion cells (ipRGCs) have emerged as the primary conduit through which light impacts non-image-forming behaviours and are a prime candidate for mediating the direct effects of light on cognition. Here, we review the current state of understanding of these effects in humans and mice, and the tools available to uncover circuit-level and photoreceptor-specific mechanisms. We also address current barriers to progress in this area. Current and future efforts to unravel the circuits through which light influences cognitive functions may inform the tailoring of lighting landscapes to optimize health and cognitive function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yalçin, M. et al. It’s about time: the circadian network as time-keeper for cognitive functioning, locomotor activity and mental health. Front. Physiol. 13, 873237 (2022).
Muscogiuri, G. et al. Exposure to artificial light at night: a common link for obesity and cancer? Eur. J. Cancer 173, 263–275 (2022).
Dollish, H. K., Tsyglakova, M. & McClung, C. A. Circadian rhythms and mood disorders: time to see the light. Neuron https://doi.org/10.1016/j.neuron.2023.09.023 (2023).
Zielinska-Dabkowska, K. M., Schernhammer, E. S., Hanifin, J. P. & Brainard, G. C. Reducing nighttime light exposure in the urban environment to benefit human health and society. Science 380, 1130–1135 (2023).
Campbell, I., Sharifpour, R. & Vandewalle, G. Light as a modulator of non-image-forming brain functions—positive and negative impacts of increasing light availability. Clocks Sleep. 5, 116–140 (2023). This work presents a review of non-image-forming vision and the effects of light, in the context of changing lighting environments.
Vandewalle, G., Maquet, P. & Dijk, D.-J. Light as a modulator of cognitive brain function. Trends Cogn. Sci. 13, 429–438 (2009).
Beier, C., Zhang, Z., Yurgel, M. & Hattar, S. Projections of ipRGCs and conventional RGCs to retinorecipient brain nuclei. J. Comp. Neurol. 529, 1863–1875 (2021).
Warthen, D. M. & Provencio, I. The role of intrinsically photosensitive retinal ganglion cells in nonimage-forming responses to light. Eye Brain 4, 43–48 (2012).
Fisk, A. S. et al. Light and cognition: roles for circadian rhythms, sleep, and arousal. Front. Neurol. 9, 56 (2018).
Brainard, G. C. & Hanifin, J. P. Photons, clocks, and consciousness. J. Biol. Rhythm. 20, 314–325 (2005).
Gaggioni, G., Maquet, P., Schmidt, C., Dijk, D. J. & Vandewalle, G. Neuroimaging, cognition, light and circadian rhythms. Front. Syst. Neurosci. 8, 126 (2014).
LeGates, T. A., Fernandez, D. C. & Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 15, 443–454 (2014). This review provides useful details about melanopsin and ipRGCs, non-image-forming visual functions and the direct and indirect pathways through which ipRGCs can influence circadian rhythmicity, mood and cognition.
Münch, M. & Bromundt, V. Light and chronobiology: implications for health and disease. Dialogues Clin. Neurosci. 14, 448–453 (2012).
Takahashi, J. S. & Zatz, M. Regulation of circadian rhythmicity. Science 217, 1104–1111 (1982).
Vitaterna, M. H., Takahashi, J. S. & Turek, F. W. Overview of circadian rhythms. Alcohol. Res. Health 25, 85–93 (2001). This work is an essential, expert introduction to circadian rhythms.
Westland, S., Pan, Q. & Lee, S. A review of the effects of colour and light on non-image function in humans. Coloration Technol. 133, 349–361 (2017).
Zeitzer, J. M., Dijk, D. J., Kronauer, R., Brown, E. & Czeisler, C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J. Physiol. 526(Pt 3), 695–702 (2000).
Chellappa, S. L., Gordijn, M. C. & Cajochen, C. Can light make us bright? Effects of light on cognition and sleep. Prog. Brain Res. 190, 119–133 (2011).
Siraji, M. A., Kalavally, V., Schaefer, A. & Haque, S. Effects of daytime electric light exposure on human alertness and higher cognitive functions: a systematic review. Front. Psychol. 12, 765750 (2021).
Barack, D. L. & Krakauer, J. W. Two views on the cognitive brain. Nat. Rev. Neurosci. 22, 359–371 (2021).
Neisser, U. Cognitive Psychology: Classic Edition (Psychology Press, 2014).
Poldrack, R. A. et al. The cognitive atlas: toward a knowledge foundation for cognitive neuroscience. Front. Neuroinform 5, 17 (2011).
Cajochen, C. Alerting effects of light. Sleep. Med. Rev. 11, 453–464 (2007).
Meyer, N., Harvey, A. G., Lockley, S. W. & Dijk, D. J. Circadian rhythms and disorders of the timing of sleep. Lancet 400, 1061–1078 (2022).
Rüger, M., Gordijn, M. C. M., Beersma, D. G. M., Vries, B. D. & Daan, S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1413–R1420 (2006).
Cajochen, C. et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 90, 1311–1316 (2005).
Chellappa, S. L. et al. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS ONE 6, e16429 (2011).
Schöllhorn, I. et al. Melanopic irradiance defines the impact of evening display light on sleep latency, melatonin and alertness. Commun. Biol. 6, 228 (2023).
Lok, R., Smolders, K., Beersma, D. G. M. & de Kort, Y. A. W. Light, alertness, and alerting effects of white light: a literature overview. J. Biol. Rhythm. 33, 589–601 (2018).
Provencio, I. et al. A novel human opsin in the inner retina. J. Neurosci. 20, 600–605 (2000).
Qiu, X. et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature 433, 745–749 (2005).
Vandewalle, G. et al. Daytime light exposure dynamically enhances brain responses. Curr. Biol. 16, 1616–1621 (2006).
Badia, P., Myers, B., Boecker, M., Culpepper, J. & Harsh, J. R. Bright light effects on body temperature, alertness, EEG and behavior. Physiol. Behav. 50, 583–588 (1991).
Souman, J. L., Tinga, A. M., Te Pas, S. F., van Ee, R. & Vlaskamp, B. N. S. Acute alerting effects of light: a systematic literature review. Behav. Brain Res. 337, 228–239 (2018).
Lockley, S. W. et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 29, 161–168 (2006).
Sahin, L. & Figueiro, M. G. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol. Behav. 116–117, 1–7 (2013).
Phipps-Nelson, J., Redman, J. R., Dijk, D.-J. & Rajaratnam, S. M. W. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep 26, 695–700 (2003).
Lok, R., Woelders, T., Gordijn, M. C. M., Hut, R. A. & Beersma, D. G. M. White light during daytime does not improve alertness in well-rested individuals. J. Biol. Rhythm. 33, 637–648 (2018). This study neatly illustrates the importance of considering the internal state of study participants, and other factors that may influence cognition.
Katsuki, F. & Constantinidis, C. Bottom-up and top-down attention: different processes and overlapping neural systems. Neuroscientist 20, 509–521 (2014).
Min, B.-K., Jung, Y.-C., Kim, E. & Park, J. Y. Bright illumination reduces parietal EEG α activity during a sustained attention task. Brain Res. 1538, 83–92 (2013).
Killgore, W. D. S. et al. Blue light exposure enhances neural efficiency of the task positive network during a cognitive interference task. Neurosci. Lett. 735, 135242 (2020).
Vandewalle, G. et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS ONE 2, e1247 (2007).
Aston-Jones, G., Chen, S., Zhu, Y. & Oshinsky, M. L. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 4, 732–738 (2001).
Gnyawali, S., Feigl, B., Adhikari, P. & Zele, A. J. The role of melanopsin photoreception on visual attention linked pupil responses. Eur. J. Neurosci. 55, 1986–2002 (2022).
Alexandre, C. et al. Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat. Med. 23, 768–774 (2017).
Åkerstedt, T., Hallvig, D. & Kecklund, G. Normative data on the diurnal pattern of the Karolinska Sleepiness Scale ratings and its relation to age, sex, work, stress, sleep quality and sickness absence/illness in a large sample of daytime workers. J. Sleep. Res. 26, 559–566 (2017).
Vandewalle, G. et al. Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J. Biol. Rhythm. 26, 249–259 (2011).
Diamond, A. Executive functions. Annu. Rev. Psychol. 64, 135–168 (2013). This work is an essential introduction to executive functions.
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001).
Morellini, F. Spatial memory tasks in rodents: what do they model? Cell Tissue Res. 354, 273–286 (2013).
Manoochehri, M. Up to the magical number seven: an evolutionary perspective on the capacity of short term memory. Heliyon 7, e06955 (2021).
Lee, H.-H., Tu, Y.-C. & Yeh, S.-L. In search of blue-light effects on cognitive control. Sci. Rep. 11, 15505 (2021).
Ferlazzo, F. et al. Effects of new light sources on task switching and mental rotation performance. J. Environ. Psychol. 39, 92–100 (2014).
Hartstein, L. E., LeBourgeois, M. K. & Berthier, N. E. Light correlated color temperature and task switching performance in preschool-age children: preliminary insights. PLoS ONE 13, e0202973 (2018).
Daneault, V. et al. Plasticity in the sensitivity to light in aging: decreased non-visual impact of light on cognitive brain activity in older individuals but no impact of lens replacement. Front. Physiol. 9, 1557 (2018).
Vandewalle, G. et al. Blue light stimulates cognitive brain activity in visually blind individuals. J. Cogn. Neurosci. 25, 2072–2085 (2013). This work is one of a series of studies using sub-behaviourally impactful light stimuli to induce changes in measures of brain activity in the absence of behavioural changes, unique in the use of visually blind participants with ostensibly intact ipRGCs.
Vandewalle, G. et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb. Cortex 17, 2788–2795 (2007).
Alkozei, A. et al. Exposure to blue light increases subsequent functional activation of the prefrontal cortex during performance of a working memory task. Sleep 39, 1671–1680 (2016).
Kandel, E. R., Dudai, Y. & Mayford, M. R. The molecular and systems biology of memory. Cell 157, 163–186 (2014). This work is an essential introduction to memory.
Kukushkin, N. V. & Carew, T. J. Memory takes time. Neuron 95, 259–279 (2017).
Korte, M. & Schmitz, D. Cellular and system biology of memory: timing, molecules, and beyond. Physiological Rev. 96, 647–693 (2016).
Hartsock, M. J. & Spencer, R. L. Memory and the circadian system: identifying candidate mechanisms by which local clocks in the brain may regulate synaptic plasticity. Neurosci. Biobehav. Rev. 118, 134–162 (2020).
Frankland, P. W., Josselyn, S. A. & Köhler, S. The neurobiological foundation of memory retrieval. Nat. Neurosci. 22, 1576–1585 (2019).
Clopath, C. Synaptic consolidation: an approach to long-term learning. Cogn. Neurodyn 6, 251–257 (2012).
Goto, A. Synaptic plasticity during systems memory consolidation. Neurosci. Res. 183, 1–6 (2022).
Alkozei, A., Smith, R., Dailey, N. S., Bajaj, S. & Killgore, W. D. S. Acute exposure to blue wavelength light during memory consolidation improves verbal memory performance. PLoS ONE 12, e0184884 (2017).
Cajochen, C. et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 110, 1432–1438 (2011).
Ober, B. A. in Encyclopedia of the Neurological Sciences 2nd edn (eds Aminoff, M. J. & Daroff, R. B.) 1042–1044 (Academic, 2014).
Jung, H.-C., Kim, J.-H. & Lee, C.-W. The effect of the illuminance of light emitting diode (LED) lamps on long-term memory. Displays 49, 1–5 (2017).
Lee, C. W. & Kim, J. H. The influence of LED lighting on attention and long-term memory. Int. J. Opt. 2020, 8652108 (2020).
Adolphs, R. Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci. 4, 165–178 (2003). This work is an essential introduction to social and emotional cognition.
Padilla-Coreano, N., Tye, K. M. & Zelikowsky, M. Dynamic influences on the neural encoding of social valence. Nat. Rev. Neurosci. 23, 535–550 (2022).
Vandewalle, G. et al. Spectral quality of light modulates emotional brain responses in humans. Proc. Natl Acad. Sci. USA 107, 19549–19554 (2010).
Sabbah, S., Worden, M. S., Laniado, D. D., Berson, D. M. & Sanes, J. N. Luxotonic signals in human prefrontal cortex as a possible substrate for effects of light on mood and cognition. Proc. Natl Acad. Sci. USA 119, e2118192119 (2022).
Mure, L. S., Vinberg, F., Hanneken, A. & Panda, S. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 366, 1251–1255 (2019).
Schmidt, T. M. & Kofuji, P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J. Neurosci. 29, 476–482 (2009).
Wong, K. Y. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J. Neurosci. 32, 11478–11485 (2012).
Bijleveld, E. & Knufinke, M. Exposure to bright light biases effort-based decisions. Behav. Neurosci. 132, 183–193 (2018).
Cawley, E. I. et al. Dopamine and light: dissecting effects on mood and motivational states in women with subsyndromal seasonal affective disorder. J. Psychiatry Neurosci. 38, 388–397 (2013).
Kombeiz, O. & Steidle, A. Facilitation of creative performance by using blue and red accent lighting in work and learning areas. Ergonomics 61, 456–463 (2018).
Summers, T. A. & Hebert, P. R. Shedding some light on store atmospherics: influence of illumination on consumer behavior. J. Bus. Res. 54, 145–150 (2001).
Guido, G., Piper, L., Prete, M. I., Mileti, A. & Trisolini, C. M. Effects of blue lighting in ambient and mobile settings on the intention to buy hedonic and utilitarian products. Psychol. Mark. 34, 215–226 (2017).
Huiberts, L. M., Smolders, K. C. H. J. & de Kort, Y. A. W. Shining light on memory: effects of bright light on working memory performance. Behav. Brain Res. 294, 234–245 (2015).
Smolders, K. C. H. J. & de Kort, Y. A. W. Bright light and mental fatigue: effects on alertness, vitality, performance and physiological arousal. J. Environ. Psychol. 39, 77–91 (2014).
Hattar, S. et al. Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 (2003).
Sonoda, T., Lee, S. K., Birnbaumer, L. & Schmidt, T. M. Melanopsin phototransduction is repurposed by iprgc subtypes to shape the function of distinct visual circuits. Neuron 99, 754–767.e4 (2018).
Zhao, X., Stafford, B. K., Godin, A. L., King, W. M. & Wong, K. Y. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J. Physiol. 592, 1619–1636 (2014).
Schmidt, T. M. & Kofuji, P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J. Neurosci. 30, 16262–16271 (2010).
Güler, A. D. et al. Melanopsin cells are the principal conduits for rod–cone input to non-image-forming vision. Nature 453, 102–105 (2008).
Fu, Y. et al. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc. Natl Acad. Sci. USA 102, 10339–10344 (2005).
Fernandez, D. C. et al. Light affects mood and learning through distinct retina–brain pathways. Cell 175, 71–84.e18 (2018).
Hattar, S. et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497, 326–349 (2006). This work is an early description of ipRGC projections, essential for hypotheses regarding ipRGC inputs to cognitive systems.
Hattar, S., Liao, H. W., Takao, M., Berson, D. M. & Yau, K. W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
Aranda, M. L. & Schmidt, T. M. Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions. Cell Mol. Life Sci. 78, 889–907 (2021). This work is an essential introduction to ipRGCs, and the tools available to interrogate ipRGC circuits and related behaviours.
Bowrey, H. E., James, M. H., Omrani, M., Mohammadkhani, A. & Aston-Jones, G. Chemogenetic stimulation a retinal circuit activates brain noradrenergic neurons, prevents apoptosis suppresses depression-like behaviors. Preprint at bioRxiv https://doi.org/10.1101/2021.04.20.440684 (2021).
LeGates, T. A. et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491, 594–598 (2012).
Nayak, S. K., Jegla, T. & Panda, S. Role of a novel photopigment, melanopsin, in behavioral adaptation to light. Cell Mol. Life Sci. 64, 144–154 (2007).
Zhang, Z., Beier, C., Weil, T. & Hattar, S. The retinal ipRGC–preoptic circuit mediates the acute effect of light on sleep. Nat. Commun. 12, 5115 (2021).
Li, J. Y. & Schmidt, T. M. Divergent projection patterns of M1 ipRGC subtypes. J. Comp. Neurol. 526, 2010–2018 (2018). The findings of this study describe distinct populations of M1 ipRGCs, candidates in many non-image-forming behaviours.
Do, M. T. H. Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior. Neuron 104, 205–226 (2019).
Sondereker, K. B., Stabio, M. E. & Renna, J. M. Crosstalk: the diversity of melanopsin ganglion cell types has begun to challenge the canonical divide between image-forming and non-image-forming vision. J. Comp. Neurol. 528, 2044–2067 (2020).
Bliss, T. V. & Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356 (1973).
Basu, J. & Siegelbaum, S. A. The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb. Perspect. Biol. 7, a021733 (2015).
Warthen, D. M., Wiltgen, B. J. & Provencio, I. Light enhances learned fear. Proc. Natl Acad. Sci. USA 108, 13788–13793 (2011).
Shan, L. L. et al. Light exposure before learning improves memory consolidation at night. Sci. Rep. 5, 15578 (2015).
Lueptow, L. M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 55718 https://doi.org/10.3791/55718 (2017).
Tam, S. K. et al. Modulation of recognition memory performance by light requires both melanopsin and classical photoreceptors. Proc. Biol. Sci. 283, 20162275 (2016).
Hasan, S. et al. Modulation of recognition memory performance by light and its relationship with cortical EEG θ and γ activities. Biochem. Pharmacol. 191, 114404 (2021).
Gevins, A., Smith, M. E., McEvoy, L. & Yu, D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb. Cortex 7, 374–385 (1997).
Pitkänen, A., Pikkarainen, M., Nurminen, N. & Ylinen, A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 911, 369–391 (2000).
Curtis, C. E. & D’Esposito, M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 7, 415–423 (2003).
Battaglia, F. P., Benchenane, K., Sirota, A., Pennartz, C. M. & Wiener, S. I. The hippocampus: hub of brain network communication for memory. Trends Cogn. Sci. 15, 310–318 (2011).
Roesler, R., Parent, M. B., LaLumiere, R. T. & McIntyre, C. K. Amygdala–hippocampal interactions in synaptic plasticity and memory formation. Neurobiol. Learn. Mem. 184, 107490 (2021).
Horner, A. J. & Doeller, C. F. Plasticity of hippocampal memories in humans. Curr. Opin. Neurobiol. 43, 102–109 (2017).
Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957).
Lisman, J. et al. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 20, 1434–1447 (2017).
Knierim, J. J., Neunuebel, J. P. & Deshmukh, S. S. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local–global reference frames. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130369 (2014).
Baddeley, A. Working memory. Science 255, 556–559 (1992). This work is an essential introduction to working memory.
Stuss, D. T. & Benson, D. F. Neuropsychological studies of the frontal lobes. Psychol. Bull. 95, 3 (1984).
Florin-Lechner, S. M., Druhan, J. P., Aston-Jones, G. & Valentino, R. J. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 742, 89–97 (1996).
Roecklein, K. et al. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 210, 150–158 (2013).
Heatherton, T. F. et al. Medial prefrontal activity differentiates self from close others. Soc. Cogn. Affect. Neurosci. 1, 18–25 (2006).
Hu, R. K. et al. An amygdala-to-hypothalamus circuit for social reward. Nat. Neurosci. 24, 831–842 (2021).
Dwortz, M. F., Curley, J. P., Tye, K. M. & Padilla-Coreano, N. Neural systems that facilitate the representation of social rank. Philos. Trans. R. Soc. Lond. B Biol. Sci. 377, 20200444 (2022).
Jobson, D. D., Hase, Y., Clarkson, A. N. & Kalaria, R. N. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 3, fcab125 (2021).
Saalmann, Y. B. & Kastner, S. Cognitive and perceptual functions of the visual thalamus. Neuron 71, 209–223 (2011).
O’Connor, D. H., Fukui, M. M., Pinsk, M. A. & Kastner, S. Attention modulates responses in the human lateral geniculate nucleus. Nat. Neurosci. 5, 1203–1209 (2002).
Baker, P. M. et al. The lateral habenula circuitry: reward processing and cognitive control. J. Neurosci. 36, 11482–11488 (2016).
Hermans, E. J. et al. How the amygdala affects emotional memory by altering brain network properties. Neurobiol. Learn. Mem. 112, 2–16 (2014).
McIntyre, C. K., McGaugh, J. L. & Williams, C. L. Interacting brain systems modulate memory consolidation. Neurosci. Biobehav. Rev. 36, 1750–1762 (2012).
Roozendaal, B., Hahn, E. L., Nathan, S. V., Dominique, J.-F. & McGaugh, J. L. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J. Neurosci. 24, 8161–8169 (2004).
Roozendaal, B., McReynolds, J. R. & McGaugh, J. L. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J. Neurosci. 24, 1385–1392 (2004).
McIntyre, C. K. et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc. Natl Acad. Sci. USA 102, 10718–10723 (2005).
Roozendaal, B., Castello, N. A., Vedana, G., Barsegyan, A. & McGaugh, J. L. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol. Learn. Mem. 90, 576–579 (2008).
Wang, G. et al. Short-term acute bright light exposure induces a prolonged anxiogenic effect in mice via a retinal ipRGC–CeA circuit. Sci. Adv. 9, eadf4651 (2023).
Raam, T. & Hong, W. Organization of neural circuits underlying social behavior: a consideration of the medial amygdala. Curr. Opin. Neurobiol. 68, 124–136 (2021).
Zhang, W.-H., Zhang, J.-Y., Holmes, A. & Pan, B.-X. Amygdala circuit substrates for stress adaptation and adversity. Biol. Psychiatry 89, 847–856 (2021).
Jiao, X., Beck, K., Myers, C., Servatius, R. & Pang, K. Altered activity of the medial prefrontal cortex and amygdala during acquisition and extinction of an active avoidance task. Front. Behav. Neurosci. 9, 249 (2015).
McCue, M. G., LeDoux, J. E. & Cain, C. K. Medial amygdala lesions selectively block aversive Pavlovian–instrumental transfer in rats. Front. Behav. Neurosci. 8, 329 (2014).
Cartoni, E., Puglisi-Allegra, S. & Baldassarre, G. The three principles of action: a Pavlovian–instrumental transfer hypothesis. Front. Behav. Neurosci. 7, 153 (2013).
Berry, M. H. et al. A melanopsin ganglion cell subtype forms a dorsal retinal mosaic projecting to the supraoptic nucleus. Nat. Commun. 14, 1492 (2023).
Hu, J. et al. Melanopsin retinal ganglion cells mediate light-promoted brain development. Cell 185, 3124–3137.e15 (2022).
Liao, P. Y., Chiu, Y. M., Yu, J. H. & Chen, S. K. Mapping central projection of oxytocin neurons in unmated mice using Cre and alkaline phosphatase reporter. Front. Neuroanat. 14, 559402 (2020).
Huang, Y.-F., Liao, P.-Y., Yu, J.-H. & Chen, S.-K. Light disrupts social memory via a retina-to-supraoptic nucleus circuit. EMBO Rep. 24, e56839 (2023). This work presents a recent finding demonstrating direct effects of light on cognition, mediated by ipRGCs, using several of the techniques discussed.
Deurveilher, S. & Semba, K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience 130, 165–183 (2005).
Breton-Provencher, V., Drummond, G. T. & Sur, M. Locus coeruleus norepinephrine in learned behavior: anatomical modularity and spatiotemporal integration in targets. Front. Neural Circuits 15, 638007 (2021).
Aston-Jones, G., Rajkowski, J. & Cohen, J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320 (1999).
Maness, E. B. et al. Role of the locus coeruleus and basal forebrain in arousal and attention. Brain Res. Bull. 188, 47–58 (2022).
Aston-Jones, G., Rajkowski, J. & Cohen, J. In Progress in Brain Research 126 (eds. Uyliǹgs H. B. M. et al.) 165–182 (Elsevier, 2000).
Záborszky, L. et al. Specific basal forebrain–cortical cholinergic circuits coordinate cognitive operations. J. Neurosci. 38, 9446–9458 (2018).
Peirson, S. N., Brown, L. A., Pothecary, C. A., Benson, L. A. & Fisk, A. S. Light and the laboratory mouse. J. Neurosci. Methods 300, 26–36 (2018). This work presents a wealth of practical information for laboratory mouse husbandry and experimental design (including a list of melatonin-deficient and sufficient lines) that takes the effects of light and ipRGC photoreception into account.
Chellappa, S. L. et al. Photic memory for executive brain responses. Proc. Natl Acad. Sci. USA 111, 6087–6091 (2014).
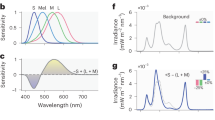
Spitschan, M. & Woelders, T. The method of silent substitution for examining melanopsin contributions to pupil control. Front. Neurol. 9, 941 (2018). This work includes detailed and accessible explanations of the fundamental concepts behind silent substitution, including an essential discussion of the overlapping spectral sensitivities of photoreceptors.
Rushton, W. A. H. Review lecture. Pigments and signals in colour vision. J. Physiol. 220, 1–31 (1972).
McDowell, R. J. et al. Beyond lux: methods for species and photoreceptor-specific quantification of ambient light for mammals. Preprint at bioRxiv https://doi.org/10.1101/2023.08.25.554794 (2023). This preprint establishes resources for the most current α-opic light measurement and reporting across species.
Hannibal, J. et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest. Ophthalmol. Vis. Sci. 45, 4202–4209 (2004).
Mure, L. S. Intrinsically photosensitive retinal ganglion cells of the human retina. Front. Neurol. 12, 636330 (2021). This ipRGC review focuses specifically on the state of knowledge of human ipRGCs.
Göz, D. et al. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE 3, e3153 (2008).
An, K. et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat. Neurosci. 23, 869–880 (2020).
Huang, L. et al. A visual circuit related to habenula underlies the antidepressive effects of light therapy. Neuron 102, 128–142.e8 (2019).
Gao, F. et al. A non-canonical retina–ipRGCs–SCN–PVT visual pathway for mediating contagious itch behavior. Cell Rep. 41, 111444 (2022).
Kennaway, D. J., Voultsios, A., Varcoe, T. J. & Moyer, R. W. Melatonin in mice: rhythms, response to light, adrenergic stimulation, and metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R358–R365 (2002).
Altimus, C. M. et al. Rods–cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc. Natl Acad. Sci. USA 105, 19998–20003 (2008).
Grillon, C., Pellowski, M., Merikangas, K. R. & Davis, M. Darkness facilitates the acoustic startle reflex in humans. Biol. Psychiatry 42, 453–460 (1997).
Thompson, S., Lupi, D., Hankins, M. W., Peirson, S. N. & Foster, R. G. The effects of rod and cone loss on the photic regulation of locomotor activity and heart rate. Eur. J. Neurosci. 28, 724–729 (2008).
Ishida, A. et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2, 297–307 (2005).
Pilorz, V. et al. Melanopsin regulates both sleep-promoting and arousal-promoting responses to light. PLoS Biol. 14, e1002482 (2016).
Huang, Y., Zhou, W. & Zhang, Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav. Brain Res. 226, 26–31 (2012).
Milosavljevic, N., Cehajic-Kapetanovic, J., Procyk, C. A. & Lucas, R. J. Chemogenetic activation of melanopsin retinal ganglion cells induces signatures of arousal and/or anxiety in mice. Curr. Biol. 26, 2358–2363 (2016).
Lucas, R. J. et al. Measuring and using light in the melanopsin age. Trends Neurosci. 37, 1–9 (2014).
Enezi, J. A. et al. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J. Biol. Rhythm. 26, 314–323 (2011).
Lucas, R. J. et al. In the eye of the beholder: measuring and standardising light for laboratory mammals. Preprint at https://www.preprints.org/manuscript/202309.1766/v1 (2023). This preprint provides the most current expert consensus on using, measuring and reporting light used with laboratory mammals, including a discussion of colour vision across species.
Vanuk, J. R. et al. Morning blue light treatment improves sleep complaints, symptom severity, and retention of fear extinction memory in post-traumatic stress disorder. Front. Behav. Neurosci. 16, 886816 (2022).
Chen, P., Ban, W., Wang, W., You, Y. & Yang, Z. The devastating effects of sleep deprivation on memory: lessons from rodent models. Clocks Sleep. 5, 276–294 (2023).
Smies, C. W., Bodinayake, K. K. & Kwapis, J. L. Time to learn: the role of the molecular circadian clock in learning and memory. Neurobiol. Learn. Mem. 193, 107651 (2022). This work presents a current discussion of circadian rhythms and memory.
Xu, S., Akioma, M. & Yuan, Z. Relationship between circadian rhythm and brain cognitive functions. Front. Optoelectron. 14, 278–287 (2021).
Diekelmann, S. & Born, J. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010).
Gerstner, J. R. & Yin, J. C. Circadian rhythms and memory formation. Nat. Rev. Neurosci. 11, 577–588 (2010).
Vartanian, G. V. et al. Melatonin suppression by light in humans is more sensitive than previously reported. J. Biol. Rhythm. 30, 351–354 (2015).
Bedrosian, T. A. & Nelson, R. J. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 7, e1017 (2017).
Krishnan, H. C. & Lyons, L. C. Synchrony and desynchrony in circadian clocks: impacts on learning and memory. Learn. Mem. 22, 426–437 (2015).
Domagalik, A. et al. Long-term reduction of short-wavelength light affects sustained attention and visuospatial working memory with no evidence for a change in circadian rhythmicity. Front. Neurosci. 14, 654 (2020).
Riemersma-van der Lek, R. F. et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 299, 2642–2655 (2008).
Yamadera, H. et al. Effects of bright light on cognitive and sleep–wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin. Neurosci. 54, 352–353 (2000).
Huang, X. et al. A visual circuit related to the nucleus reuniens for the spatial-memory-promoting effects of light treatment. Neuron 109, 347–362.e7 (2021).
Milosavljevic, N., Brown, T. M. & Lucas, R. J. A bright idea for improving spatial memory. Neuron 109, 197–199 (2021).
Miller, H. V., Barnes, J. C. & Beaver, K. M. Self-control and health outcomes in a nationally representative sample. Am. J. Health Behav. 35, 15–27 (2011).
La Morgia, C. et al. Multimodal investigation of melanopsin retinal ganglion cells in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 10, 918–932 (2023).
La Morgia, C. et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 79, 90–109 (2016).
Oh, A. J. et al. Pupillometry evaluation of melanopsin retinal ganglion cell function and sleep–wake activity in pre-symptomatic Alzheimer’s disease. PLoS ONE 14, e0226197 (2019).
Myers, B. L. & Badia, P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci. Biobehav. Rev. 19, 553–571 (1995).
Murman, D. L. The impact of age on cognition. Semin. Hear. 36, 111–121 (2015).
Vugler, A. A., Joseph, A. & Jeffery, G. Survival and remodeling of melanopsin cells during retinal dystrophy. Vis. Neurosci. 25, 125–138 (2008).
Cui, Q., Ren, C., Sollars, P., Pickard, G. & So, K.-F. The injury resistant ability of melanopsin-expressing intrinsically photosensitive retinal ganglion cells. Neuroscience 284, 845–853 (2015).
Turner, P. L. & Mainster, M. A. Circadian photoreception: ageing and the eye’s important role in systemic health. Br. J. Ophthalmol. 92, 1439–1444 (2008).
Esquiva, G., Lax, P., Pérez-Santonja, J. J., García-Fernández, J. M. & Cuenca, N. Loss of melanopsin-expressing ganglion cell subtypes and dendritic degeneration in the aging human retina. Front. Aging Neurosci. 9, 79 (2017).
Cajochen, C., Münch, M., Knoblauch, V., Blatter, K. & Wirz-Justice, A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol. Int. 23, 461–474 (2006).
Beier, C. et al. Divergent outer retinal circuits drive image and non-image visual behaviors. Cell Rep. 39, 111003 (2022).
Maruani, J. & Geoffroy, P. A. Multi-level processes and retina–brain pathways of photic regulation of mood. J. Clin. Med. 11, 448 (2022).
Rupp, A. C. et al. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. eLife 8, e44358 (2019).
Gooley, J. J., Lu, J., Fischer, D. & Saper, C. B. A broad role for melanopsin in nonvisual photoreception. J. Neurosci. 23, 7093–7106 (2003).
Desgranges, B., Baron, J. C. & Eustache, F. The functional neuroanatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage 8, 198–213 (1998).
Nakajima, M. & Schmitt, L. I. Understanding the circuit basis of cognitive functions using mouse models. Neurosci. Res. 152, 44–58 (2020). This work presents an indispensable in-depth discussion of techniques available to dissect cognitive functions in mouse models, including behavioural tasks, circuit dissection and neural recording techniques.
Arakawa, H. & Iguchi, Y. Ethological and multi-behavioral analysis of learning and memory performance in laboratory rodent models. Neurosci. Res. 135, 1–12 (2018).
Ghafarimoghadam, M. et al. A review of behavioral methods for the evaluation of cognitive performance in animal models: current techniques and links to human cognition. Physiol. Behav. 244, 113652 (2022).
Isik, S. & Unal, G. Open-source software for automated rodent behavioral analysis. Front. Neurosci. 17, 1149027 (2023).
Guenthner, C. J., Miyamichi, K., Yang, H. H., Heller, H. C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013).
Patel, A. A., McAlinden, N., Mathieson, K. & Sakata, S. Simultaneous electrophysiology and fiber photometry in freely behaving mice. Front. Neurosci. 14, 148 (2020).
Bush, G., Shin, L. M., Holmes, J., Rosen, B. R. & Vogt, B. A. The multi-source interference task: validation study with fMRI in individual subjects. Mol. Psychiatry 8, 60–70 (2003).
Einhäuser, W. in Computational and Cognitive Neuroscience of Vision (ed. Zhao, Q.) 141–169 (Springer, 2017).
Owen, A. M., McMillan, K. M., Laird, A. R. & Bullmore, E. n-Back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 25, 46–59 (2005).
Yi, A. in Encyclopedia of Clinical Neuropsychology (eds Kreutzer, J. S., DeLuca, J. & Caplan, B.) 475–476 (Springer, 2011).
Karayanidis, F. & McKewen, M. in Psychology of Learning and Motivation Vol. 74 (ed. Federmeier, K. D.) 141–193 (Academic, 2021).
Emmer, K. M., Russart, K. L. G., Walker, W. H., Nelson, R. J. & DeVries, A. C. Effects of light at night on laboratory animals and research outcomes. Behav. Neurosci. 132, 302–314 (2018).
Hofstetter, J. R., Hofstetter, A. R., Hughes, A. M. & Mayeda, A. R. Intermittent long-wavelength red light increases the period of daily locomotor activity in mice. J. Circadian Rhythm. 3, 8 (2005).
Roedel, A., Storch, C., Holsboer, F. & Ohl, F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab. Anim. 40, 371–381 (2006).
Dauchy, R. T. & Blask, D. E. Vivarium lighting as an important extrinsic factor influencing animal-based research. J. Am. Assoc. Lab. Anim. Sci. 62, 3–25 (2023).
Chellappa, S. L., Steiner, R., Oelhafen, P. & Cajochen, C. Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Sci. Rep. 7, 14215 (2017).
Levine, D. A. et al. Sex differences in cognitive decline among US adults. JAMA Netw. Open. 4, e210169 (2021).
Yagi, S. & Galea, L. A. M. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44, 200–213 (2019).
Cowan, R. et al. Sex differences in response to red and blue light in human primary visual cortex: a bold fMRI study. Psychiatry Res. 100, 129–138 (2001).
Krizo, J. A. & Mintz, E. M. Sex differences in behavioral circadian rhythms in laboratory rodents. Front. Endocrinol. 5, 234 (2014).
Acknowledgements
The authors are grateful to J. Bhoi for edits improving the clarity and content of the manuscript with valuable suggestions and insight on neuromodulation.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Christian Cajochen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Circadian rhythms
-
Natural rhythms that oscillate over a period of approximately 24 h.
- Cognitive inhibition
-
(Also known as inhibitory control). An element of executive function that refers to the ability to control behaviour, thoughts and emotions, and to apply selective attention while tuning out irrelevant stimuli.
- Cognitive neurobehavioural performance
-
A subject’s ability to complete cognitive tasks, which may be relatively improved or diminished by various stimuli and states.
- Declarative memory
-
Memory of facts and events, sometimes referred to as explicit memory.
- Electroencephalography
-
(EEG). A technique used to measure brain activity, in which electrical fields are detected at the scalp surface.
- Emotional cognition
-
(Closely tied to social cognition). The ability to recognize and understand the feelings of others and the self.
- Functional connectivity
-
Neurons within a circuit that synaptically communicate.
- Functional magnetic resonance imaging
-
(fMRI). A brain imaging method that maps brain metabolism, or blood oxygen level-dependent (BOLD) signals.
- Light therapy
-
(Also called phototherapy). The use of artificial or natural light as a treatment, most commonly for seasonal depression.
- Melanopsin
-
A light-sensitive opsin protein (encoded by the gene OPN4) that is expressed in intrinsically photosensitive retinal ganglion cells (ipRGCs).
- Melatonin
-
A sleep-promoting hormone that is synthesized in and released by the pineal gland. Melatonin levels both follow and have a role in synchronizing circadian rhythms.
- Neuromodulatory systems
-
Systems (including the cholinergic, dopaminergic, noradrenergic and serotonergic systems) that regulate other systems by using neurotransmitters to signal throughout the brain.
- Non-declarative memory
-
(Also referred to as procedural or implicit memory). Memory for skills, habits, procedures and other types of non-associative conditioning.
- Photoreceptors
-
Light-sensitive cells that, in mammals, are found in the retina.
- Principle of univariance
-
A principle stating that activation of an opsin molecule by a single photon of any wavelength will result in an identical cellular response.
- Recognition memory
-
The ability to recognize and recall previously encountered stimuli.
- Reward processing
-
The engagement of neurobiological pathways enabling the experience of reward, association with rewarding stimuli and subsequent shaping of behaviour.
- Theory of mind
-
The ability to infer or attribute mental states, such as desires, intentions and thoughts, of the self and others.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahoney, H.L., Schmidt, T.M. The cognitive impact of light: illuminating ipRGC circuit mechanisms. Nat. Rev. Neurosci. 25, 159–175 (2024). https://doi.org/10.1038/s41583-023-00788-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00788-5