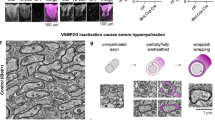
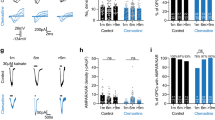
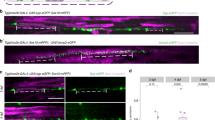
Abstract
Extracellular vesicles (EVs) have recently emerged as versatile elements of cell communication in the nervous system, mediating tissue homeostasis. EVs influence the physiology of their target cells via horizontal transfer of molecular cargo between cells and by triggering signalling pathways. In this Review, we discuss recent work revealing that EVs mediate interactions between oligodendrocytes and neurons, which are relevant for maintaining the structural integrity of axons. In response to neuronal activity, myelinating oligodendrocytes release EVs, which are internalized by neurons and provide axons with key factors that improve axonal transport, stress resistance and energy homeostasis. Glia-to-neuron transfer of EVs is thus a crucial facet of axonal preservation. When glial support is impaired, axonal integrity is progressively lost, as observed in myelin-related disorders, other neurodegenerative diseases and with normal ageing. We highlight the mechanisms that oligodendroglial EVs use to sustain axonal integrity and function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fields, R. D. et al. Glial biology in learning and cognition. Neuroscientist 20, 426–431 (2014).
Allen, N. J. & Lyons, D. A. Glia as architects of central nervous system formation and function. Science 362, 181–185 (2018).
Stassart, R. M., Möbius, W., Nave, K.-A. & Edgar, J. M. The axon–myelin unit in development and degenerative disease. Front. Neurosci. 12, 467 (2018).
Duncan, G. J., Simkins, T. J. & Emery, B. Neuron-oligodendrocyte interactions in the structure and integrity of axons. Front. Cell Dev. Biol. 9, 653101 (2021).
Nave, K.-A. & Werner, H. B. Ensheathment and myelination of axons: evolution of glial functions. Annu. Rev. Neurosci. 44, 197–219 (2021).
Zuchero, J. B. & Barres, B. A. Glia in mammalian development and disease. Development 142, 3805–3809 (2015).
Nave, K.-A. & Werner, H. B. Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell Dev. Biol. 30, 503–533 (2014).
Stadelmann, C., Timmler, S., Barrantes-Freer, A. & Simons, M. Myelin in the central nervous system: structure, function, and pathology. Physiol. Rev. 99, 1381–1431 (2019).
Lubetzki, C., Sol-Foulon, N. & Desmazières, A. Nodes of Ranvier during development and repair in the CNS. Nat. Rev. Neurol. 16, 426–439 (2020).
Tasaki, I. The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. Am. J. Physiol. Leg. Content 127, 211–227 (1939).
Hartline, D. K. & Colman, D. R. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 17, R29–R35 (2007).
Zalc, B. & Colman, D. R. Origins of vertebrate success. Science 288, 271–272 (2000).
Weil, M.-T. et al. Axonal ensheathment in the nervous system of lamprey: implications for the evolution of myelinating glia. J. Neurosci. 38, 6586–6596 (2018).
Verkhratsky, A., Ho, M. S. & Parpura, V. Evolution of neuroglia. Adv. Exp. Med. Biol. 1175, 15–44 (2019).
Bittern, J. et al. Neuron–glia interaction in the Drosophila nervous system. Dev. Neurobiol. 81, 438–452 (2021).
Cuadras, J. & Marti-Subirana, A. Glial cells of the crayfish and their relationships with neurons. An ultrastructural study. J. Physiol. 82, 196–217 (1987).
Volkenhoff, A. et al. Glial glycolysis is essential for neuronal survival in Drosophila. Cell Metab. 22, 437–447 (2015).
Bourke, A. M., Schwarz, A. & Schuman, E. M. De-centralizing the central dogma: mRNA translation in space and time. Mol. Cell 83, 452–468 (2023).
Frühbeis, C., Fröhlich, D., Kuo, W. P. & Krämer-Albers, E.-M. Extracellular vesicles as mediators of neuron–glia communication. Front. Cell Neurosci. 7, 182 (2013).
Tytell, M., Lasek, R. J. & Gainer, H. Axonal maintenance, glia, exosomes, and heat shock proteins. F1000Res 5, F1000 Faculty Rev-205 (2016).
Buchheit, T. E. & Tytell, M. Transfer of molecules from glia to axon in the squid may be mediated by glial vesicles. J. Neurobiol. 23, 217–230 (1992). This study provided the first evidence of vesicular transfer of molecules from ensheathing glia to giant axons in squid.
Saab, A. S. & Nave, K.-A. Myelin dynamics: protecting and shaping neuronal functions. Curr. Opin. Neurobiol. 47, 104–112 (2017).
Philips, T. & Rothstein, J. D. Oligodendroglia: metabolic supporters of neurons. J. Clin. Investig. 127, 3271–3280 (2017).
Welte, M. A. Expanding roles for lipid droplets. Curr. Biol. 25, R470–R481 (2015).
Smolič, T., Zorec, R. & Vardjan, N. Pathophysiology of lipid droplets in neuroglia. Antioxidants 11, 22 (2021).
Olguín-Albuerne, M. & Morán, J. Redox signaling mechanisms in nervous system development. Antioxid. Redox Signal. 28, 1603–1625 (2018).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Lo Cicero, A., Stahl, P. D. & Raposo, G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr. Opin. Cell Biol. 35, 69–77 (2015).
van Niel, G. et al. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382 (2022).
Budnik, V., Ruiz-Cañada, C. & Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 17, 160–172 (2016).
Krämer-Albers, E.-M. & Hill, A. F. Extracellular vesicles: interneural shuttles of complex messages. Curr. Opin. Neurobiol. 39, 101–107 (2016).
Holm, M. M., Kaiser, J. & Schwab, M. E. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci. 41, 360–372 (2018).
Schnatz, A., Müller, C., Brahmer, A. & Krämer-Albers, E.-M. Extracellular vesicles in neural cell interaction and CNS homeostasis. FASEB Bioadv. 3, 577–592 (2021).
Saint-Pol, J., Gosselet, F., Duban-Deweer, S., Pottiez, G. & Karamanos, Y. Targeting and crossing the blood–brain barrier with extracellular vesicles. Cells 9, E851 (2020).
Krämer-Albers, E.-M. Extracellular vesicles at CNS barriers: mode of action. Curr. Opin. Neurobiol. 75, 102569 (2022).
Korkut, C. et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 77, 1039–1046 (2013).
Pastuzyn, E. D. et al. The neuronal gene arc encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Cell 172, 275–288.e18 (2018).
Ashley, J. et al. Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172, 262–274.e11 (2018).
Men, Y. et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 10, 4136 (2019).
Delpech, J.-C., Herron, S., Botros, M. B. & Ikezu, T. Neuroimmune crosstalk through extracellular vesicles in health and disease. Trends Neurosci. 42, 361–372 (2019).
Dickens, A. M. et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal. 10, eaai7696 (2017).
Hill, A. F. Extracellular vesicles and neurodegenerative diseases. J. Neurosci. 39, 9269–9273 (2019).
Croese, T. & Furlan, R. Extracellular vesicles in neurodegenerative diseases. Mol. Asp. Med. 60, 52–61 (2018).
O’Hara, B. A., Morris-Love, J., Gee, G. V., Haley, S. A. & Atwood, W. J. JC virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor. PLoS Pathog. 16, e1008371 (2020).
Bello-Morales, R. et al. Role of microvesicles in the spread of herpes simplex virus 1 in oligodendrocytic cells. J. Virol. 92, e00088-18 (2018).
Rufino-Ramos, D. et al. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J. Control. Rel. 262, 247–258 (2017).
Cheng, L. & Hill, A. F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 21, 379–399 (2022).
Rasband, M. N. & Peles, E. Mechanisms of node of Ranvier assembly. Nat. Rev. Neurosci. 22, 7–20 (2021).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Frühbeis, C. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS Biol. 11, e1001604 (2013). This study demonstrates exosomal oligodendrocyte-to-neuron transfer of cargo that exerts biological activity in receiving cells.
Hsu, C. et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232 (2010).
Krämer-Albers, E.-M. et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteom. Clin. Appl. 1, 1446–1461 (2007).
Edgar, J. M. et al. Río-Hortega’s drawings revisited with fluorescent protein defines a cytoplasm-filled channel system of CNS myelin. J. Anat. 239, 1241–1255 (2021).
Káradóttir, R., Cavelier, P., Bergersen, L. H. & Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005).
Micu, I. et al. The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 276, 41–50 (2016).
Alix, J. J. P., Dolphin, A. C. & Fern, R. Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation. J. Physiol. 586, 4069–4089 (2008).
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Micu, I., Plemel, J. R., Caprariello, A. V., Nave, K.-A. & Stys, P. K. Axo-myelinic neurotransmission: a novel mode of cell signalling in the central nervous system. Nat. Rev. Neurosci. 19, 49–58 (2018).
Savina, A., Fader, C. M., Damiani, M. T. & Colombo, M. I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6, 131–143 (2005).
Verweij, F. J. et al. ER membrane contact sites support endosomal small GTPase conversion for exosome secretion. J. Cell Biol. 221, e202112032 (2022).
Feldmann, A. et al. Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7. J. Neurosci. 31, 5659–5672 (2011).
Lam, M. et al. CNS myelination requires VAMP2/3-mediated membrane expansion in oligodendrocytes. Nat. Commun. 13, 5583 (2022).
Yergert, K. M., Doll, C.A., O’Rouke, R., Hines, J. H. & Appel, B. Identification of 3′ UTR motifs required for mRNA localization to myelin sheaths in vivo. PLoS Biol. https://doi.org/10.1371/journal.pbio.3001053 (2021).
Thakurela, S. et al. The transcriptome of mouse central nervous system myelin. Sci. Rep. 6, 25828 (2016).
Ainger, K. et al. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J. Cell Biol. 123, 431–441 (1993).
Waxman, S. G. & Sims, T. J. Specificity in central myelination: evidence for local regulation of myelin thickness. Brain Res. 292, 179–185 (1984).
White, R. et al. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J. Cell Biol. 181, 579–586 (2008).
Wake, H., Lee, P. R. & Fields, R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011).
Krämer-Albers, E.-M. & White, R. From axon–glial signalling to myelination: the integrating role of oligodendroglial Fyn kinase. Cell Mol. Life Sci. 68, 2003–2012 (2011).
Saab, A. S. et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016).
Looser, Z. J. et al. Potassium regulates axon-oligodendrocyte signaling and metabolic coupling in white matter. Preprint at bioRxiv https://doi.org/10.1101/2022.11.08.515614 (2022).
Baietti, M. F. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685 (2012).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Larios, J., Mercier, V., Roux, A. & Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 219, e201904113 (2020).
van Niel, G. et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 21, 708–721 (2011).
Mukherjee, C. et al. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab. 32, 259–272 (2020). This study identifies ferritin as an exosomal cargo that is conserved between insect ensheathing glia and mammalian oligodendrocytes and facilitates antioxidant defence to prevent neurodegeneration.
Coetzee, T., Suzuki, K. & Popko, B. New perspectives on the function of myelin galactolipids. Trends Neurosci. 21, 126–130 (1998).
Villarroya-Beltri, C. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980 (2013).
Kunadt, M. et al. Extracellular vesicle sorting of α-synuclein is regulated by sumoylation. Acta Neuropathol. 129, 695–713 (2015).
Carnino, J. M., Ni, K. & Jin, Y. Post-translational modification regulates formation and cargo-loading of extracellular vesicles. Front. Immunol. 11, 948 (2020).
Perez-Hernandez, D. et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 288, 11649–11661 (2013).
Frühbeis, C. et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 18, e3000621 (2020). This study shows how impaired oligodendroglial exosomes fail to support axonal transport and axonal preservation in two mouse models of severe neurodegenerative disease in humans.
Iraci, N. et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat. Chem. Biol. 13, 951–955 (2017).
Fröhlich, D. et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130510 (2014).
Simons, M., Krämer, E. M., Thiele, C., Stoffel, W. & Trotter, J. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J. Cell Biol. 151, 143–154 (2000).
Werner, H. B. et al. A critical role for the cholesterol-associated proteolipids PLP and M6B in myelination of the central nervous system. GLIA 61, 567–586 (2013).
Werner, H. B. et al. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J. Neurosci. 27, 7717–7730 (2007).
Snaidero, N. et al. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 18, 314–323 (2017).
Edgar, J. et al. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J. Cell Biol. 166, 121–131 (2004).
Chamberlain, K. A. et al. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 109, 3456–3472.e8 (2021). This study reveals that exosomal oligodendrocyte-to-axon transfer of SIRT2 increases axonal energy production by deacetylation of mitochondrial proteins ANT1 and ANT2.
Krämer-Albers, E.-M. Superfood for axons: glial exosomes boost axonal energetics by delivery of SIRT2. Neuron 109, 3397–3400 (2021).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Gargareta, V. et al. Conservation and divergence of myelin proteome and oligodendrocyte transcriptome profiles between humans and mice. eLife 11, e77019 (2022).
Liu, G. et al. Loss of NAD-dependent protein deacetylase Sirtuin-2 alters mitochondrial protein acetylation and dysregulates mitophagy. Antioxid. Redox Signal. 26, 849–863 (2017).
Fourcade, S. et al. Loss of SIRT2 leads to axonal degeneration and locomotor disability associated with redox and energy imbalance. Aging Cell 16, 1404–1413 (2017).
Tytell, M., Greenberg, S. G. & Lasek, R. J. Heat shock-like protein is transferred from glia to axon. Brain Res. 363, 161–164 (1986).
Wei, Z., Batagov, A. O., Carter, D. R. F. & Krichevsky, A. M. Fetal bovine serum RNA interferes with the cell culture derived extracellular RNA. Sci. Rep. 6, 31175 (2016).
Auber, M., Fröhlich, D., Drechsel, O., Karaulanov, E. & Krämer-Albers, E.-M. Serum-free media supplements carry miRNAs that co-purify with extracellular vesicles. J. Extracell. Vesicles 8, 1656042 (2019).
Verweij, F. J. et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat. Methods 18, 1013–1026 (2021).
Wan, C., Stowell, M. H. B. & Shen, J. Progress and gaps of extracellular vesicle-mediated intercellular cargo transfer in the central nervous system. Commun. Biol. 5, 1223 (2022).
Saugier-Veber, P. et al. X-linked spastic paraplegia and Pelizaeus–Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat. Genet. 6, 257–262 (1994).
Garbern, J. Y. et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125, 551–561 (2002).
Novarino, G. et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science 343, 506–511 (2014).
Lossos, A. et al. Myelin-associated glycoprotein gene mutation causes Pelizaeus–Merzbacher disease-like disorder. Brain 138, 2521–2536 (2015).
Steyer, A. M. et al. Focused ion beam-scanning electron microscopy links pathological myelin outfoldings to axonal changes in mice lacking Plp1 or Mag. Glia 71, 509–523 (2023).
Lappe-Siefke, C. et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33, 366–374 (2003).
Edgar, J. M. et al. Early ultrastructural defects of axons and axon–glia junctions in mice lacking expression of Cnp1. GLIA 57, 1815–1824 (2009).
Patzig, J. et al. Septin/anillin filaments scaffold central nervous system myelin to accelerate nerve conduction. eLife 5, e17119 (2016).
Al-Abdi, L. et al. CNP deficiency causes severe hypomyelinating leukodystrophy in humans. Hum. Genet. 139, 615–622 (2020).
Jahn, O. et al. The CNS myelin proteome: deep profile and persistence after post-mortem delay. Front. Cell. Neurosci. 14, 239 (2020).
Trapp, B. D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998).
Chen, J.-F. et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 109, 2292–2307.e5 (2021).
Depp, C. et al. Ageing-associated myelin dysfunction drives amyloid deposition in mouse models of Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2021.07.31.454562 (2021).
Nguyen, M. V. C. et al. Oligodendrocyte lineage cells contribute unique features to Rett syndrome neuropathology. J. Neurosci. 33, 18764–18774 (2013).
Kang, S. H. et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 16, 571–579 (2013).
Baecher-Allan, C., Kaskow, B. J. & Weiner, H. L. Multiple sclerosis: mechanisms and immunotherapy. Neuron 97, 742–768 (2018).
Franklin, R. J. M. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 3, 705–714 (2002).
Franklin, R. J. M. & Ffrench-Constant, C. Regenerating CNS myelin — from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18, 753–769 (2017).
Lubetzki, C., Zalc, B., Williams, A., Stadelmann, C. & Stankoff, B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19, 678–688 (2020).
Osorio-Querejeta, I., Alberro, A., Muñoz-Culla, M., Mäger, I. & Otaegui, D. Therapeutic potential of extracellular vesicles for demyelinating diseases; challenges and opportunities. Front. Mol. Neurosci. 11, 434 (2018).
Zhang, J. et al. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 347, 113895 (2022).
Osorio-Querejeta, I. et al. MiR-219a-5p enriched extracellular vesicles induce OPC differentiation and EAE improvement more efficiently than liposomes and polymeric nanoparticles. Pharmaceutics 12, E186 (2020).
Casella, G. et al. Extracellular vesicles containing IL-4 modulate neuroinflammation in a mouse model of multiple sclerosis. Mol. Ther. 26, 2107–2118 (2018).
Casella, G. et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci. Transl. Med. 12, eaba0599 (2020).
Tanabe, K. et al. Generation of functional human oligodendrocytes from dermal fibroblasts by direct lineage conversion. Development 149, dev199723 (2022).
Ehrlich, M. et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl Acad. Sci. USA 114, E2243–E2252 (2017).
Zhuang, X. et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19, 1769–1779 (2011).
Philips, T. et al. MCT1 deletion in oligodendrocyte lineage cells causes late-onset hypomyelination and axonal degeneration. Cell Rep. 34, 108610 (2021).
Trevisiol, A. et al. Structural myelin defects are associated with low axonal ATP levels but rapid recovery from energy deprivation in a mouse model of spastic paraplegia. PLoS Biol. 18, e3000943 (2020).
Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 (1998).
Moffett, J. R., Ross, B., Arun, P., Madhavarao, C. N. & Namboodiri, A. M. A. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 81, 89–131 (2007).
Mersmann, N. et al. Aspartoacylase-lacZ knockin mice: an engineered model of Canavan disease. PLoS ONE 6, e20336 (2011).
Kassmann, C. M. et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 39, 969–976 (2007).
Buscham, T. J. et al. Progressive axonopathy when oligodendrocytes lack the myelin protein CMTM5. eLife 11, e75523 (2022).
De Monasterio-Schrader, P. et al. Uncoupling of neuroinflammation from axonal degeneration in mice lacking the myelin protein tetraspanin-2. GLIA 61, 1832–1847 (2013).
Peters, A. The effects of normal aging on myelin and nerve fibers: a review. J. Neurocytol. 31, 581–593 (2002).
Sturrock, R. R. Changes in neuroglia and myelination in the white matter of aging mice. J. Gerontol. 31, 513–522 (1976).
Lasiene, J., Matsui, A., Sawa, Y., Wong, F. & Horner, P. J. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 8, 201–213 (2009).
Marshall-Phelps, K. L. H. et al. Neuronal activity disrupts myelinated axon integrity in the absence of NKCC1b. J. Cell Biol. 219, e201909022 (2020).
Neusch, C. et al. Lack of the Kir4.1 channel subunit abolishes K + buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K + regulation. J. Neurophysiol. 95, 1843–1852 (2006).
Kapell, H. et al. Neuron–oligodendrocyte potassium shuttling at nodes of Ranvier protects against inflammatory demyelination. J. Clin. Invest. 133, e164223 (2023).
Xin, W. et al. Oligodendrocytes support neuronal glutamatergic transmission via expression of glutamine synthetase. Cell Rep. 27, 2262–2271.e5 (2019).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Fernandez, P. A. et al. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron 28, 81–90 (2000).
Goebbels, S. et al. A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat. Neurosci. 20, 10–15 (2017).
Krämer-Albers, E.-M. Extracellular vesicles in the oligodendrocyte microenvironment. Neurosci. Lett. 725, 134915 (2020).
Singer, M. & Salpeter, M. M. Transport of tritium-labelled l-histidine through the Schwann and myelin sheaths into the axon of peripheral nerves. Nature 210, 1225–1227 (1966).
Giuditta, A., Dettbarn, W. D. & Brzin, M. Protein synthesis in the isolated giant axon of the squid. Proc. Natl Acad. Sci. USA 59, 1284–1287 (1968).
Gainer, H., Tasaki, I. & Lasek, R. J. Evidence for the glia–neuron protein transfer hypothesis from intracellular perfusion studies of squid giant axons. J. Cell Biol. 74, 524–530 (1977).
Lasek, R. J., Gainer, H. & Barker, J. L. Cell-to-cell transfer of glial proteins to the squid giant axon. The glia–neuron protein transfer hypothesis. J. Cell Biol. 74, 501–523 (1977).
Tytell, M. & Lasek, R. J. Glial polypeptides transferred into the squid giant axon. Brain Res. 324, 223–232 (1984).
Mathur, C. et al. Demonstration of ion channel synthesis by isolated squid giant axon provides functional evidence for localized axonal membrane protein translation. Sci. Rep. 8, 2207 (2018).
Hafner, A.-S., Donlin-Asp, P. G., Leitch, B., Herzog, E. & Schuman, E. M. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science 364, eaau3644 (2019).
Biever, A. et al. Monosomes actively translate synaptic mRNAs in neuronal processes. Science 367, eaay4991 (2020).
Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Colombo, M., Raposo, G. & Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018). This study establishes a technical guideline for conducting rigorous and reproducible extracellular vesicle research.
Krämer-Albers, E.-M. & Frühbeis, C. Delivery on call: exosomes as ‘care packages’ from glial cells for stressed neurons. e-Neuroforum 19, 85–91 (2013).
Acknowledgements
The authors dedicate this article to the memory of Marie T. Filbin and Steven E. Pfeiffer in appreciation of their pioneering work on the cell biology of oligodendrocytes. The authors thank J. Trotter and K.-A. Nave for discussions. E.-M.K.-A. is supported by the Deutsche Forschungsgemeinschaft (grants KR 3668/1-2 and KR 3668/2-2). H.B.W. is supported by the Deutsche Forschungsgemeinschaft (grants WE 2720/2-2, WE 2720/4-1 and WE 2720/5-1).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Peer review
Referee accreditation
Nature Reviews Neuroscience thanks D. Lecca; B. Zuchero, who co-reviewed with M. Lam; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- 2′,3′-Cyclic nucleotide 3′-phosphodiesterase
-
(CNP). The third-most abundant protein of CNS myelin.
- Endosomal sorting complex required for transport
-
A multicomponent protein complex facilitating various steps of membrane remodelling.
- Exosomal escape
-
A mechanism by which exosomal cargo fuses with and integrates into the target cell and acquires biological activity.
- Exosomes
-
A subclass of extracellular vesicles released by fusion of multivesicular endosomes with the plasma membrane and are the endosomal intraluminal vesicles that are formed by inward budding of the limiting membrane.
- Extracellular vesicles
-
(EVs). Umbrella term for different types of vesicle released by cells comprising cytoplasmic content and membrane surface-expressed epitopes.
- Fluorescence resonance energy transfer-sensor imaging
-
Biosensors that allow visualization of dynamic molecular events in living cells, including the generation of ATP from ADP or changes in cytosolic calcium levels.
- Hereditary spastic paraplegia
-
(SPG). A heterogenous group of progressive gait disorders caused by dysfunction or degeneration of long-projecting axons in the spinal cord.
- Hypomyelinating leukodystrophy
-
(HLD). Umbrella term for a heterogenous group of severe neurodegenerative disorders primarily affecting white matter physiology.
- Multivesicular endosomes
-
Spherical organelles of the late endosomal system that are packed with internal vesicles and can either fuse with lysosomes to degrade the internal material or fuse with the plasma membrane to release their content into the extracellular space.
- Non-compacted cytoplasmic myelin subcompartment
-
Cytosolic sub-compartments of myelin including the adaxonal myelin layer, paranodal myelin and myelinic channels through otherwise compact myelin.
- Periaxonal space
-
Extracellular space between the plasma membrane of an axon and the adjacent adaxonal myelin membrane.
- Proteolipid protein
-
(PLP). A cholesterol-associated, tetraspan-transmembrane protein; the most abundant protein of CNS myelin.
- Reducing equivalent
-
Chemical species that transfer electrons in a redox reaction.
- Sirtuin-2
-
(SIRT2). An NAD+-dependent deacetylase that is highly expressed in oligodendrocytes.
- Sphingomyelinase
-
An enzyme that cleaves the phosphodiester bond of sphingomyelin generating ceramide and phosphocholine.
- Tetraspanins
-
A protein family containing four transmembrane domains and two extracellular domains that organize proteins and lipids in membrane subdomains (tetraspanin-enriched microdomains).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krämer-Albers, EM., Werner, H.B. Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles. Nat. Rev. Neurosci. 24, 474–486 (2023). https://doi.org/10.1038/s41583-023-00711-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00711-y