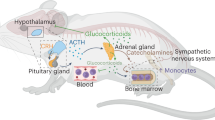
Abstract
Stress-linked psychiatric disorders, including anxiety and major depressive disorder, are associated with systemic inflammation. Recent studies have reported stress-induced alterations in haematopoiesis that result in monocytosis, neutrophilia, lymphocytopenia and, consequently, in the upregulation of pro-inflammatory processes in immunologically relevant peripheral tissues. There is now evidence that this peripheral inflammation contributes to the development of psychiatric symptoms as well as to common co-morbidities of psychiatric disorders such as metabolic syndrome and immunosuppression. Here, we review the specific brain and spinal regions, and the neuronal populations within them, that respond to stress and transmit signals to peripheral tissues via the autonomic nervous system or neuroendocrine pathways to influence immunological function. We comprehensively summarize studies that have employed retrograde tracing to define neurocircuits linking the brain to the bone marrow, spleen, gut, adipose tissue and liver. Moreover, we highlight studies that have used chemogenetic or optogenetic manipulation or intracerebroventricular administration of peptide hormones to control somatic immune responses. Collectively, this growing body of literature illustrates potential mechanisms through which stress signals are conveyed from the CNS to immune cells to regulate stress-relevant behaviours and comorbid pathophysiology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
O’Connor, D. B., Thayer, J. F. & Vedhara, K. Stress and health: a review of psychobiological processes. Annu. Rev. Psychol. 72, 663–688 (2021).
Savitz, J. & Harrison, N. A. Interoception and inflammation in psychiatric disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 514–524 (2018).
GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiat. 9, 137–150 (2022).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 (2008).
Dantzer, R. Neuroimmune Interactions: from the brain to the immune system and vice versa. Physiol. Rev. 98, 477–504 (2018).
Hodes, G. E., Kana, V., Menard, C., Merad, M. & Russo, S. J. Neuroimmune mechanisms of depression. Nat. Neurosci. 18, 1386–1393 (2015).
Menard, C. et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760 (2017).
Oines, E., Murison, R., Mrdalj, J., Grønli, J. & Milde, A. M. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol. Behav. 105, 1058–1066 (2012).
Margaretten, M., Julian, L., Katz, P. & Yelin, E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int. J. Clin. Rheumatol. 6, 617–623 (2011).
Halaris, A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr. Top. Behav. Neurosci. 31, 45–70 (2017).
Chan, K. L., Cathomas, F. & Russo, S. J. Central and peripheral inflammation link metabolic syndrome and major depressive disorder. Physiology 34, 123–133 (2019).
Barberio, B., Zamani, M., Black, C. J., Savarino, E. V. & Ford, A. C. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 6, 359–370 (2021).
Katze, M. G., He, Y. & Gale, M. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2, 675–687 (2002).
Renault, P. F. et al. Psychiatric complications of long-term interferon alfa therapy. Arch. Intern. Med. 147, 1577–1580 (1987).
Benros, M. E. et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70, 812–820 (2013).
Wolfe, F. & Michaud, K. Predicting depression in rheumatoid arthritis: the signal importance of pain extent and fatigue, and comorbidity. Arthritis Rheum. 61, 667–673 (2009).
Bzdok, D. & Dunbar, R. I. M. Social isolation and the brain in the pandemic era. Nat. Hum. Behav. 6, 1333–1343 (2022).
Blume, J., Douglas, S. D. & Evans, D. L. Immune suppression and immune activation in depression. Brain Behav. Immun. 25, 221–229 (2011).
Andersson, N. W. et al. Depression and the risk of severe infections: prospective analyses on a nationwide representative sample. Int. J. Epidemiol. 45, 131–139 (2016).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (APA, 2013).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457 (2010).
Leighton, S. P. et al. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol. Psychiatry 23, 48–58 (2018).
Maes, M. et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9, 853–858 (1997).
Simon, N. M. et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur. Neuropsychopharmacol. 18, 230–233 (2008).
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA 111, 16136–16141 (2014).
Pace, T. W. W. et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 163, 1630–1633 (2006).
Brydon, L. et al. Psychological stress activates interleukin-1beta gene expression in human mononuclear cells. Brain Behav. Immun. 19, 540–546 (2005).
Stewart, A. M. et al. Cytokine and endocrine parameters in mouse chronic social defeat: implications for translational ‘cross-domain’ modeling of stress-related brain disorders. Behav. Brain Res. 276, 84–91 (2015).
Tagliari, B. et al. Chronic variable stress alters inflammatory and cholinergic parameters in hippocampus of rats. Neurochem. Res. 36, 487–493 (2011).
Deonaraine, K. K. et al. Sex-specific peripheral and central responses to stress-induced depression and treatment in a mouse model. J. Neurosci. Res. 98, 2541–2553 (2020).
Harpaz, I. et al. Chronic exposure to stress predisposes to higher autoimmune susceptibility in C57BL/6 mice: glucocorticoids as a double-edged sword. Eur. J. Immunol. 43, 758–769 (2013).
Gao, X. et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl Acad. Sci. USA 115, E2960–E2969 (2018).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
van der Kooij, M. A. et al. Chronic social stress-induced hyperglycemia in mice couples individual stress susceptibility to impaired spatial memory. Proc. Natl Acad. Sci. USA 115, E10187–E10196 (2018).
Felten, D. L. & Felten, S. Y. Sympathetic noradrenergic innervation of immune organs. Brain Behav. Immun. 2, 293–300 (1988).
Saleeba, C., Dempsey, B., Le, S., Goodchild, A. & McMullan, S. A student’s guide to neural circuit tracing. Front. Neurosci. 13, 897 (2019).
Dénes, A. et al. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience 134, 947–963 (2005).
Wee, N. K. Y., Lorenz, M. R., Bekirov, Y., Jacquin, M. F. & Scheller, E. L. Shared autonomic pathways connect bone marrow and peripheral adipose tissues across the central neuraxis. Front. Endocrinol. 10, 668 (2019).
Zhong, P. et al. HCN2 channels in the ventral tegmental area regulate behavioral responses to chronic stress. eLife 7, e32420 (2018).
Russo, S. J. & Nestler, E. J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625 (2013).
Kumar, P. et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 43, 1581–1588 (2018).
Nestler, E. J. & Carlezon, W. A. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159 (2006).
Li, L. et al. Social trauma engages lateral septum circuitry to occlude social reward. Nature 613, 696–703 (2023).
Hahn, M. K. & Bannon, M. J. Stress-induced C-fos expression in the rat locus coeruleus is dependent on neurokinin 1 receptor activation. Neuroscience 94, 1183–1188 (1999).
Zhang, H. et al. α1- and β3-adrenergic receptor-mediated mesolimbic homeostatic plasticity confers resilience to social stress in susceptible mice. Biol. Psychiatry 85, 226–236 (2019).
Isingrini, E. et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci. 19, 560–563 (2016).
Senba, E., Matsunaga, K., Tohyama, M. & Noguchi, K. Stress-induced c-fos expression in the rat brain: activation mechanism of sympathetic pathway. Brain Res. Bull. 31, 329–344 (1993).
de Medeiros, M. A., Carlos Reis, L. & Eugênio Mello, L. Stress-induced c-Fos expression is differentially modulated by dexamethasone, diazepam and imipramine. Neuropsychopharmacology 30, 1246–1256 (2005).
Gomes-de-Souza, L., Costa-Ferreira, W., Mendonça, M. M., Xavier, C. H. & Crestani, C. C. Lateral hypothalamus involvement in control of stress response by bed nucleus of the stria terminalis endocannabinoid neurotransmission in male rats. Sci. Rep. 11, 16133 (2021).
Yuan, Y. et al. Reward inhibits paraventricular CRH neurons to relieve stress. Curr. Biol. 29, 1243–1251 (2019).
Kwon, M.-S. et al. The differential effects of emotional or physical stress on pain behaviors or on c-Fos immunoreactivity in paraventricular nucleus or arcuate nucleus. Brain Res. 1190, 122–131 (2008).
Herman, J. P. et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6, 603–621 (2016).
Lebow, M. A. & Chen, A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016).
Numa, C. et al. Social defeat stress-specific increase in c-Fos expression in the extended amygdala in mice: involvement of dopamine D1 receptor in the medial prefrontal cortex. Sci. Rep. 9, 16670 (2019).
Keifer, O. P., Hurt, R. C., Ressler, K. J. & Marvar, P. J. The physiology of fear: reconceptualizing the role of the central amygdala in fear learning. Physiology 30, 389–401 (2015).
Engler, H., Bailey, M. T., Engler, A. & Sheridan, J. F. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 148, 106–115 (2004).
Pfau, M. L. et al. Role of monocyte-derived microRNA106b∼25 in resilience to social stress. Biol. Psychiatry 86, 474–482 (2019).
Seidel, A. et al. Major depressive disorder is associated with elevated monocyte counts. Acta Psychiatr. Scand. 94, 198–204 (1996).
McKim, D. B. et al. Social stress mobilizes hematopoietic stem cells to establish persistent splenic myelopoiesis. Cell Rep. 25, 2552–2562.e3 (2018).
Hanoun, M., Maryanovich, M., Arnal-Estapé, A. & Frenette, P. S. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 86, 360–373 (2015).
Cosentino, M., Marino, F. & Maestroni, G. J. M. Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives. Front. Cell. Neurosci. 9, 302 (2015).
Eash, K. J., Means, J. M., White, D. W. & Link, D. C. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113, 4711–4719 (2009).
Chong, S. Z. et al. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. J. Exp. Med. 213, 2293–2314 (2016).
Jung, H., Mithal, D. S., Park, J. E. & Miller, R. J. Localized CCR2 activation in the bone marrow niche mobilizes monocytes by desensitizing CXCR4. PLoS ONE 10, e0128387 (2015).
Niraula, A., Wang, Y., Godbout, J. P. & Sheridan, J. F. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J. Neurosci. 38, 2328–2340 (2018).
Won, E. & Kim, Y.-K. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr. Neuropharmacol. 14, 665–673 (2016).
Jones, B. E. & Yang, T. Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 242, 56–92 (1985).
Kenney, M. J., Weiss, M. L. & Haywood, J. R. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol. Scand. 177, 7–15 (2003).
Poller, W. C. et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 607, 578–584 (2022). Identifies brain circuits controlling monocyte, neutrophil and lymphocyte retention and mobilization from the bone marrow into circulation during acute stress.
Wohleb, E. S., McKim, D. B., Sheridan, J. F. & Godbout, J. P. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 8, 447 (2014).
Sawant, K. V. et al. Chemokine CXCL1 mediated neutrophil recruitment: role of glycosaminoglycan interactions. Sci. Rep. 6, 33123 (2016).
Ben-Shaanan, T. L. et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944 (2016). Shows that stimulating VTA neurons enhances peripheral bactericidal function and improves social behaviour.
Kaster, M. P., Gadotti, V. M., Calixto, J. B., Santos, A. R. S. & Rodrigues, A. L. S. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology 62, 419–426 (2012).
Dudek, K. A. et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc. Natl Acad. Sci. USA 117, 3326–3336 (2020).
Garcia-Oscos, F. et al. Vagal nerve stimulation blocks interleukin 6-dependent synaptic hyperexcitability induced by lipopolysaccharide-induced acute stress in the rodent prefrontal cortex. Brain Behav. Immun. 43, 149–158 (2015).
Russo, S. et al. Peripheral immune-derived matrix metalloproteinase promotes stress susceptibility. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-1647827/v1 (2023).
Zhang, K. et al. Splenic NKG2D confers resilience versus susceptibility in mice after chronic social defeat stress: beneficial effects of (R)-ketamine. Eur. Arch. Psychiatry Clin. Neurosci. 271, 447–456 (2021).
De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015).
Wei, Y. et al. Brain-spleen axis in health and diseases: a review and future perspective. Brain Res. Bull. 182, 130–140 (2022).
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020). Shows that optogenetic stimulation of stress-responsive CNS regions influences splenic plasma cell production.
Cano, G., Sved, A. F., Rinaman, L., Rabin, B. S. & Card, J. P. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol. 439, 1–18 (2001).
Strehl, C., Ehlers, L., Gaber, T. & Buttgereit, F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front. Immunol. 10, 1744 (2019).
Kressel, A. M. et al. Identification of a brainstem locus that inhibits tumor necrosis factor. Proc. Natl Acad. Sci. USA 117, 29803–29810 (2020). Investigates the role of parasympathetic spleen innervation in regulating TNF production to limit inflammation.
Breit, S., Kupferberg, A., Rogler, G. & Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 9, 44 (2018).
Monteiro, S. et al. Splenic sympathetic signaling contributes to acute neutrophil infiltration of the injured spinal cord. J. Neuroinflammation 17, 282 (2020).
Geerling, J. C., Shin, J.-W., Chimenti, P. C. & Loewy, A. D. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J. Comp. Neurol. 518, 1460–1499 (2010).
Gray, T. S. & Magnuson, D. J. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J. Comp. Neurol. 262, 365–374 (1987).
Abe, C. et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 20, 700–707 (2017).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
He, Z.-G. et al. Neuroanatomical autonomic substrates of brainstem-gut circuitry identified using transsynaptic tract-tracing with pseudorabies virus recombinants. Am. J. Clin. Exp. Immunol. 7, 16–24 (2018).
Muller, P. A. et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 583, 441–446 (2020).
Parker, C. G., Dailey, M. J., Phillips, H. & Davis, E. A. Central sensory-motor crosstalk in the neural gut-brain axis. Auton. Neurosci. Basic. Clin. 225, 102656 (2020).
Matsuda, S. et al. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci. Res. 26, 157–170 (1996).
Foster, J. A., Rinaman, L. & Cryan, J. F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol. Stress 7, 124–136 (2017).
Zhu, X. et al. Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nat. Immunol. 24, 625–636 (2023).
Fadgyas-Stanculete, M., Buga, A.-M., Popa-Wagner, A. & Dumitrascu, D. L. The relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestations. J. Mol. Psychiatry 2, 4 (2014).
Million, M., Taché, Y. & Anton, P. Susceptibility of lewis and fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am. J. Physiol. 276, G1027–G1036 (1999).
Sherman, J. E. & Kalin, N. H. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol. Biochem. Behav. 30, 801–807 (1988).
Yamada, H., Tanno, S., Takakusaki, K. & Okumura, T. Intracisternal injection of orexin-A prevents ethanol-induced gastric mucosal damage in rats. J. Gastroenterol. 42, 336–341 (2007).
Schneider, K. M. et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell 186, 2823–2838.e20 (2023). Characterizes an HPA axis-based pathway in which enteric glia become activated by stress to exacerbate DSS-induced colitis.
Koren, T. et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 184, 5902–5915.e17 (2021). Demonstrates that the insular cortex can encode and re-activate immunological memory of peripheral inflammation in the gut.
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Xu, H. et al. Transcriptional atlas of intestinal immune cells reveals that neuropeptide α-CGRP modulates group 2 innate lymphoid cell responses. Immunity 51, 696–708 (2019).
Gross Margolis, K. et al. Enteric serotonin and oxytocin: endogenous regulation of severity in a murine model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G386–G398 (2017).
Udit, S., Blake, K. & Chiu, I. M. Somatosensory and autonomic neuronal regulation of the immune response. Nat. Rev. Neurosci. 23, 157–171 (2022).
Santos, J., Yang, P. C., Söderholm, J. D., Benjamin, M. & Perdue, M. H. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 48, 630–636 (2001).
Demaude, J., Salvador-Cartier, C., Fioramonti, J., Ferrier, L. & Bueno, L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut 55, 655–661 (2006).
Stevens, B. R. et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 57, 1555–1557 (2017).
Vaure, C. & Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 5, 316 (2014).
Costi, S. et al. Peripheral immune cell reactivity and neural response to reward in patients with depression and anhedonia. Transl. Psychiatry 11, 565 (2021).
Hung, Y.-Y., Kang, H.-Y., Huang, K.-W. & Huang, T.-L. Association between toll-like receptors expression and major depressive disorder. Psychiatry Res 220, 283–286 (2014).
Dunbar, J. A. et al. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care 31, 2368–2373 (2008).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Grant, R. W. & Dixit, V. D. Adipose tissue as an immunological organ. Obesity 23, 512–518 (2015).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Talukdar, S. et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 (2012).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009).
O’Sullivan, T. E. et al. Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity 45, 428–441 (2016).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011).
Wu, H. et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115, 1029–1038 (2007).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Misumi, I. et al. Obesity expands a distinct population of t cells in adipose tissue and increases vulnerability to infection. Cell Rep. 27, 514–524 (2019).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Li, P. et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 285, 15333–15345 (2010).
Motoyama, S. et al. Social stress increases vulnerability to high-fat diet-induced insulin resistance by enhancing neutrophil elastase activity in adipose tissue. Cells 9, 996 (2020).
Guilherme, A., Henriques, F., Bedard, A. H. & Czech, M. P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 15, 207–225 (2019).
Adler, E. S., Hollis, J. H., Clarke, I. J., Grattan, D. R. & Oldfield, B. J. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J. Neurosci. 32, 15913–15921 (2012).
Stanley, S. et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl Acad. Sci. USA 107, 7024–7029 (2010).
Shi, H. & Bartness, T. J. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res. Bull. 54, 375–385 (2001).
Tang, L. et al. Sympathetic nerve activity maintains an anti-inflammatory state in adipose tissue in male mice by inhibiting TNF-α gene expression in macrophages. Endocrinology 156, 3680–3694 (2015).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Cardoso, F. et al. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature 597, 410–414 (2021). Identifies a role for the PVH in regulating adipose tissue ILC2s and metabolism.
Marik, P. E. & Bellomo, R. Stress hyperglycemia: an essential survival response! Crit. Care Med. 41, e93–e94 (2013).
Liu, Y.-Z. et al. Chronic stress induces steatohepatitis while decreases visceral fat mass in mice. BMC Gastroenterol. 14, 106 (2014).
Swain, M. G. I. Stress and hepatic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G1135–G1138 (2000).
Shukla, P. K. et al. Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis. Sci. Rep. 11, 826 (2021).
Morinaga, H. et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes 64, 1120–1130 (2015).
Kreier, F. et al. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 147, 1140–1147 (2006).
Mizuno, K. & Ueno, Y. Autonomic nervous system and the liver. Hepatol. Res. 47, 160–165 (2017).
Tsuneki, H. et al. Hypothalamic orexin prevents non-alcoholic steatohepatitis and hepatocellular carcinoma in obesity. Cell Rep. 41, 111497 (2022). Shows that administration of intracerebroventricular orexin or chemogenetic activation of orexin-expressing neurons in the LH dampens hepatic endoplasmic reticulum stress and non-alcoholic steatohepatitis.
Tsuneki, H. et al. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 64, 459–470 (2015).
Lutter, M. et al. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J. Neurosci. 28, 3071–3075 (2008).
Chung, H.-S., Kim, J.-G., Kim, J.-W., Kim, H.-W. & Yoon, B.-J. Orexin administration to mice that underwent chronic stress produces bimodal effects on emotion-related behaviors. Regul. Pept. 194–195, 16–22 (2014).
Kim, J. G., Ea, J. Y. & Yoon, B.-J. Orexinergic neurons modulate stress coping responses in mice. Front. Mol. Neurosci. 16, 1140672 (2023).
Dergacheva, O., Yamanaka, A., Schwartz, A. R., Polotsky, V. Y. & Mendelowitz, D. Direct projections from hypothalamic orexin neurons to brainstem cardiac vagal neurons. Neuroscience 339, 47–53 (2016).
Nishio, T. et al. Hepatic vagus nerve regulates Kupffer cell activation via α7 nicotinic acetylcholine receptor in nonalcoholic steatohepatitis. J. Gastroenterol. 52, 965–976 (2017). Demonstrates that vagal stimulation of the liver decreases hepatic macrophage counts and cytokine expression in a model of non-alcoholic steatohepatitis.
Hur, M. H. et al. Chemogenetic stimulation of the parasympathetic nervous system lowers hepatic lipid accumulation and inflammation in a nonalcoholic steatohepatitis mouse model. Life Sci. 321, 121533 (2023).
Segerstrom, S. C. & Miller, G. E. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 130, 601–630 (2004).
Kiank, C. et al. Stress susceptibility predicts the severity of immune depression and the failure to combat bacterial infections in chronically stressed mice. Brain. Behav. Immun. 20, 359–368 (2006).
Luo, Z. et al. Novel insights into stress-induced susceptibility to influenza: corticosterone impacts interferon-β responses by Mfn2-mediated ubiquitin degradation of MAVS. Signal. Transduct. Target. Ther. 5, 202 (2020).
Dhabhar, F. S. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16, 300–317 (2009).
Russo, S. J., Murrough, J. W., Han, M.-H., Charney, D. S. & Nestler, E. J. Neurobiology of resilience. Nat. Neurosci. 15, 1475–1484 (2012).
Quatrini, L. & Ugolini, S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell. Mol. Immunol. 18, 269–278 (2021).
Li, S.-B. et al. Hypothalamic circuitry underlying stress-induced insomnia and peripheral immunosuppression. Sci. Adv. 6, eabc2590 (2020). Profiles circulating leukocyte populations following PVH stimulation and reports an immunosuppressed phenotypic switch.
Bourhy, L. et al. Silencing of amygdala circuits during sepsis prevents the development of anxiety-related behaviours. Brain J. Neurol. 145, 1391–1409 (2022).
Chai, H.-H. et al. The chemokine CXCL1 and its receptor CXCR2 contribute to chronic stress-induced depression in mice. FASEB J. 33, 8853–8864 (2019).
Korolkova, O. Y., Myers, J. N., Pellom, S. T., Wang, L. & M’Koma, A. E. Characterization of serum cytokine profile in predominantly colonic inflammatory bowel disease to delineate ulcerative and Crohn’s colitides. Clin. Med. Insights Gastroenterol. 8, 29–44 (2015).
Singh, U. P. et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 77, 44–49 (2016).
Sanchez-Munoz, F., Dominguez-Lopez, A. & Yamamoto-Furusho, J.-K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 14, 4280–4288 (2008).
Tatsuki, M. et al. Serological cytokine signature in paediatric patients with inflammatory bowel disease impacts diagnosis. Sci. Rep. 10, 14638 (2020).
Martinez-Fierro, M. L. et al. Serum cytokine, chemokine, and growth factor profiles and their modulation in inflammatory bowel disease. Medicine 98, e17208 (2019).
Alex, P. et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 15, 341–352 (2009).
Xing, L. et al. The anti-inflammatory effect of bovine bone-gelatin-derived peptides in LPS-induced RAW264.7 macrophages cells and dextran sulfate sodium-induced C57BL/6 mice. Nutrients 14, 1479 (2022).
Aygun, A. D., Gungor, S., Ustundag, B., Gurgoze, M. K. & Sen, Y. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediators Inflamm. 2005, 180–183 (2005).
Bastard, J. P. et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 85, 3338–3342 (2000).
Catalán, V. et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes. Surg. 17, 1464–1474 (2007).
Jung, C., Gerdes, N., Fritzenwanger, M. & Figulla, H. R. Circulating levels of interleukin-1 family cytokines in overweight adolescents. Mediators Inflamm. 2010, 958403 (2010).
Schmidt, F. M. et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 10, e0121971 (2015).
Kim, C.-S. et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 30, 1347–1355 (2006).
Kopasov, A. E., Blokhin, S. N., Volkova, E. N. & Morozov, S. G. Chemokine expression in neutrophils and subcutaneous adipose tissue cells obtained during abdominoplasty from patients with obesity and normal body weight. Bull. Exp. Biol. Med. 167, 728–731 (2019).
Kim, K.-A., Gu, W., Lee, I.-A., Joh, E.-H. & Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 7, e47713 (2012).
Chavey, C. et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 9, 339–349 (2009).
Nunemaker, C. S. et al. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J. Endocrinol. 222, 267–276 (2014).
O’Neill, C. M. et al. Circulating levels of IL-1B+IL-6 cause ER stress and dysfunction in islets from prediabetic male mice. Endocrinology 154, 3077–3088 (2013).
Kępczyńska, M. A. et al. Circulating levels of the cytokines IL10, IFNγ and resistin in an obese mouse model of developmental programming. J. Dev. Orig. Health Dis. 4, 491–498 (2013).
Takahashi, K. et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J. Biol. Chem. 278, 46654–46660 (2003).
Xu, Y. et al. Ginsenoside Rg1 protects against non-alcoholic fatty liver disease by ameliorating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation. Biol. Pharm. Bull. 41, 1638–1644 (2018).
Min, X. et al. Serum cytokine profile in relation to the severity of coronary artery disease. BioMed. Res. Int. 2017, 4013685 (2017).
Mirhafez, S. R. et al. Relationship between serum cytokine and growth factor concentrations and coronary artery disease. Clin. Biochem. 48, 575–580 (2015).
Hasdai, D. et al. Increased serum concentrations of interleukin-1 beta in patients with coronary artery disease. Heart 76, 24–28 (1996).
Koh, S. J. et al. Association of serum RANTES concentrations with established cardiovascular risk markers in middle-aged subjects. Int. J. Cardiol. 132, 102–108 (2009).
Ye, Y. et al. Serum chemokine CCL17/thymus activation and regulated chemokine is correlated with coronary artery diseases. Atherosclerosis 238, 365–369 (2015).
Versteylen, M. O. et al. CC chemokine ligands in patients presenting with stable chest pain: association with atherosclerosis and future cardiovascular events. Neth. Heart J. 24, 722–729 (2016).
Yin, M., Zhang, L., Sun, X., Mao, L. & Pan, J. Lack of apoE causes alteration of cytokines expression in young mice liver. Mol. Biol. Rep. 37, 2049–2054 (2010).
Naura, A. S. et al. High-fat diet induces lung remodeling in ApoE-deficient mice: an association with an increase in circulatory and lung inflammatory factors. Lab. Invest. 89, 1243–1251 (2009).
Lv, J. et al. Amygdalin ameliorates the progression of atherosclerosis in LDL receptor-deficient mice. Mol. Med. Rep. 16, 8171–8179 (2017).
Soehnlein, O. et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol. Med. 5, 471–481 (2013).
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006).
Bondi, C. O., Rodriguez, G., Gould, G. G., Frazer, A. & Morilak, D. A. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33, 320–331 (2008).
Wallace, D. L. et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci. 12, 200–209 (2009).
Takahashi, A. et al. Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 7, 12838 (2017).
LeClair, K. B. et al. Individual history of winning and hierarchy landscape influence stress susceptibility in mice. eLife 10, e71401 (2021).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Labonté, B. et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111 (2017).
Chuang, J.-C. et al. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol. Psychiatry 67, 1075–1082 (2010).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
McCullough, K. M. et al. Nucleus accumbens medium spiny neuron subtypes differentially regulate stress-associated alterations in sleep architecture. Biol. Psychiatry 89, 1138–1149 (2021).
Warren, B. L., Mazei-Robison, M. S., Robison, A. J. & Iñiguez, S. D. Can I get a witness? Using vicarious defeat stress to study mood-related illnesses in traditionally understudied populations. Biol. Psychiatry 88, 381–391 (2020).
Harris, A. Z. et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43, 1276–1283 (2018).
Newman, E. L. et al. Fighting females: neural and behavioral consequences of social defeat stress in female mice. Biol. Psychiatry 86, 657–668 (2019).
Barrot, M. et al. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc. Natl Acad. Sci. USA 102, 8357–8362 (2005).
Scarpa, J. R. et al. Shared transcriptional signatures in major depressive disorder and mouse chronic stress models. Biol. Psychiatry 88, 159–168 (2020).
Hodes, G. E. et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 35, 16362–16376 (2015).
Pothula, S. et al. Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons. Neuropsychopharmacology 46, 799–808 (2021).
Liu, J., Dietz, K., Hodes, G. E., Russo, S. J. & Casaccia, P. Widespread transcriptional alternations in oligodendrocytes in the adult mouse brain following chronic stress. Dev. Neurobiol. 78, 152–162 (2018).
Willner, P. Reliability of the chronic mild stress model of depression: a user survey. Neurobiol. Stress. 6, 68–77 (2017).
Ducottet, C. & Belzung, C. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav. Brain Res. 156, 153–162 (2005).
Acknowledgements
We thank all authors who contributed to the work collected and summarized in this Review. This Review was supported by a Postdoctoral Fellowship from the Canadian Institutes of Health Research (201811MFE-414896-231226), a NARSAD Young Investigator Award from the Brain and Behaviour Research Foundation (30894), and a Pathway to Independence Award from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K99DK137037) to K.L.C., a research grant from the Cure Alzheimer’s Fund to W.C.P., National Institutes of Health grants R35HL135752, P01HL131478 and 1P01HL142494 to F.K.S., and R01MH104559 and R01MH127820 to S.J.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
K.L.C. and S.J.R. researched data for the article and wrote the article. All authors contributed substantially to the discussion of content and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Jonathan Godbout and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Chemogenetics
-
An approach in which specific cellular pathways are activated or inhibited using engineered protein receptors that respond to previously unrecognized small molecules.
- Chemokines
-
Chemotactic cytokines that stimulate the migration of cells.
- Cytokines
-
Secreted proteins that act as signalling molecules for the immune system.
- Granulocyte
-
Leukocytes containing cytoplasmic secretory granules such as neutrophils, basophils and eosinophils.
- Leukocytes
-
A type of blood cell made in the bone marrow and found within blood and lymphoid tissue as part of the immune system.
- Lymphocytopenia
-
A reduction in the number of lymphocytes in the blood.
- Lymphopoiesis
-
The production of lymphocytes from progenitor cells.
- Monocytosis
-
An increase in the number of monocytes in the blood.
- Myelopoiesis
-
The production of myeloid cells from progenitor cells.
- Neutrophilia
-
An increase in the number of neutrophils in the blood.
- Optogenetics
-
An approach in which light-sensitive ion channels, pumps or enzymes are used to regulate the activity of specific neurons in the brain or periphery.
- Plasma cells
-
Effector B lymphocytes that produce antibodies.
- Resilience
-
The ability to maintain normal physiological and behavioural function in the face of severe stress.
- Reward
-
A positive emotional stimulus. In psychological terms, a reward is reinforcing — it promotes repeated responses to obtain the same stimulus.
- Splenomegaly
-
An enlargement of the spleen.
- Susceptible
-
Having increased vulnerability to succumb to the deleterious effects of stress.
- Ventral tegmental area
-
VTA. A ventral midbrain site containing dopaminergic neurons that are an essential component of the reward circuitry in the brain.
- Viral tracing
-
The use of trans-synaptic self-replicating viruses to identify neural pathways.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chan, K.L., Poller, W.C., Swirski, F.K. et al. Central regulation of stress-evoked peripheral immune responses. Nat. Rev. Neurosci. 24, 591–604 (2023). https://doi.org/10.1038/s41583-023-00729-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00729-2