Abstract
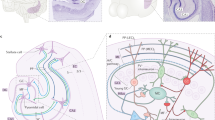
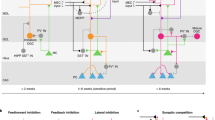
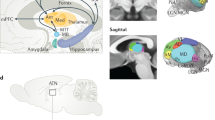
There has been considerable speculation regarding the function of the dentate gyrus (DG) — a subregion of the mammalian hippocampus — in learning and memory. In this Perspective article, we compare leading theories of DG function. We note that these theories all critically rely on the generation of distinct patterns of activity in the region to signal differences between experiences and to reduce interference between memories. However, these theories are divided by the roles they attribute to the DG during learning and recall and by the contributions they ascribe to specific inputs or cell types within the DG. These differences influence the information that the DG is thought to impart to downstream structures. We work towards a holistic view of the role of DG in learning and memory by first developing three critical questions to foster a dialogue between the leading theories. We then evaluate the extent to which previous studies address our questions, highlight remaining areas of conflict, and suggest future experiments to bridge these theories.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Amaral, D. G., Scharfman, H. E. & Lavenex, P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163, 3–22 (2007).
Cameron, H., Woolley, C., McEwen, B. & Gould, E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56, 337–344 (1993).
Eriksson, P. S. et al. Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 (1998).
Kuhn, H. G., Toda, T. & Gage, F. H. Adult hippocampal neurogenesis: a coming-of-age story. J. Neurosci. 38, 10401–10410 (2018).
Paredes, M. F. et al. Does adult neurogenesis persist in the human hippocampus? Cell Stem Cell 23, 780–781 (2018).
Kempermann, G. et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23, 25–30 (2018).
Ohara, S. et al. Local projections of layer Vb-to-Va are more prominent in lateral than in medial entorhinal cortex. eLife 10, e67262 (2021).
Ming, G. & Song, H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 (2011).
Marr, D. A theory of cerebellar cortex. J. Physiol. 202, 437–470 (1969).
Eccles, J., Ito, M. & Szentagothai, J. The Cerebellum as a Neuronal Machine 335 (Springer, 1967).
Rolls, E. T. in Neural Models of Plasticity 240–265 (Elsevier, 1989).
McNaughton, B. L. & Morris, R. G. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415 (1987).
McNaughton, B. L. & Nadel, L. in Neuroscience and Connectionist Theory (eds Gluck, M. A. & Rumelhart, D. E.) 1–63 (Lawrence Erlbaum, 1990).
O’reilly, R. C. & McClelland, J. L. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade‐off. Hippocampus 4, 661–682 (1994).
Myers, C. E. & Scharfman, H. E. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus 21, 1190–1215 (2011).
Myers, C. E. & Scharfman, H. E. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus 19, 321–337 (2009).
Amaral, D., Ishizuka, N. & Claiborne, B. Neurons, numbers and the hippocampal network. Prog. Brain Res. 83, 1–11 (1990).
Yassa, M. A. & Stark, C. E. Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525 (2011).
Mishkin, M., Suzuki, W. A., Gadian, D. G. & Vargha-Khadem, F. Hierarchical organization of cognitive memory. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 352, 1461–1467 (1997).
Manns, J. R. & Eichenbaum, H. Evolution of declarative memory. Hippocampus 16, 795–808 (2006).
Knierim, J. J., Lee, I. & Hargreaves, E. L. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus 16, 755–764 (2006).
Reagh, Z. M. & Yassa, M. A. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc. Natl Acad. Sci. USA 111, E4264–E4273 (2014).
Doan, T. P., Lagartos-Donate, M. J., Nilssen, E. S., Ohara, S. & Witter, M. P. Convergent projections from perirhinal and postrhinal cortices suggest a multisensory nature of lateral, but not medial, entorhinal cortex. Cell Rep. 29, 617–627 (2019).
Nilssen, E. S., Doan, T. P., Nigro, M. J., Ohara, S. & Witter, M. P. Neurons and networks in the entorhinal cortex: a reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus 29, 1238–1254 (2019).
Burwell, R. D. & Amaral, D. G. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J. Comp. Neurol. 391, 293–321 (1998).
Knierim, J. J., Neunuebel, J. P. & Deshmukh, S. S. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local–global reference frames. Philos. Trans. R. Soc. B Biol. Sci. 369, 20130369 (2014).
Neunuebel, J. P., Yoganarasimha, D., Rao, G. & Knierim, J. J. Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J. Neurosci. 33, 9246–9258 (2013).
O’Keefe, J. O. & Nadel, L. The Hippocampus as a Cognitive Map (Clarendon, 1978).
O’Keefe, J. & Nadel, L. Précis of O’Keefe & Nadel’s The hippocampus as a cognitive map. Behav. Brain Sci. 2, 487–494 (1979).
Rueckemann, J. W., Sosa, M., Giocomo, L. M. & Buffalo, E. A. The grid code for ordered experience. Nat. Rev. Neurosci. 22, 637–649 (2021).
Lee, J. W. & Jung, M. W. Separation or binding? Role of the dentate gyrus in hippocampal mnemonic processing. Neurosci. Biobehav. Rev. 75, 183–194 (2017).
Vinogradova, O. The hippocampus and the orienting reflex. Neuronal Mech. Orient. Reflex. 128, 154 (1975).
Amaral, D. G. & Campbell, M. Transmitter systems in the primate dentate gyrus. Hum. Neurobiol. 5, 169–180 (1986).
Prince, L. Y., Bacon, T. J., Tigaret, C. M. & Mellor, J. R. Neuromodulation of the feedforward dentate gyrus-CA3 microcircuit. Front. Synaptic Neurosci. 8, 32 (2016).
Harley, C. W. Norepinephrine and the dentate gyrus. Prog. Brain Res. 163, 299–318 (2007).
Straube, T., Korz, V., Balschun, D. & Uta Frey, J. Requirement of β‐adrenergic receptor activation and protein synthesis for LTP‐reinforcement by novelty in rat dentate gyrus. J. Physiol. 552, 953–960 (2003).
Brown, R. A., Walling, S. G., Milway, J. S. & Harley, C. W. Locus ceruleus activation suppresses feedforward interneurons and reduces β-γ electroencephalogram frequencies while it enhances θ frequencies in rat dentate gyrus. J. Neurosci. 25, 1985–1991 (2005).
Kitchigina, V., Vankov, A., Harley, C. & Sara, S. J. Novelty‐elicited, noradrenaline‐dependent enhancement of excitability in the dentate gyrus. Eur. J. Neurosci. 9, 41–47 (1997).
Ogando, M. B. et al. Cholinergic modulation of dentate gyrus processing through dynamic reconfiguration of inhibitory circuits. Cell Rep. 36, 109572 (2021).
Hofmann, M. E. & Frazier, C. J. Muscarinic receptor activation modulates the excitability of hilar mossy cells through the induction of an afterdepolarization. Brain Res. 1318, 42–51 (2010).
Duffy, A. M., Schaner, M. J., Chin, J. & Scharfman, H. E. Expression of c‐fos in hilar mossy cells of the dentate gyrus in vivo. Hippocampus 23, 649–655 (2013).
Bernstein, H. L., Lu, Y.-L., Botterill, J. J. & Scharfman, H. E. Novelty and novel objects increase c-Fos immunoreactivity in mossy cells in the mouse dentate gyrus. Neural Plast. https://doi.org/10.1155/2019/1815371 (2019).
Ewell, L. A. & Jones, M. V. Frequency-tuned distribution of inhibition in the dentate gyrus. J. Neurosci. 30, 12597–12607 (2010).
Buzsàki, G. & Eidelberg, E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 230, 346–350 (1981).
Li, Y. et al. Molecular layer perforant path-associated cells contribute to feed-forward inhibition in the adult dentate gyrus. Proc. Natl Acad. Sci. USA 110, 9106–9111 (2013).
Sloviter, R. & Brisman, J. Lateral inhibition and granule cell synchrony in the rat hippocampal dentate gyrus. J. Neurosci. 15, 811–820 (1995).
Scharfman, H. E. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J. Neurophysiol. 74, 179–194 (1995).
Scharfman, H., Kunkel, D. & Schwartzkroin, P. A. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience 37, 693–707 (1990).
Otis, T. & Mody, I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience 49, 13–32 (1992).
Staley, K. J., Otis, T. S. & Mody, I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J. Neurophysiol. 67, 1346–1358 (1992).
Espinoza, C., Guzman, S. J., Zhang, X. & Jonas, P. Parvalbumin + interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat. Commun. 9, 4605 (2018).
Hsu, D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog. Brain Res. 163, 601–613 (2007).
Josselyn, S. A., Köhler, S. & Frankland, P. W. Finding the engram. Nat. Rev. Neurosci. 16, 521–534 (2015).
Hainmueller, T. & Bartos, M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci. 21, 153–168 (2020).
Sun, X. et al. Functionally distinct neuronal ensembles within the memory engram. Cell 181, 410–423 (2020).
Ramirez, S. et al. Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339 (2015).
Lacagnina, A. F. et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci. 22, 753–761 (2019).
Denny, C. A., Lebois, E. & Ramirez, S. From engrams to pathologies of the brain. Front. Neural Circuits 11, 23 (2017).
Teyler, T. J. & DiScenna, P. The hippocampal memory indexing theory. Behav. Neurosci. 100, 147 (1986).
Miller, S. M. & Sahay, A. Functions of adult-born neurons in hippocampal memory interference and indexing. Nat. Neurosci. 22, 1565–1575 (2019).
Piatti, V. C., Espósito, M. S. & Schinder, A. F. The timing of neuronal development in adult hippocampal neurogenesis. Neuroscientist 12, 463–468 (2006).
Espósito, M. S. et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 25, 10074–10086 (2005).
Schmidt-Hieber, C., Jonas, P. & Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187 (2004).
Gu, Y. et al. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci. 15, 1700–1706 (2012).
Tashiro, A., Makino, H. & Gage, F. H. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci. 27, 3252–3259 (2007).
Rangel, L. et al. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat. Commun. 5, 3181 (2014).
Park, S.-B. et al. The fasciola cinereum subregion of the hippocampus is important for the acquisition of visual contextual memory. Prog. Neurobiol. 210, 102217 (2022).
Mau, W., Hasselmo, M. E. & Cai, D. J. The brain in motion: how ensemble fluidity drives memory-updating and flexibility. eLife 9, e63550 (2020).
Aimone, J. B., Wiles, J. & Gage, F. H. Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 9, 723–727 (2006).
Aimone, J. B., Wiles, J. & Gage, F. H. Computational influence of adult neurogenesis on memory encoding. Neuron 61, 187–202 (2009).
Lisman, J. E. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate–CA3 interactions. Neuron 22, 233–242 (1999).
Aimone, J. B., Deng, W. & Gage, F. H. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70, 589–596 (2011).
Nolan, C. R., Wyeth, G., Milford, M. & Wiles, J. The race to learn: spike timing and STDP can coordinate learning and recall in CA3. Hippocampus 21, 647–660 (2011).
Legéndy, C. R. On the ‘data stirring’ role of the dentate gyrus of the hippocampus. Rev. Neurosci. 28, 599–615 (2017).
Sahay, A. & Hen, R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 10, 1110–1115 (2007).
Cayco-Gajic, N. A. & Silver, R. A. Re-evaluating circuit mechanisms underlying pattern separation. Neuron 101, 584–602 (2019).
Hamlyn, L. Electron microscopy of mossy fibre endings in Ammon’s horn. Nature 190, 645–646 (1961).
Chicurel, M. E. & Harris, K. M. Three‐dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 325, 169–182 (1992).
Rollenhagen, A. et al. Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci. 27, 10434–10444 (2007).
Laatsch, R. H. & Cowan, W. Electron microscopic studies of the dentate gyrus of the rat. I. Normal structure with special reference to synaptic organization. J. Comp. Neurol. 128, 359–395 (1966).
Henze, D. A., Wittner, L. & Buzsáki, G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 5, 790–795 (2002).
Scharfman, H. E. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 7, 348–354 (2007).
Treves, A. & Rolls, E. T. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2, 189–199 (1992).
Marr, D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81 (1971).
Cerasti, E. & Treves, A. How informative are spatial CA3 representations established by the dentate gyrus? PLoS Computat. Biol. 6, e1000759 (2010).
McClelland, J. L., McNaughton, B. L. & O’Reilly, R. C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419 (1995).
O’Reilly, R. C., Bhattacharyya, R., Howard, M. D. & Ketz, N. Complementary learning systems. Cogn. Sci. 38, 1229–1248 (2014).
Lee, H., GoodSmith, D. & Knierim, J. J. Parallel processing streams in the hippocampus. Curr. Opin. Neurobiol. 64, 127–134 (2020).
Jarrard, L. E., Okaichi, H., Steward, O. & Goldschmidt, R. B. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav. Neurosci. 98, 946 (1984).
McLamb, R. L., Mundy, W. R. & Tilson, H. A. Intradentate colchicine impairs acquisition of a two-way active avoidance response in a Y-maze. Neurosci. Lett. 94, 338–342 (1988).
Mcnaughton, B. L., Barnes, C. A., Meltzer, J. & Sutherland, R. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp. Brain Res. 76, 485–496 (1989).
Nanry, K. P., Mundy, W. R. & Tilson, H. A. Colchicine-induced alterations of reference memory in rats: role of spatial versus non-spatial task components. Behav. Brain Res. 35, 45–53 (1989).
Xavier, G. F., Oliveira‐Filho, F. J. & Santos, A. M. Dentate gyrus‐selective colchicine lesion and disruption of performance in spatial tasks: difficulties in ‘place strategy’ because of a lack of flexibility in the use of environmental cues? Hippocampus 9, 668–681 (1999).
Gilbert, P. E., Kesner, R. P. & Lee, I. Dissociating hippocampal subregions: a double dissociation between dentate gyrus and CA1. Hippocampus 11, 626–636 (2001).
Lee, I. & Kesner, R. P. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav. Neurosci. 117, 1044 (2003).
Lee, I. & Kesner, R. P. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus 14, 66–76 (2004).
Lee, I. & Kesner, R. P. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear‐conditioning. Hippocampus 14, 301–310 (2004).
Lee, I., Hunsaker, M. R. & Kesner, R. P. The role of hippocampal subregions in detecting spatial novelty. Behav. Neurosci. 119, 145 (2005).
Hernández-Rabaza, V. et al. The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol. Learn. Mem. 90, 553–559 (2008).
Lee, C.-H. & Lee, I. Impairment of pattern separation of ambiguous scenes by single units in the CA3 in the absence of the dentate gyrus. J. Neurosci. 40, 3576–3590 (2020).
Lee, I. & Solivan, F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learn. Mem. 15, 357–367 (2008).
Ahn, J. & Lee, I. Intact CA3 in the hippocampus is only sufficient for contextual behavior based on well‐learned and unaltered visual background. Hippocampus 24, 1081–1093 (2014).
Walsh, T. J., Schulz, D. W., Tilson, H. A. & Schmechel, D. E. Cochicine-induced granule cell loss in rat hippocampus: selective behavioral and histological alterations. Brain Res. 398, 23–36 (1986).
Bernier, B. E. et al. Dentate gyrus contributes to retrieval as well as encoding: evidence from context fear conditioning, recall, and extinction. J. Neurosci. 37, 6359–6371 (2017).
Lassalle, J.-M., Bataille, T. & Halley, H. Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiol. Learn. Mem. 73, 243–257 (2000).
Madroñal, N. et al. Rapid erasure of hippocampal memory following inhibition of dentate gyrus granule cells. Nat. Commun. 7, 10923 (2016).
Lee, I. & Solivan, F. Dentate gyrus is necessary for disambiguating similar object-place representations. Learn. Mem. 17, 252–258 (2010).
O’Keefe, J. & Dostrovsky, J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971).
Eichenbaum, H., Dudchenko, P., Wood, E., Shapiro, M. & Tanila, H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23, 209–226 (1999).
van Dijk, M. T. & Fenton, A. A. On how the dentate gyrus contributes to memory discrimination. Neuron 98, 832–845 (2018).
Senzai, Y. & Buzsáki, G. Physiological properties and behavioral correlates of hippocampal granule cells and mossy cells. Neuron 93, 691–704 (2017).
GoodSmith, D. et al. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron 93, 677–690 (2017).
Knierim, J. J. & Neunuebel, J. P. Tracking the flow of hippocampal computation: pattern separation, pattern completion, and attractor dynamics. Neurobiol. Learn. Mem. 129, 38–49 (2016).
GoodSmith, D., Lee, H., Neunuebel, J. P., Song, H. & Knierim, J. J. Dentate gyrus mossy cells share a role in pattern separation with dentate granule cells and proximal CA3 pyramidal cells. J. Neurosci. 39, 9570–9584 (2019).
Neunuebel, J. P. & Knierim, J. J. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427 (2014).
Neunuebel, J. P. & Knierim, J. J. Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J. Neurosci. 32, 3848–3858 (2012).
Hainmueller, T. & Bartos, M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558, 292–296 (2018).
Jung, D. et al. Dentate granule and mossy cells exhibit distinct spatiotemporal responses to local change in a one-dimensional landscape of visual-tactile cues. Sci. Rep. 9, 9545 (2019).
Fredes, F. & Shigemoto, R. The role of hippocampal mossy cells in novelty detection. Neurobiol. Learn. Mem. 183, 107486 (2021).
Fredes, F. et al. Ventro-dorsal hippocampal pathway gates novelty-induced contextual memory formation. Curr. Biol. 31, 25–38 (2021).
Kirschen, G. W. et al. Active dentate granule cells encode experience to promote the addition of adult-born hippocampal neurons. J. Neurosci. 37, 4661–4678 (2017).
Kim, S., Jung, D. & Royer, S. Place cell maps slowly develop via competitive learning and conjunctive coding in the dentate gyrus. Nat. Commun. 11, 4550 (2020).
Woods, N. I. et al. The dentate gyrus classifies cortical representations of learned stimuli. Neuron 107, 173–184 (2020).
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013).
Guo, N. et al. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med. 24, 438–449 (2018).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017).
Josselyn, S. A. & Tonegawa, S. Memory engrams: recalling the past and imagining the future. Science 367, eaaw4325 (2020).
Guskjolen, A. et al. Recovery of ‘lost’ infant memories in mice. Curr. Biol. 28, 2283–2290 (2018).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015).
Denny, C. A. et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014).
Richards, B. A. & Frankland, P. W. The conjunctive trace. Hippocampus 23, 207–212 (2013).
Routtenberg, A. Lifetime memories from persistently supple synapses. Hippocampus 23, 202–206 (2013).
Stark, C. E. & Okado, Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J. Neurosci. 23, 6748–6753 (2003).
Guzman, S. J. et al. How connectivity rules and synaptic properties shape the efficacy of pattern separation in the entorhinal cortex–dentate gyrus–CA3 network. Nat. Comput. Sci. 1, 830–842 (2021).
Chambers, R. A., Potenza, M. N., Hoffman, R. E. & Miranker, W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology 29, 747–758 (2004).
Becker, S. A computational principle for hippocampal learning and neurogenesis. Hippocampus 15, 722–738 (2005).
Wiskott, L., Rasch, M. J., & Kempermann, G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus 16, 329–343 (2006).
Deisseroth, K. et al. Excitation–neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42, 535–552 (2004).
Weisz, V. I. & Argibay, P. F. Neurogenesis interferes with the retrieval of remote memories: forgetting in neurocomputational terms. Cognition 125, 13–25 (2012).
Tran, L. M., Josselyn, S. A., Richards, B. A. & Frankland, P. W. Forgetting at biologically realistic levels of neurogenesis in a large-scale hippocampal model. Behav. Brain Res. 376, 112180 (2019).
Chambers, R. A. & Conroy, S. K. Network modeling of adult neurogenesis: shifting rates of neuronal turnover optimally gears network learning according to novelty gradient. J. Cogn. Neurosci. 19, 1–12 (2007).
Jinde, S. et al. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron 76, 1189–1200 (2012).
Scharfman, H. E. The enigmatic mossy cell of the dentate gyrus. Nat. Rev. Neurosci. 17, 562–575 (2016).
Botterill, J. J. et al. Bidirectional regulation of cognitive and anxiety-like behaviors by dentate gyrus mossy cells in male and female mice. J. Neurosci. 41, 2475–2495 (2021).
Nakashiba, T. et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201 (2012).
Clelland, C. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009).
Sahay, A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011).
Masachs, N. et al. The temporal origin of dentate granule neurons dictates their role in spatial memory. Mol. Psychiatry 26, 7130–7140 (2021).
Saxe, M. D. et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl Acad. Sci. USA 103, 17501–17506 (2006).
Morris, A. M., Curtis, B. J., Churchwell, J. C., Maasberg, D. W. & Kesner, R. P. Temporal associations for spatial events: the role of the dentate gyrus. Behav. Brain Res. 256, 250–256 (2013).
Akers, K. G. et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014).
Epp, J. R. et al. Neurogenesis-mediated forgetting minimizes proactive interference. Nat. Commun. 7, 10838 (2016).
Cuartero, M. I. et al. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J. Clin. Investig. 129, 1536–1550 (2019).
Ishikawa, R., Fukushima, H., Frankland, P. W. & Kida, S. Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. eLife 5, e17464 (2016).
Gao, A. et al. Elevation of hippocampal neurogenesis induces a temporally graded pattern of forgetting of contextual fear memories. J. Neurosci. 38, 3190–3198 (2018).
Ishikawa, R., Uchida, C., Kitaoka, S., Furuyashiki, T. & Kida, S. Improvement of PTSD-like behavior by the forgetting effect of hippocampal neurogenesis enhancer memantine in a social defeat stress paradigm. Mol. Brain 12, 68 (2019).
Scott, G. A. et al. Adult neurogenesis mediates forgetting of multiple types of memory in the rat. Mol. Brain 14, 97 (2021).
Ryan, T. J. & Frankland, P. W. Forgetting as a form of adaptive engram cell plasticity. Nat. Rev. Neurosci. 23, 173–186 (2022).
Fricke, R. A. & Prince, D. A. Electrophysiology of dentate gyrus granule cells. J. Neurophysiol. 51, 195–209 (1984).
Scharfman, H. E. Blockade of excitation reveals inhibition of dentate spiny hilar neurons recorded in rat hippocampal slices. J. Neurophysiol. 68, 978–984 (1992).
Staley, K. J. & Mody, I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol. 68, 197–212 (1992).
Danielson, N. B. et al. In vivo imaging of dentate gyrus mossy cells in behaving mice. Neuron 93, 552–559 (2017).
Botterill, J. J. et al. An excitatory and epileptogenic effect of dentate gyrus mossy cells in a mouse model of epilepsy. Cell Rep. 29, 2875–2889 (2019).
Nitz, D. & McNaughton, B. Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J. Neurophysiol. 91, 863–872 (2004).
Rangel, L. M., Chiba, A. A. & Quinn, L. K. Theta and beta oscillatory dynamics in the dentate gyrus reveal a shift in network processing state during cue encounters. Front. Syst. Neurosci. 9, 96 (2015).
Trimper, J. B., Galloway, C. R., Jones, A. C., Mandi, K. & Manns, J. R. Gamma oscillations in rat hippocampal subregions dentate gyrus, CA3, CA1, and subiculum underlie associative memory encoding. Cell Rep. 21, 2419–2432 (2017).
Sasaki, T. et al. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nat. Neurosci. 21, 258–269 (2018).
Sullivan, D. et al. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci. 31, 8605–8616 (2011).
Luna, V. M. et al. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science 364, 578–583 (2019).
Piatti, V. C., Ewell, L. A. & Leutgeb, J. K. Neurogenesis in the dentate gyrus: carrying the message or dictating the tone. Front. Neurosci. 7, 50 (2013).
Ikrar, T. et al. Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circ. 7, 204 (2013).
Trinchero, M. F., Giacomini, D. & Schinder, A. F. Dynamic interplay between GABAergic networks and developing neurons in the adult hippocampus. Curr. Opin. Neurobiol. 69, 124–130 (2021).
Drew, L. J. et al. Activation of local inhibitory circuits in the dentate gyrus by adult‐born neurons. Hippocampus 26, 763–778 (2016).
Lacefield, C. O., Itskov, V., Reardon, T., Hen, R. & Gordon, J. A. Effects of adult‐generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus 22, 106–116 (2012).
Berdugo-Vega, G. et al. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun. 11, 135 (2020).
McHugh, S. B. et al. Adult-born dentate granule cells promote hippocampal population sparsity. Nat. Neurosci. 25, 1481–1491 (2022).
Aimone, J. B. et al. Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 94, 991–1026 (2014).
Scharfman, H. E., Goodman, J. H. & Sollas, A. L. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J. Neurosci. 20, 6144–6158 (2000).
Cho, K.-O. et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 6, 6606 (2015).
GoodSmith, D. et al. Flexible encoding of objects and space in single cells of the dentate gyrus. Curr. Biol. 32, 1088–1101 (2022).
Sahay, A., Wilson, D. A. & Hen, R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588 (2011).
Si, B. & Treves, A. The role of competitive learning in the generation of DG fields from EC inputs. Cogn. Neurodyn. 3, 177–187 (2009).
Wang, C. et al. Egocentric coding of external items in the lateral entorhinal cortex. Science 362, 945–949 (2018).
Morris, A. M., Weeden, C. S., Churchwell, J. C. & Kesner, R. P. The role of the dentate gyrus in the formation of contextual representations. Hippocampus 23, 162–168 (2013).
Leutgeb, J. K., Leutgeb, S., Moser, M.-B. & Moser, E. I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007).
Alme, C. B. et al. Place cells in the hippocampus: eleven maps for eleven rooms. Proc. Natl Acad. Sci. USA 111, 18428–18435 (2014).
Deng, W., Mayford, M. & Gage, F. H. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife 2, e00312 (2013).
Kim, S. H. et al. Global remapping in granule cells and mossy cells of the mouse dentate gyrus. Cell Rep. 42, 112334 (2023).
Hafting, T., Fyhn, M., Molden, S., Moser, M.-B. & Moser, E. I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005).
Keene, C. S. et al. Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. J. Neurosci. 36, 3660–3675 (2016).
Wang, C., Chen, X. & Knierim, J. J. Egocentric and allocentric representations of space in the rodent brain. Curr. Opin. Neurobiol. 60, 12–20 (2020).
Tsao, A. et al. Integrating time from experience in the lateral entorhinal cortex. Nature 561, 57–62 (2018).
Dvorak, D., Chung, A., Park, E. H. & Fenton, A. A. Dentate spikes and external control of hippocampal function. Cell Rep. 36, 109497 (2021).
Fernández-Ruiz, A. et al. Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 372, eabf3119 (2021).
Jung, M. W., Wiener, S. I. & McNaughton, B. L. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 14, 7347–7356 (1994).
Chawla, M. et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus 15, 579–586 (2005).
Lu, L., Igarashi, K. M., Witter, M. P., Moser, E. I. & Moser, M.-B. Topography of place maps along the CA3-to-CA2 axis of the hippocampus. Neuron 87, 1078–1092 (2015).
Van Strien, N., Cappaert, N. & Witter, M. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci. 10, 272–282 (2009).
Royer, S., Sirota, A., Patel, J. & Buzsáki, G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J. Neurosci. 30, 1777–1787 (2010).
Jung, M. W., Wiener, S. I. & McNaughton, B. L. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 14, 7347–7356 (1994).
Kjelstrup, K. B. et al. Finite scale of spatial representation in the hippocampus. Science 321, 140–143 (2008).
Keinath, A. T. et al. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus 24, 1533–1548 (2014).
Jinno, S. & Kosaka, T. Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus 20, 829–840 (2010).
Tanaka, K. F., Samuels, B. A. & Hen, R. Serotonin receptor expression along the dorsal–ventral axis of mouse hippocampus. Philos. Trans. R. Soc. B: Biol. Sci. 367, 2395–2401 (2012).
Christensen, T., Bisgaard, C., Nielsen, H. B. & Wiborg, O. Transcriptome differentiation along the dorso–ventral axis in laser-captured microdissected rat hippocampal granular cell layer. Neuroscience 170, 731–741 (2010).
Fanselow, M. S. & Dong, H.-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 (2010).
Witter, M. P. & Amaral, D. G. in The Rat Nervous System 635–704 (Elsevier, 2004).
Paxinos, G. The Rat Nervous System (Gulf Professional Publishing, 2004).
Amaral, D. G. & Witter, M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591 (1989).
Scharfman, H. E., Sollas, A. L., Smith, K. L., Jackson, M. B. & Goodman, J. H. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J. Comp. Neurol. 454, 424–439 (2002).
Witter, M. P. Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn. Mem. 14, 705–713 (2007).
Lee, H., Wang, C., Deshmukh, S. S. & Knierim, J. J. Neural population evidence of functional heterogeneity along the CA3 transverse axis: pattern completion versus pattern separation. Neuron 87, 1093–1105 (2015).
Scharfman, H. E. Spiny neurons of area CA3c in rat hippocampal slices have similar electrophysiological characteristics and synaptic responses despite morphological variation. Hippocampus 3, 9–28 (1993).
Claiborne, B. J., Amaral, D. G. & Cowan, W. M. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 246, 435–458 (1986).
Ishizuka, N., Weber, J. & Amaral, D. G. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J. Comp. Neurol. 295, 580–623 (1990).
Li, X., Somogyi, P., Ylinen, A. & Buzsáki, G. The hippocampal CA3 network: an in vivo intracellular labeling study. J. Comp. Neurol. 339, 181–208 (1994).
Lee, I., Yoganarasimha, D., Rao, G. & Knierim, J. J. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature 430, 456–459 (2004).
Tosches, M. A. et al. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888 (2018).
Bingman, V. P. & Muzio, R. N. Reflections on the structural–functional evolution of the hippocampus: what is the big deal about a dentate gyrus. Brain Behav. Evol. 90, 53–61 (2017).
Treves, A., Tashiro, A., Witter, M. P. & Moser, E. I. What is the mammalian dentate gyrus good for? Neuroscience 154, 1155–1172 (2008).
Amrein, I., Slomianka, L. & Lipp, H. Granule cell number, cell death and cell proliferation in the dentate gyrus of wild‐living rodents. Eur. J. Neurosci. 20, 3342–3350 (2004).
Amrein, I. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb. Perspect. Biol. 7, a021295 (2015).
Fiedler, J., De Leonibus, E. & Treves, A. Has the hippocampus really forgotten about space? Curr. Opin. Neurobiol. 71, 164–169 (2021).
Augusto-Oliveira, M., Arrifano, G. P., Malva, J. O. & Crespo-Lopez, M. E. Adult hippocampal neurogenesis in different taxonomic groups: possible functional similarities and striking controversies. Cells 8, 125 (2019).
Rodrı́guez, F. et al. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 22, 2894–2903 (2002).
Allegra, M., Posani, L., Gómez-Ocádiz, R. & Schmidt-Hieber, C. Differential relation between neuronal and behavioral discrimination during hippocampal memory encoding. Neuron 108, 1103–1112 (2020).
Stefanini, F. et al. A distributed neural code in the dentate gyrus and in CA1. Neuron 107, 703–716 (2020).
Lin, B., Chen, T. & Schild, D. Cell type‐specific relationships between spiking and [Ca2+] i in neurons of the Xenopus tadpole olfactory bulb. J. Physiol. 582, 163–175 (2007).
Kropff, E., Yang, S. M. & Schinder, A. F. Dynamic role of adult-born dentate granule cells in memory processing. Curr. Opin. Neurobiol. 35, 21–26 (2015).
Snyder, J. S. Recalibrating the relevance of adult neurogenesis. Trends Neurosci. 42, 164–178 (2019).
Danielson, N. B. et al. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron 90, 101–112 (2016).
Alvarez, D. D. et al. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science 354, 459–465 (2016).
Acknowledgements
This work was partially supported by the NSF DMS-1042134, NIH R03MH120406, NIH R01 NS039456, NRF 2021R1A4A2001803, NRF 2019R1A2C2088799, NRF 2022M3E5E8017723, the NIH Institute of Neural Computation/Biology T32 MH020002 and the Kavli Institute for Brain and Mind. The authors gratefully thank D. Nitz, L.K. Quinn, J.W. Rueckemann, T. Johnson, P. Riviere and C. Heyman for insightful discussions and editorial assistance.
Author information
Authors and Affiliations
Contributions
The authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks A. Schinder and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borzello, M., Ramirez, S., Treves, A. et al. Assessments of dentate gyrus function: discoveries and debates. Nat. Rev. Neurosci. 24, 502–517 (2023). https://doi.org/10.1038/s41583-023-00710-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00710-z
This article is cited by
-
Distinguishing examples while building concepts in hippocampal and artificial networks
Nature Communications (2024)
-
Expression of GCaMP6s in the dentate gyrus induces tonic–clonic seizures
Scientific Reports (2024)
-
Natural killer cells in the central nervous system
Cell Communication and Signaling (2023)