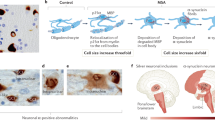
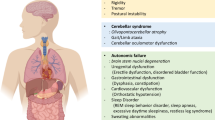
Abstract
Multiple system atrophy (MSA) is a rare oligodendroglial α-synucleinopathy characterized by neurodegeneration in striatonigral and olivopontocerebellar regions and autonomic brain centres. It causes complex cumulative motor and non-motor disability with fast progression and effective therapy is currently lacking. The difficulties in the diagnosis and treatment of MSA are largely related to the incomplete understanding of the pathogenesis of the disease. The MSA pathogenic landscape is complex, and converging findings from genetic and neuropathological studies as well as studies in experimental models of MSA have indicated the involvement of genetic and epigenetic changes; α-synuclein misfolding, aggregation and spreading; and α-synuclein strain specificity. These studies also indicate the involvement of myelin and iron dyshomeostasis, neuroinflammation, mitochondrial dysfunction and other cell-specific aspects that are relevant to the fast progression of MSA. In this Review, we discuss these findings and emphasize the implications of the complexity of the multifactorial pathogenic cascade for future translational research and its impact on biomarker discovery and treatment target definitions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fanciulli, A. & Wenning, G. K. Multiple-system atrophy. N. Engl. J. Med. 372, 249–263 (2015).
Wenning, G. K. et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov. Disord. 37, 1131–1148 (2022).
O’Sullivan, S. S. et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131, 1362–1372 (2008).
Poewe, W. et al. Multiple system atrophy. Nat. Rev. Dis. Prim. 8, 56 (2022). This article provides a contemporary clinical understanding of MSA.
Goedert, M. & Spillantini, M. G. Lewy body diseases and multiple system atrophy as alpha-synucleinopathies. Mol. Psychiatry 3, 462–465 (1998).
Högl, B. et al. Rapid eye movement sleep behaviour disorder: past, present, and future. J. Sleep. Res. 31, e13612 (2022).
Goldstein, D. S., Isonaka, R., Lamotte, G. & Kaufmann, H. Different phenoconversion pathways in pure autonomic failure with versus without Lewy bodies. Clin. Auton. Res. 31, 677–684 (2021).
Asi, Y. T. et al. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia 62, 964–970 (2014).
Watts, J. C. et al. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl Acad. Sci. USA 110, 19555–19560 (2013).
Peelaerts, W. et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015).
Djelloul, M. et al. Alpha-synuclein expression in the oligodendrocyte lineage: an in vitro and in vivo study using rodent and human models. Stem Cell Rep. 5, 174–184 (2015).
Kaji, S. et al. BCAS1-positive immature oligodendrocytes are affected by the α-synuclein-induced pathology of multiple system atrophy. Acta Neuropathol. Commun. 8, 120 (2020).
Ahmed, Z., Asi, Y. T., Lees, A. J., Revesz, T. & Holton, J. L. Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol. 23, 263–273 (2013).
Peng, C. et al. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature 557, 558–563 (2018). This study shows that α-synuclein strains of MSA are generated by the specific intracellular milieu of oligodendrocytes, but the different strains of α-synuclein show no cell-type preference in seeding of α-synuclein pathology.
Mavroeidi, P. et al. Endogenous oligodendroglial alpha-synuclein and TPPP/p25α orchestrate alpha-synuclein pathology in experimental multiple system atrophy models. Acta Neuropathol. 138, 415–441 (2019).
Herrera-Vaquero, M. et al. Signs of early cellular dysfunction in multiple system atrophy. Neuropathol. Appl. Neurobiol. 47, 268–282 (2021). This study shows that early predisposition and mitochondrial deficits dictate the cellular susceptibility to oxidative stress in MSA.
Monzio Compagnoni, G. et al. Mitochondrial dysregulation and impaired autophagy in iPSC-derived dopaminergic neurons of multiple system atrophy. Stem Cell Rep. 11, 1185–1198 (2018).
Nakamoto, F. K. et al. The pathogenesis linked to coenzyme Q10 insufficiency in iPSC-derived neurons from patients with multiple-system atrophy. Sci. Rep. 8, 14215 (2018).
Valera, E. et al. MicroRNA-101 modulates autophagy and oligodendroglial alpha-synuclein accumulation in multiple system atrophy. Front. Mol. Neurosci. 10, 329 (2017).
Song, Y. J. et al. p25α relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am. J. Pathol. 171, 1291–1303 (2007). This study shows that cellular dislocation of p25α/TPPP occurs early in MSA and contributes to abnormalities in myelin and subsequent α-synuclein aggregation in oligodendrocytes.
Ota, K. et al. Relocation of p25α/tubulin polymerization promoting protein from the nucleus to the perinuclear cytoplasm in the oligodendroglia of sporadic and COQ2 mutant multiple system atrophy. Acta Neuropathol. Commun. 2, 136 (2014).
Mavroeidi, P. et al. Autophagy mediates the clearance of oligodendroglial SNCA/alpha-synuclein and TPPP/p25A in multiple system atrophy models. Autophagy 18, 2104–2133 (2022).
Don, A. S. et al. Altered lipid levels provide evidence for myelin dysfunction in multiple system atrophy. Acta Neuropathol. Commun. 2, 150 (2014).
Bleasel, J. M., Wong, J. H., Halliday, G. M. & Kim, W. S. Lipid dysfunction and pathogenesis of multiple system atrophy. Acta Neuropathol. Commun. 2, 15 (2014).
Wenning, G. K., Stefanova, N., Jellinger, K. A., Poewe, W. & Schlossmacher, M. G. Multiple system atrophy: a primary oligodendrogliopathy. Ann. Neurol. 64, 239–246 (2008).
Nakamura, K. et al. Filamentous aggregations of phosphorylated alpha-synuclein in Schwann cells (Schwann cell cytoplasmic inclusions) in multiple system atrophy. Acta Neuropathol. Commun. 3, 29 (2015).
Donadio, V. et al. Phosphorylated α-synuclein in skin Schwann cells: a new biomarker for multiple system atrophy. Brain https://doi.org/10.1093/brain/awac124 (2022). This study provides an indication of α-synuclein pathology in Schwann cells that expands the specific vulnerability of myelinating cells in MSA and suggests a novel biomarker to differentiate MSA and Lewy body disorders.
Schweighauser, M. et al. Structures of α-synuclein filaments from multiple system atrophy. Nature https://doi.org/10.1038/s41586-020-2317-6 (2020). In this study, cryo-electron microscopy is used to define the specific structure of MSA α-synuclein filaments.
Wenning, G. K. & Jellinger, K. A. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 109, 129–140 (2005).
Wenning, G. K., Quinn, N., Magalhăes, M., Mathias, C. & Daniel, S. E. ‘Minimal change’ multiple system atrophy. Mov. Disord. 9, 161–166 (1994). This study reveals how cases of ‘minimal change’ MSA indicate that GCI pathology precedes the neuronal loss and causes neuronal dysfunction linked to atypical parkinsonism.
Wakabayashi, K. et al. An autopsy case of early (‘minimal change’) olivopontocerebellar atrophy (multiple system atrophy-cerebellar). Acta Neuropathol. 110, 185–190 (2005).
Ling, H. et al. Minimal change multiple system atrophy: an aggressive variant? Mov. Disord. 30, 960–967 (2015).
Kon, T., Mori, F., Tanji, K., Miki, Y. & Wakabayashi, K. An autopsy case of preclinical multiple system atrophy (MSA-C). Neuropathology 33, 667–672 (2013).
Koga, S. & Dickson, D. W. ‘Minimal change’ multiple system atrophy with limbic-predominant α-synuclein pathology. Acta Neuropathol. 137, 167–169 (2019).
Fujishiro, H. et al. Glial cytoplasmic inclusions in neurologically normal elderly: prodromal multiple system atrophy? Acta Neuropathol. 116, 269–275 (2008).
Stefanova, N. A mouse model of multiple system atrophy: bench to bedside. Neurotherapeutics https://doi.org/10.1007/s13311-022-01287-8 (2022).
Cykowski, M. D. et al. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138, 2293–2309 (2015).
Hass, E. W. et al. Robust α-synuclein pathology in select brainstem neuronal populations is a potential instigator of multiple system atrophy. Acta Neuropathol. Commun. 9, 80 (2021).
Araki, K. et al. The secondary structural difference between Lewy body and glial cytoplasmic inclusion in autopsy brain with synchrotron FTIR micro-spectroscopy. Sci. Rep. 10, 19423 (2020).
Yamasaki, T. R. et al. Parkinson’s disease and multiple system atrophy have distinct α-synuclein seed characteristics. J. Biol. Chem. 294, 1045–1058 (2019).
Shahnawaz, M. et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578, 273–277 (2020).
Bargar, C. et al. Discrimination of MSA-P and MSA-C by RT-QuIC analysis of olfactory mucosa: the first assessment of assay reproducibility between two specialized laboratories. Mol. Neurodegener. 16, 82 (2021).
Martinez-Valbuena, I. et al. Alpha-synuclein seeding shows a wide heterogeneity in multiple system atrophy. Transl Neurodegener. 11, 7 (2022).
Melki, R. Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J. Parkinsons Dis. 5, 217–227 (2015).
Teil, M. et al. Brain injections of glial cytoplasmic inclusions induce a multiple system atrophy-like pathology. Brain https://doi.org/10.1093/brain/awab374 (2022).
Torre-Muruzabal, T. et al. Host oligodendrogliopathy and ɑ-synuclein strains dictate disease severity in multiple system atrophy. Brain https://doi.org/10.1093/brain/awac061 (2022).
Van der Perren, A. et al. The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. https://doi.org/10.1007/s00401-020-02157-3 (2020).
Luk, K. C. et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Rey, N. L. et al. Spread of aggregates after olfactory bulb injection of α-synuclein fibrils is associated with early neuronal loss and is reduced long term. Acta Neuropathol. 135, 65–83 (2018).
Uemura, N. et al. Slow progressive accumulation of oligodendroglial alpha-synuclein (α-Syn) pathology in synthetic α-Syn fibril-induced mouse models of synucleinopathy. J. Neuropathol. Exp. Neurol. 78, 877–890 (2019).
Kaindlstorfer, C. et al. The relevance of iron in the pathogenesis of multiple system atrophy: a viewpoint. J. Alzheimers Dis. 61, 1253–1273 (2018).
Reyes, J. F. et al. Binding of α-synuclein oligomers to Cx32 facilitates protein uptake and transfer in neurons and oligodendrocytes. Acta Neuropathol. 138, 23–47 (2019).
Dutta, S. et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol. 142, 495–511 (2021).
Scheiblich, H. et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 184, 5089–5106 (2021).
Heras-Garvin, A. et al. Anle138b modulates α-synuclein oligomerization and prevents motor decline and neurodegeneration in a mouse model of multiple system atrophy. Mov. Disord. 34, 255–263 (2019).
Lemos, M. et al. Targeting α-synuclein by PD03 AFFITOPE® and Anle138b rescues neurodegenerative pathology in a model of multiple system atrophy: clinical relevance. Transl Neurodegener. 9, 38 (2020).
Heras-Garvin, A. et al. ATH434 reduces α-synuclein-related neurodegeneration in a murine model of multiple system atrophy. Mov. Disord. https://doi.org/10.1002/mds.28714 (2021).
Mandler, M. et al. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol. Neurodegener. 10, 10 (2015).
Pagano, G. et al. Trial of prasinezumab in early-stage Parkinson’s disease. N. Engl. J. Med. 387, 421–432 (2022).
Lang, A. E. et al. Trial of cinpanemab in early Parkinson’s disease. N. Engl. J. Med. 387, 408–420 (2022).
Ishizawa, K. et al. Microglial activation parallels system degeneration in multiple system atrophy. J. Neuropathol. Exp. Neurol. 63, 43–52 (2004).
Ahmed, Z. et al. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol. Appl. Neurobiol. 38, 4–24 (2012).
Fellner, L., Jellinger, K. A., Wenning, G. K. & Stefanova, N. Glial dysfunction in the pathogenesis of α-synucleinopathies: emerging concepts. Acta Neuropathol. 121, 675–693 (2011). This study shows that glial cells have a prominent role in the pathogenesis of α-synucleinopathies including neuroinflammatory signalling and disease-specific misfolding in MSA.
Salvesen, L. et al. Changes in total cell numbers of the basal ganglia in patients with multiple system atrophy — a stereological study. Neurobiol. Dis. 74, 104–113 (2015).
Salvesen, L. et al. Neocortical neuronal loss in patients with multiple system atrophy: a stereological study. Cereb. Cortex 27, 400–410 (2017).
Fellner, L. et al. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360 (2013).
Díaz, E. F. et al. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes. Glia 67, 1598–1619 (2019).
Song, Y. J. et al. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J. Neuropathol. Exp. Neurol. 68, 1073–1083 (2009).
Nykjaer, C. H., Brudek, T., Salvesen, L. & Pakkenberg, B. Changes in the cell population in brain white matter in multiple system atrophy. Mov. Disord. 32, 1074–1082 (2017).
Refolo, V. et al. Progressive striatonigral degeneration in a transgenic mouse model of multiple system atrophy: translational implications for interventional therapies. Acta Neuropathol. Commun. 6, 2 (2018). This study shows that oligodendroglial α-synuclein aggregation causes progressive nigral and striatal degeneration in a mouse model of MSA.
Stefanova, N. et al. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov. Disord. 22, 2196–2203 (2007).
Gerhard, A. et al. [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology 61, 686–689 (2003).
Kubler, D. et al. Widespread microglial activation in multiple system atrophy. Mov. Disord. 34, 564–568 (2019).
Jucaite, A. et al. Glia imaging differentiates multiple system atrophy from Parkinson’s disease: a positron emission tomography study with [(11)C]PBR28 and machine learning analysis. Mov. Disord. 37, 119–129 (2022). This study shows that signal distribution in [(11)C]PBR28 PET imaging discloses stronger glial activation in MSA compared with PD.
Stefanova, N. Microglia in Parkinson’s disease. J. Parkinsons Dis. https://doi.org/10.3233/jpd-223237 (2022).
Dansokho, C. & Heneka, M. T. Neuroinflammatory responses in Alzheimer’s disease. J. Neural Transm. 125, 771–779 (2018).
Pike, A. F. et al. Synuclein evokes NLRP3 inflammasome-mediated IL-1β secretion from primary human microglia. Glia 69, 1413–1428 (2021).
Trudler, D. et al. Soluble α-synuclein-antibody complexes activate the NLRP3 inflammasome in hiPSC-derived microglia. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2025847118 (2021).
Stefanova, N., Georgievska, B., Eriksson, H., Poewe, W. & Wenning, G. K. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox. Res. 21, 393–404 (2012).
Guo, M. et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 143, 1476–1497 (2020).
Stefanova, N. et al. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 179, 954–963 (2011).
Shadrin, A. A. et al. Shared genetics of multiple system atrophy and inflammatory bowel disease. Mov. Disord. 36, 449–459 (2021). This study shows that overlapping genetic backgrounds between MSA and inflammatory bowel disease implicate immune stress and gut dysfunction in the aetiology of MSA.
Williams, G. P. et al. T cell infiltration in both human multiple system atrophy and a novel mouse model of the disease. Acta Neuropathol. 139, 855–874 (2020).
Kaufmann, M. et al. Identification of early neurodegenerative pathways in progressive multiple sclerosis. Nat. Neurosci. 25, 944–955 (2022).
Ubhi, K. et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J. Neurosci. 30, 6236–6246 (2010).
Levin, J. et al. Generation of ferric iron links oxidative stress to α-synuclein oligomer formation. J. Parkinsons Dis. 1, 205–216 (2011).
Dexter, D. T., Jenner, P., Schapira, A. H. & Marsden, C. D. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann. Neurol. 32, S94–S100 (1992).
Tanji, K. et al. Alteration of autophagosomal proteins in the brain of multiple system atrophy. Neurobiol. Dis. 49, 190–198 (2013).
Sailer, A. et al. A genome-wide association study in multiple system atrophy. Neurology 87, 1591–1598 (2016).
Federoff, M. et al. Genome-wide estimate of the heritability of multiple system atrophy. Parkinsonism Relat. Disord. 22, 35–41 (2016).
Funayama, M., Nishioka, K., Li, Y. & Hattori, N. Molecular genetics of Parkinson’s disease: contributions and global trends. J. Hum. Genet. https://doi.org/10.1038/s10038-022-01058-5 (2022).
Ozawa, T. et al. No mutation in the entire coding region of the alpha-synuclein gene in pathologically confirmed cases of multiple system atrophy. Neurosci. Lett. 270, 110–112 (1999).
Mokretar, K. et al. Somatic copy number gains of α-synuclein (SNCA) in Parkinson’s disease and multiple system atrophy brains. Brain 141, 2419–2431 (2018). In this study, somatic α-synuclein copy-number gains in substantia nigra in MSA and PD are found, which suggest that mosaicism may be a risk factor in sporadic α-synucleinopathies.
Perez-Rodriguez, D. et al. Investigation of somatic CNVs in brains of synucleinopathy cases using targeted SNCA analysis and single cell sequencing. Acta Neuropathol. Commun. 7, 219 (2019).
Yang, Y. et al. New SNCA mutation and structures of α-synuclein filaments from juvenile-onset synucleinopathy. Preprint at bioRxiv https://doi.org/10.1101/2022.11.23.517690 (2022).
Srulijes, K. et al. No association of GBA mutations and multiple system atrophy. Eur. J. Neurol. 20, e61–e62 (2013).
Segarane, B. et al. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology 72, 1185–1186 (2009).
Wernick, A. I. et al. GBA variation and susceptibility to multiple system atrophy. Parkinsonism Relat. Disord. 77, 64–69 (2020).
Brettschneider, J. et al. Converging patterns of α-synuclein pathology in multiple system atrophy. J. Neuropathol. Exp. Neurol. 77, 1005–1016 (2018).
Hernandez, D. et al. The dardarin G 2019 S mutation is a common cause of Parkinson’s disease but not other neurodegenerative diseases. Neurosci. Lett. 389, 137–139 (2005).
Ozelius, L. J. et al. G2019S mutation in the leucine-rich repeat kinase 2 gene is not associated with multiple system atrophy. Mov. Disord. 22, 546–549 (2007).
Riboldi, G. M. et al. Early-onset pathologically proven multiple system atrophy with LRRK2 G2019S mutation. Mov. Disord. 34, 1080–1082 (2019).
Ando, S. et al. A patient clinically diagnosed as multiple system atrophy harboring LRRK2 p.G2019S. Clin. Parkinsonism Relat. Disord. 1, 100–101 (2019).
Heckman, M. G. et al. LRRK2 exonic variants and risk of multiple system atrophy. Neurology 83, 2256–2261 (2014).
Soma, H. et al. Associations between multiple system atrophy and polymorphisms of SLC1A4, SQSTM1, and EIF4EBP1 genes. Mov. Disord. 23, 1161–1167 (2008).
Combarros, O., Infante, J., Llorca, J. & Berciano, J. Interleukin-1A (-889) genetic polymorphism increases the risk of multiple system atrophy. Mov. Disord. 18, 1385–1386 (2003).
Infante, J., Llorca, J., Berciano, J. & Combarros, O. Interleukin-8, intercellular adhesion molecule-1 and tumour necrosis factor-alpha gene polymorphisms and the risk for multiple system atrophy. J. Neurol. Sci. 228, 11–13 (2005).
Nishimura, M. et al. Contribution of the interleukin-1β gene polymorphism in multiple system atrophy. Mov. Disord. 17, 808–811 (2002).
Nishimura, M., Kuno, S., Kaji, R. & Kawakami, H. Influence of a tumor necrosis factor gene polymorphism in Japanese patients with multiple system atrophy. Neurosci. Lett. 374, 218–221 (2005).
Collaboration., T. M.-S. A. R. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N. Engl. J. Med. 369, 233–244 (2013). This study shows that COQ2 mutations are linked to MSA in the Japanese population.
Hopfner, F. et al. Common variants near ZIC1 and ZIC4 in autopsy-confirmed multiple system atrophy. Mov. Disord. 37, 2110–2121 (2022).
Gilman, S. et al. The North American Multiple System Atrophy Study Group. J. Neural Transm. 112, 1687–1694 (2005).
Wenning, G. K. et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 12, 264–274 (2013).
Low, P. A. et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 14, 710–719 (2015).
Matsushima, M. et al. Multiple system atrophy in Hokkaido, Japan: a prospective registry study of natural history and symptom assessment scales followed for 5 years. BMJ Open 11, e045100 (2021).
Watanabe, H. et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 125, 1070–1083 (2002).
Ozawa, T. et al. The phenotype spectrum of Japanese multiple system atrophy. J. Neurol. Neurosurg. Psychiatry 81, 1253–1255 (2010).
Gu, X. et al. Analysis of GWAS-linked variants in multiple system atrophy. Neurobiol. Aging 67, 201.e1–201.e4 (2018).
Ogaki, K. et al. Analysis of COQ2 gene in multiple system atrophy. Mol. Neurodegener. 9, 44 (2014).
Schottlaender, L. V. & Houlden, H. Mutant COQ2 in multiple-system atrophy. N. Engl. J. Med. 371, 81 (2014).
Sharma, M., Wenning, G. & Kruger, R. Mutant COQ2 in multiple-system atrophy. N. Engl. J. Med. 371, 80–81 (2014).
Chen, Y. P. et al. Mutation scanning of the COQ2 gene in ethnic Chinese patients with multiple-system atrophy. Neurobiol. Aging 36, 1222.e7–1222.e11 (2015).
Wullner, U. et al. Probable multiple system atrophy in a German family. J. Neurol. Neurosurg. Psychiatry 75, 924–925 (2004).
Hara, K. et al. Multiplex families with multiple system atrophy. Arch. Neurol. 64, 545–551 (2007).
Leys, F. et al. Family history for neurodegeneration in multiple system atrophy: does it indicate susceptibility? Mov. Disord. https://doi.org/10.1002/mds.29202 (2022).
Gwinn-Hardy, K. et al. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol. 99, 663–672 (2000).
Fanciulli, A. et al. A multiplex pedigree with pathologically confirmed multiple system atrophy and Parkinson’s disease with dementia. Brain Commun. 4, fcac175 (2022).
Singleton, A. B. et al. α-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841 (2003).
Ross, O. A. et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann. Neurol. 63, 743–750 (2008).
Kiely, A. P. et al. α-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta Neuropathol. 125, 753–769 (2013).
King, A., Lee, Y. K., Jones, S. & Troakes, C. A pathologically confirmed case of combined amyotrophic lateral sclerosis with C9orf72 mutation and multiple system atrophy. Neuropathology 42, 302–308 (2022).
Goldman, J. S. et al. Multiple system atrophy and amyotrophic lateral sclerosis in a family with hexanucleotide repeat expansions in C9orf72. JAMA Neurol. 71, 771–774 (2014).
Schottlaender, L. V. et al. Analysis of C9orf72 repeat expansions in a large series of clinically and pathologically diagnosed cases with atypical parkinsonism. Neurobiol. Aging 36, 1221.e1–1226.e6 (2015).
Scholz, S. W. et al. Multiple system atrophy is not caused by C9orf72 hexanucleotide repeat expansions. Neurobiol. Aging 36, 1223.e1–1223.e2 (2015).
Gilman, S. et al. Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann. Neurol. 39, 241–255 (1996).
Nirenberg, M. J., Libien, J., Vonsattel, J. P. & Fahn, S. Multiple system atrophy in a patient with the spinocerebellar ataxia 3 gene mutation. Mov. Disord. 22, 251–254 (2007).
Wan, L. et al. Biallelic intronic AAGGG expansion of RFC1 is related to multiple system atrophy. Ann. Neurol. 88, 1132–1143 (2020).
Record, C. J. et al. Severe distinct dysautonomia in RFC1-related disease associated with Parkinsonism. J. Peripher. Nerv. Syst. 27, 311–315 (2022).
Goutman, S. A. et al. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 21, 465–479 (2022).
Bettencourt, C. et al. White matter DNA methylation profiling reveals deregulation of HIP1, LMAN2, MOBP, and other loci in multiple system atrophy. Acta Neuropathol. 139, 135–156 (2020).
Koga, S., Sekiya, H., Kondru, N., Ross, O. A. & Dickson, D. W. Neuropathology and molecular diagnosis of synucleinopathies. Mol. Neurodegener. 16, 83 (2021).
Kontopoulos, E., Parvin, J. D. & Feany, M. B. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 15, 3012–3023 (2006).
Sturm, E. et al. Neuroprotection by epigenetic modulation in a transgenic model of multiple system atrophy. Neurotherapeutics 13, 871–879 (2016).
Schafferer, S. et al. Changes in the miRNA–mRNA regulatory network precede motor symptoms in a mouse model of multiple system atrophy: clinical implications. PLoS ONE 11, e0150705 (2016).
Kim, T., Valera, E. & Desplats, P. Alterations in striatal microRNA–mRNA networks contribute to neuroinflammation in multiple system atrophy. Mol. Neurobiol. 56, 7003–7021 (2019).
Vanacore, N. et al. Case–control study of multiple system atrophy. Mov. Disord. 20, 158–163 (2005).
Ozawa, T. et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain 127, 2657–2671 (2004).
Campese, N. et al. Neuropathology of multiple system atrophy: Kurt Jellinger’s legacy. J. Neural Transm. 128, 1481–1494 (2021).
Uchihara, T. et al. Silver stainings distinguish Lewy bodies and glial cytoplasmic inclusions: comparison between Gallyas–Braak and Campbell–Switzer methods. Acta Neuropathol. 110, 255–260 (2005).
Koga, S. et al. Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov. Disord. 32, 405–413 (2017).
Wakabayashi, K., Miki, Y., Tanji, K. & Mori, F. Neuropathology of multiple system atrophy, a glioneuronal degenerative disease. Cerebellum https://doi.org/10.1007/s12311-022-01407-2 (2022).
Doppler, K. et al. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov. Disord. 30, 1688–1692 (2015).
Haga, R. et al. Clinical utility of skin biopsy in differentiating between Parkinson’s disease and multiple system atrophy. Parkinsons Dis. 2015, 167038 (2015).
Overk, C. et al. Multiple system atrophy: experimental models and reality. Acta Neuropathol. 135, 33–47 (2018).
Stefanova, N., Tison, F., Reindl, M., Poewe, W. & Wenning, G. K. Animal models of multiple system atrophy. Trends Neurosci. 28, 501–506 (2005).
Kahle, P. J. et al. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 3, 583–588 (2002).
Shults, C. W. et al. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J. Neurosci. 25, 10689–10699 (2005).
Yazawa, I. et al. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron 45, 847–859 (2005).
Tanji, K. et al. A mouse model of adult-onset multiple system atrophy. Neurobiol. Dis. 127, 339–349 (2019).
Marmion, D. J. et al. Viral-based rodent and nonhuman primate models of multiple system atrophy: fidelity to the human disease. Neurobiol. Dis. 148, 105184 (2021).
Stefanova, N., Kaufmann, W. A., Humpel, C., Poewe, W. & Wenning, G. K. Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol. 124, 51–65 (2012).
Woerman, A. L. et al. Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines. Acta Neuropathol. 137, 437–454 (2019).
Ding, X. et al. Propagation of pathological α-synuclein from the urogenital tract to the brain initiates MSA-like syndrome. iScience 23, 101166 (2020).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.S. reports consultancy fees from Inhibikase Therapeutics and Astellas Pharma and research grants from the Austrian Science Fund (FWF), Alterity Therapeutics and the US MSA Coalition. G.K.W. reports consultancy and lecture fees from Biohaven, Inhibikase, Ono and Takeda and research grants from the Austrian Science Fund (FWF), the Austrian National Bank, the US MSA Coalition, Parkinson Funds Austria and International Parkinson and Movement Disorder Society outside the submitted work.
Peer review
Peer review information
Nature Reviews Neuroscience thanks C. Proukakis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stefanova, N., Wenning, G.K. Multiple system atrophy: at the crossroads of cellular, molecular and genetic mechanisms. Nat Rev Neurosci 24, 334–346 (2023). https://doi.org/10.1038/s41583-023-00697-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00697-7
This article is cited by
-
Multiple system atrophy: an update and emerging directions of biomarkers and clinical trials
Journal of Neurology (2024)
-
Neuronal SNCA transcription during Lewy body formation
Acta Neuropathologica Communications (2023)
-
Mild cognitive impairment in multiple system atrophy: a brain network disorder
Journal of Neural Transmission (2023)
-
Vagus nerve inflammation contributes to dysautonomia in COVID-19
Acta Neuropathologica (2023)