Abstract
People living with HIV are affected by the chronic consequences of neurocognitive impairment (NCI) despite antiretroviral therapies that suppress viral replication, improve health and extend life. Furthermore, viral suppression does not eliminate the virus, and remaining infected cells may continue to produce viral proteins that trigger neurodegeneration. Comorbidities such as diabetes mellitus are likely to contribute substantially to CNS injury in people living with HIV, and some components of antiretroviral therapy exert undesirable side effects on the nervous system. No treatment for HIV-associated NCI has been approved by the European Medicines Agency or the US Food and Drug Administration. Historically, roadblocks to developing effective treatments have included a limited understanding of the pathophysiology of HIV-associated NCI and heterogeneity in its clinical manifestations. This heterogeneity might reflect multiple underlying causes that differ among individuals, rather than a single unifying neuropathogenesis. Despite these complexities, accelerating discoveries in HIV neuropathogenesis are yielding potentially druggable targets, including excessive immune activation, metabolic alterations culminating in mitochondrial dysfunction, dysregulation of metal ion homeostasis and lysosomal function, and microbiome alterations. In addition to drug treatments, we also highlight the importance of non-pharmacological interventions. By revisiting mechanisms implicated in NCI and potential interventions addressing these mechanisms, we hope to supply reasons for optimism in people living with HIV affected by NCI and their care providers.
Key points
-
The pathogenesis of neurocognitive impairment (NCI) in people living with HIV is complex, and no disease-modifying therapies are currently available.
-
Neuroinflammatory and metabolic changes are hallmarks of HIV-associated NCI and are potential therapeutic targets.
-
The neuropathogenesis of HIV-associated NCI in diverse ethnic and racial groups warrants further exploration.
-
Examples of promising targets for the treatment of NCI in people living with HIV include human growth hormone-releasing hormone (hGHRH) and the enzyme phosphatidylinositol-glycan-specific phospholipase D (also known as GPLD1).
-
Non-pharmacological interventions that might enhance cognitive function in people living with HIV include increased physical activity, improved quality of sleep and nutritional treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
23 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41582-023-00895-y
References
Heaton, R. K. et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17, 3–16 (2011).
HIV.gov. Global HIV/AIDS Overview https://www.who.int/data/gho/data/themes/hiv-aids (2023).
Ellis, R. J. et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin. Infect. Dis. 58, 1015–1022 (2014).
Wright, E. J. Neurological disease: the effects of HIV and antiretroviral therapy and the implications for early antiretroviral therapy initiation. Curr. Opin. HIV AIDS 4, 447–452 (2009).
Vecchio, A. et al. Neurocognitive effects of antiretroviral initiation among people living with HIV in rural Uganda. J. Acquir. Immune Defic. Syndr. 84, 534–542 (2020).
Gao, C. et al. Antiretroviral therapy improves neurocognitive impairment in people living with HIV? A meta-analysis. Int. J. Nurs. Sci. 7, 238–247 (2020).
Coban, H. et al. Impact of aging on neurocognitive performance in previously antiretroviral-naive HIV-infected individuals on their first suppressive regimen. AIDS 31, 1565–1571 (2017).
Simioni, S. et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24, 1243–1250 (2010).
Brew, B. J. et al. Factors in AIDS dementia complex trial design: results and lessons from the abacavir trial. PLoS Clin. Trials 2, e13 (2007).
Antinori, A. et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799 (2007).
Nightingale, S. et al. Moving on From HAND: why we need new criteria for cognitive impairment in persons living with human immunodeficiency virus and a proposed way forward. Clin. Infect. Dis. 73, 1113–1118 (2021).
Nightingale, S. et al. A new approach to cognitive impairment in people with HIV. Lancet HIV 9, e815–e817 (2022).
Nightingale, S. et al. Cognitive impairment in people living with HIV: consensus recommendations for a new approach. Nat. Rev. Neurol. 19, 424–433 (2023).
Alakkas, A. et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J. Neurovirol. 25, 32–41 (2019).
Eden, A. et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 11, e0157160 (2016).
Ellis, R. J. et al. Higher cerebrospinal fluid biomarkers of neuronal injury in HIV-associated neurocognitive impairment. J. Neurovirol. 28, 438–445 (2022).
Heaton, R. K. et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096 (2010).
Chetty, L., Cobbing, S. & Chetty, V. Physical activity and exercise for older people living with HIV: a protocol for a scoping review. Syst. Rev. 9, 60 (2020).
Saloner, R. & Cysique, L. A. HIV-associated neurocognitive disorders: a global perspective. J. Int. Neuropsychol. Soc. 23, 860–869 (2017).
Anuradha, S. et al. Factors influencing adherence to ART: new insights from a center providing free ART under the national program in Delhi, India. J. Int. Assoc. Provid. AIDS Care 12, 195–201 (2013).
Benedict, R. H., Mezhir, J. J., Walsh, K. & Hewitt, R. G. Impact of human immunodeficiency virus type-1-associated cognitive dysfunction on activities of daily living and quality of life. Arch. Clin. Neuropsychol. 15, 535–544 (2000).
Jones, J. D. et al. Changes in cognition precede changes in HRQoL among HIV+ males: longitudinal analysis of the multicenter AIDS cohort study. Neuropsychology 33, 370–378 (2019).
Pinheiro, C. A. et al. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz. J. Med. Biol. Res. 49, e5344 (2016).
Laverick, R. et al. Self-reported decline in everyday function, cognitive symptoms, and cognitive function in people with HIV. J. Acquir. Immune Defic. Syndr. 76, e74–e83 (2017).
Sevigny, J. J. et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch. Neurol. 64, 97–102 (2007).
Valcour, V. G., Shikuma, C. M., Watters, M. R. & Sacktor, N. C. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS 18, S79–86 (2004).
Wendelken, L. A. & Valcour, V. Impact of HIV and aging on neuropsychological function. J. Neurovirol. 18, 256–263 (2012).
Becker, J. T., Lopez, O. L., Dew, M. A. & Aizenstein, H. J. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 18 (Suppl. 1), S11–18 (2004).
Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372, 293–299 (2008).
Ellis, R. J. et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 25, 1747–1751 (2011).
Munoz-Moreno, J. A. et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res. Hum. Retroviruses 24, 1301–1307 (2008).
Grant, I. et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 82, 2055–2062 (2014).
Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2015–2019 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (2021).
Manly, J. J. et al. The effect of African-American acculturation on neuropsychological test performance in normal and HIV-positive individuals. The HIV Neurobehavioral Research Center (HNRC) Group. J. Int. Neuropsychol. Soc. 4, 291–302 (1998).
Vo, Q. T. et al. Neuropsychological test performance before and after HIV-1 seroconversion: the Multicenter AIDS Cohort Study. J. Neurovirol. 19, 24–31 (2013).
Winston, A. et al. Neurocognitive function in HIV Infected patients on antiretroviral therapy. PLoS One 8, e61949 (2013).
Watson, C. W.-M. et al. Ethnic/racial disparities in longitudinal neurocognitive decline in people with HIV. J. Acquir. Immune Defic. Syndr. 90, 97–105 (2022).
Tan, Y. W., Burgess, G. H. & Green, R. J. The effects of acculturation on neuropsychological test performance: a systematic literature review. Clin. Neuropsychol. 35, 541–571 (2021).
Wojna, V. et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J. Neurovirol. 12, 356–364 (2006).
Kamalyan, L. et al. Neurocognitive impairment in Spanish-speaking Latinos living with HIV in the US: application of the neuropsychological norms for the US-Mexico border region in Spanish (NP-NUMBRS). Clin. Neuropsychol. 35, 433–452 (2021).
Ruhanya, V. et al. HIV-1 subtype C Vpr amino acid residue 45y and specific conserved fragments are associated with neurocognitive impairment and markers of viral load. AIDS Res. Hum. Retroviruses 39, 166–175 (2023).
Aderinto, N. HIV-associated neurocognitive disorders in Africa: an emerging challenge: a correspondence. IJS Glob. Health 6, e0146 (2023).
Sacktor, N., Nakasujja, N., Robertson, K. & Clifford, D. B. HIV-associated cognitive impairment in sub-Saharan Africa–the potential effect of clade diversity. Nat. Clin. Pract. Neurol. 3, 436–443 (2007).
Gray, L. R. et al. CNS-specific regulatory elements in brain-derived HIV-1 strains affect responses to latency-reversing agents with implications for cure strategies. Mol. Psychiatry 21, 574–584 (2016).
Nath, A. Eradication of human immunodeficiency virus from brain reservoirs. J. Neurovirol. 21, 227–234 (2015).
Ellis, R. & Letendre, S. L. Update and new directions in therapeutics for neurological complications of HIV infections. Neurotherapeutics 13, 471–476 (2016).
Desplats, P. et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 80, 1415–1423 (2013).
Perez-Valero, I. et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS 33, 475–481 (2019).
Mukerji, S. S. et al. Temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J. Acquir. Immune Defic. Syndr. 75, 246–255 (2017).
Trunfio, M. et al. Cerebrospinal fluid HIV-1 escape according to different thresholds and underlying comorbidities: is it time to assess the definitions. AIDS 33, 759–762 (2019).
Manesh, A. et al. Symptomatic HIV CNS viral escape among patients on effective cART. Int. J. Infect. Dis. 84, 39–43 (2019).
Cochrane, C. R. et al. Intact HIV proviruses persist in the brain despite viral suppression with ART. Ann. Neurol. 92, 532–544 (2022).
Oliveira, M. F. et al. Evaluation of archival HIV DNA in brain and lymphoid tissues. J. Virol. 97, e0054323 (2023).
Sanna, P. P., Repunte-Canonigo, V., Masliah, E. & Lefebvre, C. Gene expression patterns associated with neurological disease in human HIV infection. PLoS One 12, e0175316 (2017).
Farhadian, S. F. et al. Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV. JCI Insight 3, e121718 (2018).
Suzuki, K. et al. Elevation of cell-associated HIV-1 transcripts in CSF CD4+ T cells, despite effective antiretroviral therapy, is linked to brain injury. Proc. Natl Acad. Sci. USA 119, e2210584119 (2022).
Spudich, S. et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J. Clin. Invest. 129, 3339–3346 (2019).
Lian, X. et al. Signatures of immune selection in intact and defective proviruses distinguish HIV-1 elite controllers. Sci. Transl. Med. 13, eabl4097 (2021).
Einkauf, K. B. et al. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell 185, 266–282.e15 (2022).
Pollack, R. A. et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 21, 494–506.e4 (2017).
Lenassi, M. et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11, 110–122 (2010).
Arakelyan, A., Fitzgerald, W., Zicari, S., Vanpouille, C. & Margolis, L. Extracellular vesicles carry HIV Env and facilitate Hiv infection of human lymphoid tissue. Sci. Rep. 7, 1695 (2017).
Chandra, P. K. et al. Latent HIV-exosomes induce mitochondrial hyperfusion due to loss of phosphorylated dynamin-related protein 1 in brain endothelium. Mol. Neurobiol. 58, 2974–2989 (2021).
Pulliam, L., Sun, B., Mustapic, M., Chawla, S. & Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 25, 702–709 (2019).
Giannessi, F., Aiello, A., Franchi, F., Percario, Z. A. & Affabris, E. The role of extracellular vesicles as allies of HIV, HCV and SARS viruses. Viruses 12, 571 (2020).
Mahajan, S. D., Ordain, N. S., Kutscher, H., Karki, S. & Reynolds, J. L. HIV neuroinflammation: the role of exosomes in cell signaling, prognostic and diagnostic biomarkers and drug delivery. Front. Cell Dev. Biol. 9, 637192 (2021).
Ojha, C. R. et al. Interplay between autophagy, exosomes and HIV-1 associated neurological disorders: new insights for diagnosis and therapeutic applications. Viruses 9, 176 (2017).
Shrivastava, S. et al. Exosome-mediated stable epigenetic repression of HIV-1. Nat. Commun. 12, 5541 (2021).
Narayanan, A. et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem. 288, 20014–20033 (2013).
Sampey, G. C. et al. Exosomes from HIV-1-infected cells stimulate production of pro-inflammatory cytokines through Trans-activating response (TAR) RNA. J. Biol. Chem. 291, 1251–1266 (2016).
Barclay, R. A. et al. Exosomes from uninfected cells activate transcription of latent HIV-1. J. Biol. Chem. 292, 14764 (2017).
Tyagi, M., Bukrinsky, M. & Simon, G. L. Mechanisms of HIV transcriptional regulation by drugs of abuse. Curr. HIV Res. 14, 442–454 (2016).
Saloner, R. et al. Benzodiazepine use is associated with an increased risk of neurocognitive impairment in people living with HIV. J. Acquir. Immune Defic. Syndr. 82, 475–482 (2019).
Sundermann, E. E. et al. The association between benzodiazepine use and greater risk of neurocognitive impairment is moderated by medical burden in people with HIV. J. Neurovirol. 28, 410–421 (2022).
Lin, A. et al. Alprazolam prompts HIV-1 transcriptional reactivation and enhances CTL response through RUNX1 inhibition and STAT5 activation. Front. Neurol. 12, 663793 (2021).
Qu, Y. et al. Legacy effect on neuropsychological function in HIV-infected men on combination antiretroviral therapy. AIDS 36, 19–27 (2022).
Rawlings, S. A., Chaillon, A., Smith, D. & Gianella, S. Scale up rapid research autopsies for tissue immunology. Nature 595, 352 (2021).
Marquine, M. J. et al. The impact of ethnicity/race on the association between the Veterans Aging Cohort Study (VACS) index and neurocognitive function among HIV-infected persons. J. Neurovirol. 22, 442–454 (2016).
Fields, J. A. et al. Alterations in brain TREM2 and amyloid-β levels are associated with neurocognitive impairment in HIV-infected persons on antiretroviral therapy. J. Neurochem. 147, 784–802 (2018).
Hulgan, T. et al. Mitochondrial DNA haplogroups and neurocognitive impairment during HIV infection. Clin. Infect. Dis. 61, 1476–1484 (2015).
Moore, D. J. et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20, 879–887 (2006).
Levine, A. J. et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J. Neurovirol. 22, 431–441 (2016).
Masliah, E. et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 42, 963–972 (1997).
Jernigan, T. L. et al. Clinical factors related to brain structure in HIV: the CHARTER study. J. Neurovirol. 17, 248–257 (2011).
Gaetani, L. et al. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 90, 870–881 (2019).
Tortelli, R. et al. Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur. J. Neurol. 22, 215–218 (2015).
Tortelli, R. et al. Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur. J. Neurol. 19, 1561–1567 (2012).
Petzold, A. et al. CSF neurofilament levels: a potential prognostic marker in Guillain-Barre syndrome. Neurology 67, 1071–1073 (2006).
Petzold, A. et al. CSF protein biomarkers for proximal axonal damage improve prognostic accuracy in the acute phase of Guillain-Barre syndrome. Muscle Nerve 40, 42–49 (2009).
Mariotto, S. et al. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J. Peripher. Nerv. Syst. 23, 174–177 (2018).
Peterson, J. et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 9, e116081 (2014).
Gardner, M. B. & Luciw, P. A. Animal models of AIDS. FASEB J. 3, 2593–2606 (1989).
Reid, W. et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl Acad. Sci. USA 98, 9271–9276 (2001).
Keppler, O. T. et al. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195, 719–736 (2002).
Klotman, P. E. & Notkins, A. L. Transgenic models of human immunodeficiency virus type-1. Curr. Top. Microbiol. Immunol. 206, 197–222 (1996).
Toggas, S. M. & Mucke, L. Transgenic models in the study of AIDS dementia complex. Curr. Top. Microbiol. Immunol. 206, 223–241 (1996).
Van Duyne, R. et al. The utilization of humanized mouse models for the study of human retroviral infections. Retrovirology 6, 76 (2009).
Thaney, V. E. et al. Transgenic mice expressing HIV-1 envelope protein gp120 in the brain as an animal model in neuroAIDS research. J. Neurovirol. 24, 156–167 (2018).
Ambrose, Z., KewalRamani, V. N., Bieniasz, P. D. & Hatziioannou, T. HIV/AIDS: in search of an animal model. Trends Biotechnol. 25, 333–337 (2007).
Clements, J. E., Anderson, M. G., Zink, M. C., Joag, S. V. & Narayan, O. The SIV model of AIDS encephalopathy. Role of neurotropic viruses in diseases. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 72, 147–157 (1994).
Olmsted, R. A. et al. Molecular cloning of feline immunodeficiency virus. Proc. Natl Acad. Sci. USA 86, 2448–2452 (1989).
Meeker, R. B., Thiede, B. A., Hall, C., English, R. & Tompkins, M. Cortical cell loss in asymptomatic cats experimentally infected with feline immunodeficiency virus. AIDS Res. Hum. Retroviruses 13, 1131–1140 (1997).
Jacobson, S. et al. Cortical neuronal cytoskeletal changes associated with FIV infection. J. Neurovirol. 3, 283–289 (1997).
Clements, J. E., Mankowski, J. L., Gama, L. & Zink, M. C. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J. Neurovirol. 14, 309–317 (2008).
Williams, R. et al. Nonhuman primate models of NeuroAIDS. J. Neurovirol. 14, 292–300 (2008).
Potash, M. J. et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl Acad. Sci. USA 102, 3760–3765 (2005).
Kim, B. H. et al. CCL2 is required for initiation but not persistence of HIV infection mediated neurocognitive disease in mice. Sci. Rep. 13, 6577 (2023).
Tyor, W. R., Power, C., Gendelman, H. E. & Markham, R. B. A model of human immunodeficiency virus encephalitis in scid mice. Proc. Natl Acad. Sci. USA 90, 8658–8662 (1993).
Persidsky, Y. et al. Human immunodeficiency virus encephalitis in SCID mice. Am. J. Pathol. 149, 1027–1053 (1996).
Poluektova, L. Y., Munn, D. H., Persidsky, Y. & Gendelman, H. E. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J. Immunol. 168, 3941–3949 (2002).
Zhang, J. et al. Human microglia extensively reconstitute in humanized-BLT mice with human interleukin-34 transgene and support HIV-1 brain infection. Front. Immunol. 12, 672415 (2021).
Dash, P. K. et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J. Neurosci. 31, 3148–3157 (2011).
Leonard, J. M. et al. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 242, 1665–1670 (1988).
Iwakura, Y. et al. The induction of cataracts by HIV-1 in transgenic mice. AIDS 6, 1069–1075 (1992).
Hanna, Z. et al. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72, 121–132 (1998).
Hanna, Z. et al. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95, 163–175 (1998).
Thomas, F. P., Chalk, C., Lalonde, R., Robitaille, Y. & Jolicoeur, P. Expression of human immunodeficiency virus type 1 in the nervous system of transgenic mice leads to neurological disease. J. Virol. 68, 7099–7107 (1994).
Toggas, S. M. et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367, 188–193 (1994).
Berrada, F. et al. Neuronal expression of human immunodeficiency virus type 1 env proteins in transgenic mice: distribution in the central nervous system and pathological alterations. J. Virol. 69, 6770–6778 (1995).
Toneatto, S., Finco, O., van der Putten, H., Abrignani, S. & Annunziata, P. Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS 13, 2343–2348 (1999).
Kim, B. O. et al. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 162, 1693–1707 (2003).
Bruce-Keller, A. J. et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia 56, 1414–1427 (2008).
Jones, G. J. et al. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J. Neurosci. 27, 3703–3711 (2007).
D’Hooge, R., Franck, F., Mucke, L. & De Deyn, P. P. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur. J. Neurosci. 11, 4398–4402 (1999).
Maung, R. et al. CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J. Immunol. 193, 1895–1910 (2014).
Imamichi, H. et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl Acad. Sci. USA 117, 3704–3710 (2020).
Ferdin, J. et al. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PLoS One 13, e0191613 (2018).
Bachani, M., Sacktor, N., McArthur, J. C., Nath, A. & Rumbaugh, J. Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders. J. Neurovirol. 19, 82–88 (2013).
Dinkins, C., Arko-Mensah, J. & Deretic, V. Autophagy and HIV. Semin. Cell Dev. Biol. 21, 712–718 (2010).
Nath, A., Conant, K., Chen, P., Scott, C. & Major, E. O. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J. Biol. Chem. 274, 17098–17102 (1999).
Nath, A., Padua, R. A. & Geiger, J. D. HIV-1 coat protein gp120-induced increases in levels of intrasynaptosomal calcium. Brain Res. 678, 200–206 (1995).
Piller, S. C., Jans, P., Gage, P. W. & Jans, D. A. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc. Natl Acad. Sci. USA 95, 4595–4600 (1998).
Rozzi, S. J., Avdoshina, V., Fields, J. A. & Mocchetti, I. Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov. 4, 8 (2018).
Sawaya, B. E., Khalili, K., Mercer, W. E., Denisova, L. & Amini, S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J. Biol. Chem. 273, 20052–20057 (1998).
Teodorof-Diedrich, C. & Spector, S. A. Human immunodeficiency virus type 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J. Virol. 92, e00993 (2018).
Thangaraj, A. et al. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy 14, 1596–1619 (2018).
Villeneuve, L. M. et al. HIV-1 transgenic rats display mitochondrial abnormalities consistent with abnormal energy generation and distribution. J. Neurovirol. 22, 564–574 (2016).
Gendelman, H. E., Lipton, S. A., Tardieu, M., Bukrinsky, M. I. & Nottet, H. S. The neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 56, 389–398 (1994).
Kato, T., Hirano, A., Llena, J. F. & Dembitzer, H. M. Neuropathology of acquired immune deficiency syndrome (AIDS) in 53 autopsy cases with particular emphasis on microglial nodules and multinucleated giant cells. Acta Neuropathol. 73, 287–294 (1987).
Michaels, J., Price, R. W. & Rosenblum, M. K. Microglia in the giant cell encephalitis of acquired immune deficiency syndrome: proliferation, infection and fusion. Acta Neuropathol. 76, 373–379 (1988).
Iskander, S., Walsh, K. A. & Hammond, R. R. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J. Neuroinflammation 1, 7 (2004).
Bryant, A. K. et al. Antiretroviral therapy reduces neurodegeneration in HIV infection. AIDS 29, 323–330 (2015).
Gonzalez-Scarano, F. & Martin-Garcia, J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 5, 69–81 (2005).
Thompson, K. A., Cherry, C. L., Bell, J. E. & McLean, C. A. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am. J. Pathol. 179, 1623–1629 (2011).
Churchill, M. J. et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neurovirol. 12, 146–152 (2006).
Ryan, S. K. et al. Neuroinflammation and EIF2 signaling persist despite antiretroviral treatment in an hiPSC tri-culture model of HIV infection. Stem Cell Rep. 14, 991 (2020).
Kraft-Terry, S. D., Buch, S. J., Fox, H. S. & Gendelman, H. E. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64, 133–145 (2009).
Gosselin, D. et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017).
Kaul, M., Garden, G. A. & Lipton, S. A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 (2001).
Saylor, D. et al. HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat. Rev. Neurol. 12, 309 (2016).
Heyes, M. P. et al. Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. FASEB J. 12, 881–896 (1998).
Smith, D. G. et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J. Neurovirol. 7, 56–60 (2001).
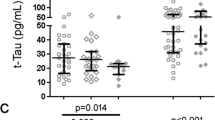
Anderson, A. M. et al. HIV, prospective memory, and cerebrospinal fluid concentrations of quinolinic acid and phosphorylated Tau. J. Neuroimmunol. 319, 13–18 (2018).
Giulian, D. et al. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J. Neurosci. 16, 3139–3153 (1996).
Borjabad, A. et al. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 7, e1002213 (2011).
Levine, A. J. et al. Systems analysis of human brain gene expression: mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer’s disease. BMC Med. Genomics 6, 4 (2013).
Borjabad, A. & Volsky, D. J. Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and multiple sclerosis. J. Neuroimmune Pharmacol. 7, 914–926 (2012).
Ginsberg, S. D. et al. Expression profiling suggests microglial impairment in HIV neuropathogenesis. Ann. Neurol. 83, 406–417 (2018).
Chivero, E. T. et al. HIV-1 tat primes and activates microglial NLRP3 inflammasome-mediated neuroinflammation. J. Neurosci. 37, 3599–3609 (2017).
Barber, S. A., Herbst, D. S., Bullock, B. T., Gama, L. & Clements, J. E. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J. Neurovirol. 10, 15–20 (2004).
Veazey, R. S. et al. Prevention of SHIV transmission by topical IFN-β treatment. Mucosal Immunol. 9, 1528–1536 (2016).
Honda, K., Takaoka, A. & Taniguchi, T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 (2006).
Alammar, L., Gama, L. & Clements, J. E. Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection. J. Immunol. 186, 4008–4018 (2011).
Zaritsky, L. A., Gama, L. & Clements, J. E. Canonical type I IFN signaling in simian immunodeficiency virus-infected macrophages is disrupted by astrocyte-secreted CCL2. J. Immunol. 188, 3876–3885 (2012).
Thaney, V. E. et al. IFNβ protects neurons from damage in a murine model of HIV-1 associated brain injury. Sci. Rep. 7, 46514 (2017).
Gelman, B. B. et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One 7, e46178 (2012).
Singh, H. et al. A pivotal role for interferon-α receptor-1 in neuronal injury induced by HIV-1. J. Neuroinflammation 17, 226 (2020).
Thaney, V. E. & Kaul, M. Type I interferons in NeuroHIV. Viral Immunol. 32, 7–14 (2019).
Bourke, N. M. et al. Control of HIV infection by IFN-α: implications for latency and a cure. Cell Mol. Life Sci. 75, 775–783 (2018).
Rivero-Juarez, A., Frias, M. & Rivero, A. Current views on interferon therapy for HIV. Expert Opin. Biol. Ther. 16, 1135–1142 (2016).
Utay, N. S. & Douek, D. C. Interferons and HIV infection: the good, the bad, and the ugly. Pathog. Immun. 1, 107–116 (2016).
Noel, N. et al. Interferon-associated therapies toward HIV control: the back and forth. Cytokine Growth Factor. Rev. 40, 99–112 (2018).
Sugawara, S., Thomas, D. L. & Balagopal, A. HIV-1 infection and type 1 interferon: navigating through uncertain waters. AIDS Res. Hum. Retroviruses 35, 25–32 (2019).
Gondim, M. V. P. et al. Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption. Sci. Transl. Med. 13, eabd8179 (2021).
Zhen, A. et al. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Invest. 127, 260–268 (2017).
Sandstrom, T. S., Ranganath, N. & Angel, J. B. Impairment of the type I interferon response by HIV-1: potential targets for HIV eradication. Cytokine Growth Factor. Rev. 37, 1–16 (2017).
Hua, S. et al. Pegylated interferon-α-induced natural killer cell activation is associated with human immunodeficiency virus-1 DNA decline in antiretroviral therapy-treated HIV-1/hepatitis C virus-coinfected patients. Clin. Infect. Dis. 66, 1910–1917 (2018).
George, J. & Mattapallil, J. J. Interferon-α subtypes as an adjunct therapeutic approach for human immunodeficiency virus functional cure. Front. Immunol. 9, 299 (2018).
Paul, S., Ricour, C., Sommereyns, C., Sorgeloos, F. & Michiels, T. Type I interferon response in the central nervous system. Biochimie 89, 770–778 (2007).
Markowitz, C. E. Interferon-beta: mechanism of action and dosing issues. Neurology 68 (Suppl. 4), S8–11 (2007).
Kitai, R., Zhao, M. L., Zhang, N., Hua, L. L. & Lee, S. C. Role of MIP-1β and RANTES in HIV-1 infection of microglia: inhibition of infection and induction by IFNβ. J. Neuroimmunol. 110, 230–239 (2000).
Ferreira, A. C. et al. From the periphery to the brain: Lipocalin-2, a friend or foe? Prog. Neurobiol. 131, 120–136 (2015).
Flo, T. H. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004).
Yang, J. et al. An iron delivery pathway mediated by a lipocalin. Mol. Cell 10, 1045–1056 (2002).
Bao, G. et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol. 6, 602–609 (2010).
Bachman, M. A., Miller, V. L. & Weiser, J. N. Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog. 5, e1000622 (2009).
Jha, M. K. et al. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci. Biobehav. Rev. 49, 135–156 (2015).
Ferreira, A. C. et al. Lipocalin-2 is involved in emotional behaviors and cognitive function. Front. Cell Neurosci. 7, 122 (2013).
Ferreira, A. C. et al. Lipocalin-2 regulates adult neurogenesis and contextual discriminative behaviours. Mol. Psychiatry 23, 1031–1039 (2018).
Mucha, M. et al. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc. Natl Acad. Sci. USA 108, 18436–18441 (2011).
Lee, S. et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J. Immunol. 179, 3231–3241 (2007).
Xing, C. et al. Neuronal production of lipocalin-2 as a help-me signal for glial activation. Stroke 45, 2085–2092 (2014).
Bi, F. et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl Acad. Sci. USA 110, 4069–4074 (2013).
Dekens, D. W. et al. Lipocalin 2 as a link between ageing, risk factor conditions and age-related brain diseases. Ageing Res. Rev. 70, 101414 (2021).
Ojeda-Juarez, D. et al. Lipocalin-2 mediates HIV-1 induced neuronal injury and behavioral deficits by overriding CCR5-dependent protection. Brain Behav. Immun. 89, 184–199 (2020).
Williams, M. E., Ipser, J. C., Stein, D. J., Joska, J. A. & Naude, P. J. W. The association of immune markers with cognitive performance in South African HIV-positive patients. J. Neuroimmune Pharmacol. 14, 679–687 (2019).
Ivey, N. S., MacLean, A. G. & Lackner, A. A. Acquired immunodeficiency syndrome and the blood-brain barrier. J. Neurovirol. 15, 111–122 (2009).
Maclean, A. G., Belenchia, G. E., Bieniemy, D. N., Moroney-Rasmussen, T. A. & Lackner, A. A. Simian immunodeficiency virus disrupts extended lengths of the blood–brain barrier. J. Med. Primatol. 34, 237–242 (2005).
Lu, T. S. et al. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. J. Immunol. 181, 6406–6416 (2008).
Eugenin, E. A. et al. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J. Leukoc. Biol. 79, 444–452 (2006).
Persidsky, Y., Zheng, J., Miller, D. & Gendelman, H. E. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J. Leukoc. Biol. 68, 413–422 (2000).
Valdebenito, S., Castellano, P., Ajasin, D. & Eugenin, E. A. Astrocytes are HIV reservoirs in the brain: a cell type with poor HIV infectivity and replication but efficient cell-to-cell viral transfer. J. Neurochem. 158, 429–443 (2021).
Churchill, M. J. et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann. Neurol. 66, 253–258 (2009).
Wahl, A. & Al-Harthi, L. HIV infection of non-classical cells in the brain. Retrovirology 20, 1 (2023).
Bertrand, L., Cho, H. J. & Toborek, M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 142, 502–511 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Fattakhov, N., Torices, S., Stangis, M., Park, M. & Toborek, M. Synergistic impairment of the neurovascular unit by HIV-1 infection and methamphetamine use: implications for HIV-1-associated neurocognitive disorders. Viruses 13, 1883 (2021).
Toborek, M. et al. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J. Neurochem. 84, 169–179 (2003).
Avraham, H. K., Jiang, S., Lee, T. H., Prakash, O. & Avraham, S. HIV-1 Tat-mediated effects on focal adhesion assembly and permeability in brain microvascular endothelial cells. J. Immunol. 173, 6228–6233 (2004).
Andras, I. E. et al. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci. Res. 74, 255–265 (2003).
Andras, I. E. et al. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J. Cereb. Blood Flow Metab. 25, 1159–1170 (2005).
de Almeida, S. M. et al. Biomarkers of chemotaxis and inflammation in cerebrospinal fluid and serum in individuals with HIV-1 subtype C versus B. J. Neurovirol. 22, 715–724 (2016).
Wang, Z., Shang, H. & Jiang, Y. Chemokines and chemokine receptors: accomplices for human immunodeficiency virus infection and latency. Front. Immunol. 8, 1274 (2017).
Anderson, A. M. et al. Cerebrospinal fluid CXCL10 is associated with the presence of low level CSF HIV during suppressive antiretroviral therapy. J. Neuroimmunol. 353, 577493 (2021).
Letendre, S. L. et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J. Neurovirol. 17, 63–69 (2011).
Alves de Lima, K. et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 21, 1421–1429 (2020).
Prieto, G. A. & Cotman, C. W. Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine Growth Factor Rev. 34, 27–33 (2017).
Harden, L. M., du Plessis, I., Poole, S. & Laburn, H. P. Interleukin (IL)-6 and IL-1β act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav. Immun. 22, 838–849 (2008).
Bourgognon, J. M. & Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 4, 2398212820979802 (2020).
Stein, D. S. et al. Predicting clinical progression or death in subjects with early-stage human immunodeficiency virus (HIV) infection: a comparative analysis of quantification of HIV RNA, soluble tumor necrosis factor type II receptors, neopterin, and β2-microglobulin. J. Infect. Dis. 176, 1161–1167 (1997).
Diez-Ruiz, A. et al. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol. 54, 1–8 (1995).
Savès, M. et al. Prognostic value of plasma markers of immune activation in patients with advanced HIV disease treated by combination antiretroviral therapy. Clin. Immunol. 99, 347–352 (2001).
Suh, J. et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J. Neuroinflammation 11, 199 (2014).
Hagberg, L. et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 7, 15 (2010).
Walsh, J. G. et al. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology 11, 35 (2014).
Mamik, M. K. et al. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J. Neuroimmune Pharmacol. 12, 233–248 (2017).
Mamik, M. K. & Power, C. Inflammasomes in neurological diseases: emerging pathogenic and therapeutic concepts. Brain 140, 2273–2285 (2017).
Sil, S. et al. Role of inflammasomes in HIV-1 and drug abuse mediated neuroinflammaging. Cells 9, 1857 (2020).
Periyasamy, P., Thangaraj, A., Bendi, V. S. & Buch, S. HIV-1 Tat-mediated microglial inflammation involves a novel miRNA-34a-NLRC5-NFκB signaling axis. Brain Behav. Immun. 80, 227–237 (2019).
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20, 3328 (2019).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Mullis, C. & Swartz, T. H. NLRP3 inflammasome signaling as a link between HIV-1 infection and atherosclerotic cardiovascular disease. Front. Cardiovasc. Med. 7, 95 (2020).
Andrade-Santos, J. L. et al. IL18 gene polymorphism and its influence on CD4+ T-cell recovery in HIV-positive patients receiving antiretroviral therapy. Infect. Genet. Evol. 75, 103997 (2019).
Howren, M. B., Lamkin, D. M. & Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186 (2009).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457 (2010).
Haapakoski, R., Mathieu, J., Ebmeier, K. P., Alenius, H. & Kivimaki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 49, 206–215 (2015).
Miller, A. H., Haroon, E., Raison, C. L. & Felger, J. C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30, 297–306 (2013).
Ding, Y. & Dai, J. Advance in stress for depressive disorder. Adv. Exp. Med. Biol. 1180, 147–178 (2019).
Juruena, M. F., Eror, F., Cleare, A. J. & Young, A. H. The role of early life stress in HPA axis and anxiety. Adv. Exp. Med. Biol. 1191, 141–153 (2020).
Smith, S. M. & Vale, W. W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 8, 383–395 (2006).
Polak, P. & Hall, M. N. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 21, 209–218 (2009).
Laplante, M. & Sabatini, D. M. mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 (2009).
Boden, G. Interaction between free fatty acids and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 5, 545–549 (2002).
Snodgrass, R. G., Huang, S., Choi, I. W., Rutledge, J. C. & Hwang, D. H. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J. Immunol. 191, 4337–4347 (2013).
Akbay, B., Shmakova, A., Vassetzky, Y. & Dokudovskaya, S. Modulation of mTORC1 signaling pathway by HIV-1. Cells 9, 1090 (2020).
Cinti, A. et al. HIV-1 enhances mTORC1 activity and repositions lysosomes to the periphery by co-opting Rag GTPases. Sci. Rep. 7, 5515 (2017).
Alirezaei, M., Kiosses, W. B. & Fox, H. S. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy 4, 963–966 (2008).
Alirezaei, M., Kiosses, W. B., Flynn, C. T., Brady, N. R. & Fox, H. S. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One 3, e2906 (2008).
Papadopoli, D. et al. mTOR as a central regulator of lifespan and aging. F1000Research 8, 998 (2019).
Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268ra179 (2014).
Westendorp, R. G. et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J. Am. Geriatr. Soc. 57, 1634–1637 (2009).
Barzilai, N., Gabriely, I., Gabriely, M., Iankowitz, N. & Sorkin, J. D. Offspring of centenarians have a favorable lipid profile. J. Am. Geriatr. Soc. 49, 76–79 (2001).
Zheng, Y. & Jiang, Y. mTOR inhibitors at a glance. Mol. Cell Pharmacol. 7, 15–20 (2015).
Avila, C. L. et al. mTOR inhibition suppresses posttransplant alloantibody production through direct inhibition of alloprimed B cells and sparing of CD8+ antibody-suppressing T cells. Transplantation 100, 1898–1906 (2016).
Achim, C. L. et al. Increased accumulation of intraneuronal amyloid β in HIV-infected patients. J. Neuroimmune Pharmacol. 4, 190–199 (2009).
Soontornniyomkij, V. et al. Cerebral β-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE ε4 carriers. AIDS 26, 2327–2335 (2012).
Green, D. A. et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19, 407–411 (2005).
Ortega, M. & Ances, B. M. Role of HIV in amyloid metabolism. J. Neuroimmune Pharmacol. 9, 483–491 (2014).
Hategan, A., Masliah, E. & Nath, A. HIV and Alzheimer’s disease: complex interactions of HIV-Tat with amyloid β peptide and Tau protein. J. Neurovirol. 25, 648–660 (2019).
Bae, M. et al. Activation of TRPML1 clears intraneuronal Aβ in preclinical models of HIV infection. J. Neurosci. 34, 11485–11503 (2014).
Andras, I. E. & Toborek, M. Amyloid beta accumulation in HIV-1-infected brain: the role of the blood brain barrier. IUBMB Life 65, 43–49 (2013).
Breen, E. C. et al. Accelerated aging with HIV begins at the time of initial HIV infection. iScience 25, 104488 (2022).
Sehl, M. E. et al. Increased rate of epigenetic aging in men living with HIV prior to treatment. Front. Genet. 12, 796547 (2021).
Gisslen, M. et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol. Neuroimmunol. Neuroinflamm. 6, e512 (2019).
Murray, J. et al. Frontal lobe microglia, neurodegenerative protein accumulation, and cognitive function in people with HIV. Acta Neuropathol. Commun. 10, 69 (2022).
Mackiewicz, M. M., Overk, C., Achim, C. L. & Masliah, E. Pathogenesis of age-related HIV neurodegeneration. J. Neurovirol. 25, 622–633 (2019).
Rodriguez-Penney, A. T. et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS 27, 5–16 (2013).
Allavena, C. et al. Antiretroviral exposure and comorbidities in an aging HIV-infected population: the challenge of geriatric patients. PLoS One 13, e0203895 (2018).
Morgello, S. et al. Frailty in medically complex individuals with chronic HIV. AIDS 33, 1603–1611 (2019).
Becker, B. W., Thames, A. D., Woo, E., Castellon, S. A. & Hinkin, C. H. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav. 15, 1888–1894 (2011).
Kamal, S. et al. The presence of human immunodeficiency virus-associated neurocognitive disorders is associated with a lower adherence to combined antiretroviral treatment. Open Forum Infect. Dis. 4, ofx070 (2017).
Tedaldi, E. M., Minniti, N. L. & Fischer, T. HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed. Res. Int. 2015, 641913 (2015).
Ijoma, U. N. et al. Health-related quality of life in people with chronic diseases managed in a low-resource setting — a study from South East Nigeria. Niger. J. Clin. Pract. 22, 1180–1188 (2019).
Morgan, E. E. et al. Synergistic effects of HIV infection and older age on daily functioning. J. Acquir. Immune Defic. Syndr. 61, 341–348 (2012).
Tozzi, V. et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int. J. STD AIDS 15, 254–259 (2004).
Duffau, P. et al. Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS 32, 1651–1660 (2018).
Banerjee, N., McIntosh, R. C. & Ironson, G. Impaired neurocognitive performance and mortality in HIV: assessing the prognostic value of the HIV-dementia scale. AIDS Behav. 23, 3482–3492 (2019).
Erlandson, K. M. et al. Frailty, neurocognitive impairment, or both in predicting poor health outcomes among adults living with human immunodeficiency virus. Clin. Infect. Dis. 68, 131–138 (2019).
Naveed, Z. et al. Neurocognitive status and risk of mortality among people living with human immunodeficiency virus: an 18-year retrospective cohort study. Sci. Rep. 11, 3738 (2021).
De Francesco, D. et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS 33, 259–268 (2019).
Smith, R. L., de Boer, R., Brul, S., Budovskaya, Y. & van Spek, H. Premature and accelerated aging: HIV or HAART. Front. Genet. 3, 328 (2012).
Guaraldi, G. et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 53, 1120–1126 (2011).
Siangphoe, U., Archer, K. J., Nguyen, C. & Lee, K. R. Associations of antiretroviral therapy and comorbidities with neurocognitive outcomes in HIV-1-infected patients. AIDS 34, 893–902 (2020).
Becker, J. T. et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 73, 1292–1299 (2009).
Wright, E. J. et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology 75, 864–873 (2010).
Ellis, R. J., Paolillo, E., Saloner, R. & Heaton, R. K. Higher comorbidity burden predicts worsening neurocognitive trajectories in people with HIV. Clin. Infect. Dis. 74, 1323–1328 (2021).
Saloner, R. et al. Effects of comorbidity burden and age on brain integrity in HIV. AIDS 33, 1175–1185 (2019).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 (1987).
Justice, A. C. et al. Veterans Aging Cohort Study (VACS): overview and description. Med. Care 44 (Suppl. 2), S13–24 (2006).
Assmann, G. & Schulte, H. The Prospective Cardiovascular Munster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am. Heart J. 116, 1713–1724 (1988).
Wong, N. D. & Levy, D. Legacy of the Framingham Heart Study: rationale, design, initial findings, and implications. Glob. Heart 8, 3–9 (2013).
Ridker, P. M., Buring, J. E., Rifai, N. & Cook, N. R. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297, 611–619 (2007).
Ellis, R. J., Paolillo, E., Saloner, R. & Heaton, R. K. Higher comorbidity burden predicts worsening neurocognitive trajectories in people with human immunodeficiency virus. Clin. Infect. Dis. 74, 1323–1328 (2022).
Bing, E. G. et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatry 58, 721–728 (2001).
Turner, B. J., Laine, C., Cosler, L. & Hauck, W. W. Relationship of gender, depression, and health care delivery with antiretroviral adherence in HIV-infected drug users. J. Gen. Intern. Med. 18, 248–257 (2003).
Bigna, J. J., Kenne, A. M., Asangbeh, S. L. & Sibetcheu, A. T. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob. Health 6, e193–e202 (2018).
Antoniou, T., Yao, Z., Raboud, J. & Gershon, A. S. Incidence of chronic obstructive pulmonary disease in people with HIV in Ontario, 1996-2015: a retrospective population-based cohort study. CMAJ Open 8, E83–E89 (2020).
Davis, K. et al. Association between HIV infection and hypertension: a global systematic review and meta-analysis of cross-sectional studies. BMC Med. 19, 105 (2021).
van Zoest, R. A., van den Born, B. H. & Reiss, P. Hypertension in people living with HIV. Curr. Opin. HIV AIDS 12, 513–522 (2017).
Cholera, R. et al. Depression and engagement in care among newly diagnosed HIV-infected adults in Johannesburg, South Africa. AIDS Behav. 21, 1632–1640 (2017).
Pence, B. W. et al. Assessing the effect of measurement-based care depression treatment on HIV medication adherence and health outcomes: rationale and design of the SLAM DUNC Study. Contemp. Clin. Trials 33, 828–838 (2012).
Pence, B. W., O’Donnell, J. K. & Gaynes, B. N. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS 26, 656–658 (2012).
Manner, I. W. et al. Markers of microbial translocation predict hypertension in HIV-infected individuals. HIV Med. 14, 354–361 (2013).
Masenga, S. K. et al. Patho-immune mechanisms of hypertension in HIV: a systematic and thematic review. Curr. Hypertens. Rep. 21, 56 (2019).
Ellis, R. J. et al. Higher levels of plasma inflammation biomarkers are associated with depressed mood and quality of life in aging, virally suppressed men, but not women, with HIV. Brain Behav. Immun. Health 7, 100121 (2020).
Morris, A. et al. HIV and chronic obstructive pulmonary disease: is it worse and why. Proc. Am. Thorac. Soc. 8, 320–325 (2011).
Lee, C. J. et al. The effects of diet alone or in combination with exercise in patients with prehypertension and hypertension: a randomized controlled trial. Korean Circ. J. 48, 637–651 (2018).
Miller, E. R. III et al. Results of the diet, exercise, and weight loss intervention trial (DEW-IT). Hypertension 40, 612–618 (2002).
Bliss, E. S., Wong, R. H., Howe, P. R. & Mills, D. E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow Metab. 41, 447–470 (2021).
Yurko-Mauro, K. Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr. Alzheimer Res. 7, 190–196 (2010).
Coca, A., Monteagudo, E., Domenech, M., Camafort, M. & Sierra, C. Can the treatment of hypertension in the middle-aged prevent dementia in the elderly. High Blood Press. Cardiovasc. Prev. 23, 97–104 (2016).
Rouch, L. et al. Blood pressure and cognitive performances in middle-aged adults: the aging, health and work longitudinal study. J. Hypertens. 37, 1244–1253 (2019).
Gupta, A. et al. Treatment of hypertension reduces cognitive decline in older adults: a systematic review and meta-analysis. BMJ Open 10, e038971 (2020).
Tadic, M., Cuspidi, C. & Hering, D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc. Disord. 16, 208 (2016).
Krishnan, S. et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 61, 381–389 (2012).
Yu, B. et al. Metabolic syndrome and neurocognitive deficits in HIV infection. J. Acquir. Immune Defic. Syndr. 81, 95–101 (2019).
McCutchan, J. A. et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 78, 485–492 (2012).
Panza, F. et al. Metabolic syndrome, mild cognitive impairment, and dementia. Curr. Alzheimer Res. 8, 492–509 (2011).
Morgan, P. K. et al. Macrophage polarization state affects lipid composition and the channeling of exogenous fatty acids into endogenous lipid pools. J. Biol. Chem. 297, 101341 (2021).
Bourgeois, C. et al. Contribution of adipose tissue to the chronic immune activation and inflammation associated with HIV infection and its treatment. Front. Immunol. 12, 670566 (2021).
Werther, G. A. et al. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121, 1562–1570 (1987).
Marks, J. L., Porte, D. Jr., Stahl, W. L. & Baskin, D. G. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127, 3234–3236 (1990).
Doré, S., Kar, S., Rowe, W. & Quirion, R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience 80, 1033–1040 (1997).
Schulingkamp, R. J., Pagano, T. C., Hung, D. & Raffa, R. B. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci. Biobehav. Rev. 24, 855–872 (2000).
Mamik, M. K. et al. Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J. Neurosci. 36, 10683–10695 (2016).
Kim, B. H. et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS 33, 973–984 (2019).
de la Monte, S. M. Intranasal insulin therapy for cognitive impairment and neurodegeneration: current state of the art. Expert Opin. Drug Deliv. 10, 1699–1709 (2013).
Hallschmid, M. Intranasal insulin for Alzheimer’s disease. CNS Drugs 35, 21–37 (2021).
Craft, S. et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 77, 1099–1109 (2020).
Chang, C. C. et al. HIV and co-infections. Immunol. Rev. 254, 114–142 (2013).
Brites, C., Borges, A. H., Sprinz, E. & Page, K. Editorial: HIV and viral co-infections. Front. Microbiol. 12, 731337 (2021).
Di Gennaro, F., Vergori, A. & Bavaro, D. F. HIV and co-infections: updates and insights. Viruses 15, 1097 (2023).
Fialho, R. et al. Cognitive impairment in HIV and HCV co-infected patients: a systematic review and meta-analysis. AIDS Care 28, 1481–1494 (2016).
Bharti, A. R. et al. Asymptomatic malaria co-infection of HIV-infected adults in nigeria: prevalence of and impact on cognition, mood, and biomarkers of systemic inflammation. J. Acquir. Immune Defic. Syndr. 86, 91–97 (2021).
Hestad, K. A. et al. Cognitive impairment in Zambians with HIV infection and pulmonary tuberculosis. J. Acquir. Immune Defic. Syndr. 80, 110–117 (2019).
Ramlall, S. et al. Neurocognitive functioning in MDR-TB patients with and without HIV in KwaZulu-Natal, South Africa. Trop. Med. Int. Health 25, 919–927 (2020).
Carlson, R. D. et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab. Brain Dis. 29, 269–279 (2014).
Letendre, S. et al. Higher anti-CMV IgG concentrations are associated with worse neurocognitive performance during suppressive antiretroviral therapy. Clin. Infect. Dis. 67, 770–777 (2018).
Brunt, S. J. et al. Short communication: do cytomegalovirus antibody levels associate with age-related syndromes in HIV patients stable on antiretroviral therapy? AIDS Res. Hum. Retroviruses 32, 567–572 (2016).
Gianella, S. & Letendre, S. Cytomegalovirus and HIV: a dangerous Pas de Deux.J. Infect. Dis. 214, S67–S74 (2016).
Roberts, E. T., Haan, M. N., Dowd, J. B. & Aiello, A. E. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol. 172, 363–371 (2010).
Wu, M. et al. HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS 32, 1803–1810 (2018).
Vance, D. E. et al. The synergistic effects of HIV, diabetes, and aging on cognition: implications for practice and research. J. Neurosci. Nurs. 46, 292 (2014).
Huck, D. M. et al. Carotid artery stiffness and cognitive decline among women with or at risk for HIV infection. J. Acquired Immune Defic. Syndromes 78, 338–347 (2018).
Freeman, M. L. et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin. Infect. Dis. 62, 392–396 (2016).
Sacre, K. et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 26, 805–814 (2012).
Lupia, T. et al. Presence of Epstein-Barr virus DNA in cerebrospinal fluid is associated with greater HIV RNA and inflammation. AIDS 34, 373–380 (2020).
van der Walt, J. M. et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am. J. Hum. Genet. 72, 804–811 (2003).
Wang, D. B. et al. Declines in Drp1 and parkin expression underlie DNA damage-induced changes in mitochondrial length and neuronal death. J. Neurosci. 33, 1357–1365 (2013).
Ye, X., Tai, W. & Zhang, D. The early events of Alzheimer’s disease pathology: from mitochondrial dysfunction to BDNF axonal transport deficits. Neurobiol. Aging 33, 1122.e1-10 (2012).
Tsunemi, T. et al. PGC-1α rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci. Transl. Med. 4, 142ra197 (2012).
Cotto, B., Natarajaseenivasan, K. & Langford, D. Astrocyte activation and altered metabolism in normal aging, age-related CNS diseases, and HAND. J. Neurovirol. 25, 722–733 (2019).
Yellen, G. Fueling thought: management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol. 217, 2235–2246 (2018).
Van den Bossche, J., O’Neill, L. A. & Menon, D. Macrophage immunometabolism: where are we (going). Trends Immunol. 38, 395–406 (2017).
Devanney, N. A., Stewart, A. N. & Gensel, J. C. Microglia and macrophage metabolism in CNS injury and disease: the role of immunometabolism in neurodegeneration and neurotrauma. Exp. Neurol. 329, 113310 (2020).
Yin, F., Sancheti, H., Patil, I. & Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 100, 108–122 (2016).
Jiang, T. & Cadenas, E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13, 1059–1067 (2014).
Swinton, M. K. et al. Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: implications for therapeutic targeting of astroglia. Neurobiol. Dis. 130, 104502 (2019).
Fields, J. A. et al. Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice. Sci. Rep. 9, 17158 (2019).
Natarajaseenivasan, K. et al. Astrocytic metabolic switch is a novel etiology for cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis. 9, 415 (2018).
Samikkannu, T., Atluri, V. S. & Nair, M. P. HIV and cocaine impact glial metabolism: energy sensor AMP-activated protein kinase role in mitochondrial biogenesis and epigenetic remodeling. Sci. Rep. 6, 31784 (2016).
Sivalingam, K., Cirino, T. J., McLaughlin, J. P. & Samikkannu, T. HIV-Tat and cocaine impact brain energy metabolism: redox modification and mitochondrial biogenesis influence NRF transcription-mediated neurodegeneration. Mol. Neurobiol. 58, 490–504 (2021).
Fields, J. A. & Ellis, R. J. HIV in the cART era and the mitochondrial: immune interface in the CNS. Int. Rev. Neurobiol. 145, 29–65 (2019).
Fields, J. A. et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol. Dis. 86, 154–169 (2015).
Avdoshina, V. et al. The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox. Res. 29, 583–593 (2016).
Vallee, K. J. & Fields, J. A. Caloric restriction mimetic 2-deoxyglucose reduces inflammatory signaling in human astrocytes: implications for therapeutic strategies targeting neurodegenerative diseases. Brain Sci. 12, 308 (2022).
Fields, J. A., Swinton, M. K., Montilla-Perez, P., Ricciardelli, E. & Telese, F. The cannabinoid receptor agonist, WIN-55212-2, suppresses the activation of pro-inflammatory genes induced by interleukin 1 beta in human astrocytes. Cannabis Cannabinoid Res. 7, 78–92 (2022).
Sheng, W. S. et al. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia 49, 211–219 (2005).
Halcrow, P. W. et al. HIV-1 gp120-induced endolysosome de-acidification leads to efflux of endolysosome iron, and increases in mitochondrial iron and reactive oxygen species. J. Neuroimmune Pharmacol. 17, 181–194 (2021).
Halcrow, P. W. et al. Heterogeneity of ferrous iron-containing endolysosomes and effects of endolysosome iron on endolysosome numbers, sizes, and localization patterns. J. Neurochem. 161, 69–83 (2022).
Halcrow, P. W., Lynch, M. L., Geiger, J. D. & Ohm, J. E. Role of endolysosome function in iron metabolism and brain carcinogenesis. Semin. Cancer Biol. 76, 74–85 (2021).
Kallianpur, A. R. et al. Hemochromatosis (HFE) gene mutations and peripheral neuropathy during antiretroviral therapy. AIDS 20, 1503–1513 (2006).
Fennema-Notestine, C. et al. Iron-regulatory genes are associated with Neuroimaging measures in HIV infection. Brain Imaging Behav. 14, 2037–2049 (2020).
Kallianpur, A. R. et al. Genetic variation in iron metabolism is associated with neuropathic pain and pain severity in HIV-infected patients on antiretroviral therapy. PLoS One 9, e103123 (2014).
Huang, J. et al. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci. Ther. 25, 796–807 (2019).
Bernardo, T. C. et al. Physical exercise and brain mitochondrial fitness: the possible role against Alzheimer’s disease. Brain Pathol. 26, 648–663 (2016).
Ruegsegger, G. N. et al. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight 4, e130681 (2019).
Cheng, A. et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 23, 128–142 (2016).
Park, H. S. et al. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology 133, 451–461 (2018).
Steiner, J. L., Murphy, E. A., McClellan, J. L., Carmichael, M. D. & Davis, J. M. Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol. 111, 1066–1071 (2011).
Ichimura, Y. et al. A ubiquitin-like system mediates protein lipidation. Nature 408, 488–492 (2000).
Kametaka, S., Okano, T., Ohsumi, M. & Ohsumi, Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 273, 22284–22291 (1998).
Baba, M., Takeshige, K., Baba, N. & Ohsumi, Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J. Cell Biol. 124, 903–913 (1994).
Takeshige, K., Baba, M., Tsuboi, S., Noda, T. & Ohsumi, Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119, 301–311 (1992).
Brier, L. W. et al. Regulation of LC3 lipidation by the autophagy-specific class III phosphatidylinositol-3 kinase complex. Mol. Biol. Cell 30, 1098–1107 (2019).
Ge, L., Baskaran, S., Schekman, R. & Hurley, J. H. The protein-vesicle network of autophagy. Curr. Opin. Cell Biol. 29, 18–24 (2014).
Fields, J. et al. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J. Neurovirol. 19, 89–101 (2013).
Fields, J. et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J. Neurosci. 35, 1921–1938 (2015).
Hui, L., Chen, X., Haughey, N. J. & Geiger, J. D. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro 4, 243–252 (2012).
Patton, S. M. et al. Cerebrospinal fluid (CSF) biomarkers of iron status are associated with CSF viral load, antiretroviral therapy, and demographic factors in HIV-infected adults. Fluids Barriers CNS 14, 11 (2017).
Abioye, A. I., Andersen, C. T., Sudfeld, C. R. & Fawzi, W. W. Anemia, iron status, and HIV: a systematic review of the evidence. Adv. Nutr. 11, 1334–1363 (2020).
Paul, B. T., Manz, D. H., Torti, F. M. & Torti, S. V. Mitochondria and iron: current questions. Expert Rev. Hematol. 10, 65–79 (2017).
Khan, N. et al. Endolysosome iron restricts Tat-mediated HIV-1 LTR transactivation by increasing HIV-1 Tat oligomerization and β-catenin expression. J. Neurovirol. 27, 755–773 (2021).
Abdel-Haq, R., Schlachetzki, J. C. M., Glass, C. K. & Mazmanian, S. K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 216, 41–59 (2019).
Fang, P., Kazmi, S. A., Jameson, K. G. & Hsiao, E. Y. The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe 28, 201–222 (2020).
Ma, Q. et al. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation 16, 53 (2019).
Byrnes, S. J. et al. Chronic immune activation and gut barrier dysfunction is associated with neuroinflammation in ART-suppressed SIV+ rhesus macaques. PLoS Pathog. 19, e1011290 (2023).
Fuchs, D. et al. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J. Acquir. Immune Defic. Syndr. 3, 873–876 (1990).
Underwood, J., Robertson, K. R. & Winston, A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 29, 253–261 (2015).
Lanman, T., Letendre, S., Ma, Q., Bang, A. & Ellis, R. CNS neurotoxicity of antiretrovirals. J. Neuroimmune Pharmacol. 16, 130–143 (2019).
Bertrand, L., Velichkovska, M. & Toborek, M. Cerebral vascular toxicity of antiretroviral therapy. J. Neuroimmune Pharmacol. 16, 74–89 (2019).
Alonso-Villaverde, C. et al. Host–pathogen interactions in the development of metabolic disturbances and atherosclerosis in HIV infection: the role of CCL2 genetic variants. Cytokine 51, 251–258 (2010).
Tarr, P. E. & Telenti, A. Genetic screening for metabolic and age-related complications in HIV-infected persons. F1000 Med. Rep. 2, 83 (2010).
Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 41, 67–76 (2009).
Mangoni, A. A. & Jackson, S. H. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br. J. Clin. Pharmacol. 57, 6–14 (2004).
Winston, A. et al. Effects of age on antiretroviral plasma drug concentration in HIV-infected subjects undergoing routine therapeutic drug monitoring. J. Antimicrob. Chemother. 68, 1354–1359 (2013).
Bertrand, L., Nair, M. & Toborek, M. Solving the blood-brain barrier challenge for the effective treatment of HIV replication in the central nervous system. Curr. Pharm. Des. 22, 5477–5486 (2016).
Nwogu, J. N. et al. Pharmacokinetic, pharmacogenetic, and other factors influencing CNS penetration of antiretrovirals. AIDS Res. Treat. 2016, 2587094 (2016).
Decloedt, E. H., Rosenkranz, B., Maartens, G. & Joska, J. Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin. Pharmacokinet. 54, 581–598 (2015).
Schifitto, G. et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS 21, 1877–1886 (2007).
Schifitto, G. et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 69, 1314–1321 (2007).
Nakasujja, N. et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 80, 196–202 (2013).
Sacktor, N. et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 77, 1135–1142 (2011).
Meulendyke, K. A. et al. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J. Neurovirol. 20, 591–602 (2014).
Sacktor, N. et al. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J. Neurovirol. 24, 16–27 (2018).
Rezaie-Majd, A. et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 22, 1194–1199 (2002).
Gerena, Y. et al. Soluble and cell-associated insulin receptor dysfunction correlates with severity of HAND in HIV-infected women. PLoS One 7, e37358 (2012).
Velichkovska, M., Surnar, B., Nair, M., Dhar, S. & Toborek, M. Targeted mitochondrial coq10 delivery attenuates antiretroviral drug-induced senescence of neural progenitor cells. Mol. Pharm. 16, 724–736 (2018).
Cross, S. A. et al. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J. Immunol. 187, 5015–5025 (2011).
Louboutin, J.-P. & Strayer, D. S. in HIV/AIDS 107–123 (Elsevier, 2018).
Rochira, V. & Guaraldi, G. Growth hormone deficiency and human immunodeficiency virus. Best Pract. Res. Clin. Endocrinol. Metab. 31, 91–111 (2017).
Stanley, T. L. et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA 312, 380–389 (2014).
Stanley, T. L. et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin. Infect. Dis. 54, 1642–1651 (2012).
Wrigley, S., Arafa, D. & Tropea, D. Insulin-like growth factor 1: at the crossroads of brain development and aging. Front. Cell Neurosci. 11, 14 (2017).
Rui-Hua, C., Yong-de, P., Xiao-Zhen, J., Chen, J. & Bin, Z. Decreased levels of serum IGF-1 and vitamin D are associated with cognitive impairment in patients with type 2 diabetes. Am. J. Alzheimers Dis. Other Demen. 34, 450–456 (2019).
Aleman, A. et al. Insulin-like growth factor-I and cognitive function in healthy older men. J. Clin. Endocrinol. Metab. 84, 471–475 (1999).
Kalmijn, S., Janssen, J. A., Pols, H. A., Lamberts, S. W. & Breteler, M. M. A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J. Clin. Endocrinol. Metab. 85, 4551–4555 (2000).
de la Monte, S. M. & Wands, J. R. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J. Alzheimers Dis. 7, 45–61 (2005).
Nazem, F., Farhangi, N. & Neshat-Gharamaleki, M. Beneficial effects of endurance exercise with rosmarinus officinalis labiatae leaves extract on blood antioxidant enzyme activities and lipid peroxidation in streptozotocin-induced diabetic rats. Can. J. Diabetes 39, 229–234 (2015).
Gleeson, M. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615 (2011).
Horowitz, A. M. et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020).
Abdolmaleki, F. & Heidarianpour, A. Endurance exercise training restores diabetes-induced alteration in circulating Glycosylphosphatidylinositol-specific phospholipase D levels in rats. Diabetol. Metab. Syndr. 12, 43 (2020).
Qin, W., Liang, Y. Z., Qin, B. Y., Zhang, J. L. & Xia, N. The clinical significance of glycoprotein phospholipase D levels in distinguishing early stage latent autoimmune diabetes in adults and type 2 diabetes. PLoS One 11, e0156959 (2016).
Baker, S. K. et al. Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 115, E9687–E9696 (2018).
Deeg, M. A. et al. Increased expression of GPI-specific phospholipase D in mouse models of type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 281, E147–154 (2001).
O’Brien, K. D., Pineda, C., Chiu, W. S., Bowen, R. & Deeg, M. A. Glycosylphosphatidylinositol-specific phospholipase D is expressed by macrophages in human atherosclerosis and colocalizes with oxidation epitopes. Circulation 99, 2876–2882 (1999).
Montoya, J. L. et al. Coagulation imbalance and neurocognitive functioning in older HIV-positive adults on suppressive antiretroviral therapy. AIDS 31, 787–795 (2017).
Lee, K. A. et al. Types of sleep problems in adults living with HIV/AIDS. J. Clin. Sleep. Med. 8, 67–75 (2012).
Rubinstein, M. L. & Selwyn, P. A. High prevalence of insomnia in an outpatient population with HIV infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19, 260–265 (1998).
Wiegand, M., Moller, A. A., Schreiber, W., Krieg, J. C. & Holsboer, F. Alterations of nocturnal sleep in patients with HIV infection. Acta Neurol. Scand. 83, 141–142 (1991).
Nokes, K. M. & Kendrew, J. Correlates of sleep quality in persons with HIV disease. J. Assoc. Nurses AIDS Care 12, 17–22 (2001).
Mahmood, Z., Hammond, A., Nunez, R. A., Irwin, M. R. & Thames, A. D. Effects of sleep health on cognitive function in HIV+ and HIV− adults. J. Int. Neuropsychol. Soc. 24, 1038–1046 (2018).
Gamaldo, C. E. et al. Evaluating sleep and cognition in HIV. J. Acquir. Immune Defic. Syndr. 63, 609–616 (2013).
Shokri-Kojori, E. et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl Acad. Sci. USA 115, 4483–4488 (2018).
Kardassis, D., Grote, L., Sjostrom, L., Hedner, J. & Karason, K. Sleep apnea modifies the long-term impact of surgically induced weight loss on cardiac function and inflammation. Obesity 21, 698–704 (2013).
Wirth, M. D. et al. Association of markers of inflammation with sleep and physical activity among people living with HIV or AIDS. AIDS Behav. 19, 1098–1107 (2015).
Vecchio, L. M. et al. The neuroprotective effects of exercise: maintaining a healthy brain throughout aging. Brain Plast. 4, 17–52 (2018).
Dufour, C. A. et al. A longitudinal analysis of the impact of physical activity on neurocognitive functioning among HIV-infected adults. AIDS Behav. 22, 1562–1572 (2018).
Fazeli, P. L. et al. Physical activity is associated with better neurocognitive and everyday functioning among older adults with HIV disease. AIDS Behav. 19, 1470–1477 (2015).
Montoya, J. L., Henry, B. & Moore, D. J. Behavioral and physical activity interventions for HAND. Curr. Top. Behav. Neurosci. 50, 479–501 (2019).
Montoya, J. L. et al. Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS 33, 931–939 (2019).
Dufour, C. A. et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J. Neurovirol. 19, 410–417 (2013).
Monroe, A. K. et al. The association between physical activity and cognition in men with and without HIV infection. HIV Med. 18, 555–563 (2017).
Henry, B. L. & Moore, D. J. Preliminary findings describing participant experience with iSTEP, an mHealth intervention to increase physical activity and improve neurocognitive function in people living with HIV. J. Assoc. Nurses AIDS Care 27, 495–511 (2016).
Vance, D. E. et al. Computerized cognitive training for the neurocognitive complications of HIV infection: a systematic review. J. Assoc. Nurses AIDS Care 30, 51–72 (2019).
Wei, J. et al. Evaluation of computerized cognitive training and cognitive and daily function in patients living with HIV: a meta-analysis. JAMA Netw. Open 5, e220970 (2022).
Abers, M. S., Shandera, W. X. & Kass, J. S. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs 28, 131–145 (2014).
Scherzer, R. & Shlipak, M. G. Risk factors: individual assessment of CKD risk in HIV-positive patients. Nat. Rev. Nephrol. 11, 392 (2015).
Rodriguez-Nóvoa, S., Alvarez, E., Labarga, P. & Soriano, V. Renal toxicity associated with tenofovir use. Expert Opin. Drug Saf. 9, 545–559 (2010).
Kakuda, T. N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22, 685–708 (2000).
Kohler, J. J. & Lewis, W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Env. Mol. Mutagen. 48, 166–172 (2007).
Lewis, W., Day, B. J. & Copeland, W. C. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat. Rev. Drug Discov. 2, 812–822 (2003).
Nooka, S. & Ghorpade, A. Organellar stress intersects the astrocyte endoplasmic reticulum, mitochondria and nucleolus in HIV associated neurodegeneration. Cell Death Dis. 9, 317 (2018).
Nagiah, S., Phulukdaree, A. & Chuturgoon, A. A. Lon protease and eiF2α are involved in acute, but not prolonged, antiretroviral induced stress response in HepG2 cells. Chem. Biol. Interact. 252, 82–86 (2016).
Stankov, M. V., Lucke, T., Das, A. M., Schmidt, R. E. & Behrens, G. M. Mitochondrial DNA depletion and respiratory chain activity in primary human subcutaneous adipocytes treated with nucleoside analogue reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 54, 280–287 (2010).
Young, M. J. Off-target effects of drugs that disrupt human mitochondrial DNA maintenance. Front. Mol. Biosci. 4, 74 (2017).
Allen Reeves, A. et al. Neurotoxicities in the treatment of HIV between dolutegravir, rilpivirine and dolutegravir/rilpivirine: a meta-analysis. Sex. Transm. Infect. 97, 261–267 (2021).
Apostolova, N. et al. Efavirenz and the CNS: what we already know and questions that need to be answered. J. Antimicrob. Chemother. 70, 2693–2708 (2015).
Blas-García, A. et al. Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: a comparison with efavirenz. J. Antimicrob. Chemother. 69, 2995–3000 (2014).
Markowitz, M. et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N. Engl. J. Med. 333, 1534–1540 (1995).
Jensen, B. K. et al. Altered oligodendrocyte maturation and myelin maintenance: the role of antiretrovirals in HIV-associated neurocognitive disorders. J. Neuropathol. Exp. Neurol. 74, 1093–1118 (2015).
Vivithanaporn, P., Asahchop, E. L., Acharjee, S., Baker, G. B. & Power, C. HIV protease inhibitors disrupt astrocytic glutamate transporter function and neurobehavioral performance. AIDS 30, 543–552 (2016).
Ekins, S. et al. α7-Nicotinic acetylcholine receptor inhibition by indinavir: implications for cognitive dysfunction in treated HIV disease. AIDS 31, 1083–1089 (2017).
Soontornniyomkij, V. et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 28, 1297 (2014).
Stern, A. L. et al. Differential effects of antiretroviral drugs on neurons in vitro: roles for oxidative stress and integrated stress response. J. Neuroimmune Pharmacol. 13, 64–76 (2018).
del Mar Gutierrez, M., Mateo, M. G., Vidal, F. & Domingo, P. Drug safety profile of integrase strand transfer inhibitors. Expert Opin. Drug Saf. 13, 431–445 (2014).
Abrams, E. & Myer, L. Lessons from dolutegravir and neural tube defects. Lancet HIV 8, e3–e4 (2021).
Gray, J. & Young, B. Acute onset insomnia associated with the initiation of raltegravir: a report of two cases and literature review. AIDS Patient Care STDS 23, 689–690 (2009).
Capetti, A. et al. Morning dosing for dolutegravir-related insomnia and sleep disorders. HIV Med. 838, e62–e63 (2017).
Latronico, T. et al. In vitro effect of antiretroviral drugs on cultured primary astrocytes: analysis of neurotoxicity and matrix metalloproteinase inhibition. J. Neurochem. 144, 271–284 (2018).
Reust, C. E. Common adverse effects of antiretroviral therapy for HIV disease. Am. Fam. Physician 83, 1443–1451 (2011).
Treisman, G. J. & Soudry, O. Neuropsychiatric effects of HIV antiviral medications. Drug Saf. 39, 945–957 (2016).
Manfredi, R. & Sabbatani, S. A novel antiretroviral class (fusion inhibitors) in the management of HIV infection. Present features and future perspectives of enfuvirtide (T-20). Curr. Med. Chem. 13, 2369–2384 (2006).
LaBonte, J., Lebbos, J. & Kirkpatrick, P. Enfuvirtide. Nat. Rev. Drug Discov. 2, 345–346 (2003).
Oldfield, V., Keating, G. M. & Plosker, G. Enfuvirtide: a review of its use in the management of HIV infection. Drugs 65, 1139–1160 (2005).
Curtis, L. et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor–naive patients. HIV Clin. Trials 15, 199–208 (2014).
Harris, M., Larsen, G. & Montaner, J. S. Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS 22, 1890–1892 (2008).
Fettiplace, A. et al. Psychiatric symptoms in patients receiving dolutegravir. J. Acquir. Immune Defic. Syndr. 74, 423–431 (2017).
Harris, M. What did we learn from the bictegravir switch studies? Lancet HIV 5, e336–e337 (2018).
Zhao, Y. et al. Memantine for AIDS dementia complex: open-label report of ACTG 301. HIV Clin. Trials 11, 59–67 (2010).
Yacoub, A. D. et al. Intranasal Insulin Improves Attention and Memory in People with HIV https://www.natap.org/2021/CROI/croi_91.htm (2021).
Yadav, A. et al. Lack of atorvastatin effect on monocyte gene expression and inflammatory markers in HIV-1-infected ART-suppressed Individuals at risk of non-AIDS comorbidities. Pathog. Immun. 6, 1–26 (2021).
Zhou, F. et al. Iptakalim alleviates rotenone-induced degeneration of dopaminergic neurons through inhibiting microglia-mediated neuroinflammation. Neuropsychopharmacology 32, 2570–2580 (2007).
Cheng, L. et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Invest. 127, 269–279 (2017).
Azzoni, L. et al. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J. Infect. Dis. 207, 213–222 (2013).
Lazi, E., Burns, J. M. & Swerdlow, R. H. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol. Aging 35, 2574–2583 (2014).
Fortier, M. et al. A ketogenic drink improves cognition in mild cognitive impairment: results of a 6-month RCT. Alzheimers Dement. 17, 543–552 (2021).
Acknowledgements
This work was supported by the U.S. National Institutes of Health grants MH128108, AG066215 (Fields); K24AG075240 (Marquine); R01DA052209, R01MH104131, R01MH087332 (Kaul); R01DA056058, R01DA043430, R01MH128869 (Ellis), and R21MH134401 (Schlachetzki).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Deanna Saylor, Bruce Brew and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ellis, R.J., Marquine, M.J., Kaul, M. et al. Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management. Nat Rev Neurol 19, 668–687 (2023). https://doi.org/10.1038/s41582-023-00879-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00879-y