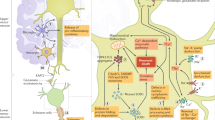
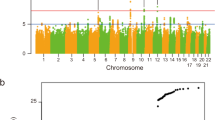
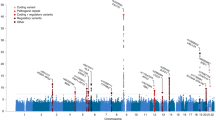
Abstract
Recent advances in sequencing technologies and collaborative efforts have led to substantial progress in identifying the genetic causes of amyotrophic lateral sclerosis (ALS). This momentum has, in turn, fostered the development of putative molecular therapies. In this Review, we outline the current genetic knowledge, emphasizing recent discoveries and emerging concepts such as the implication of distinct types of mutation, variability in mutated genes in diverse genetic ancestries and gene–environment interactions. We also propose a high-level model to synthesize the interdependent effects of genetics, environmental and lifestyle factors, and ageing into a unified theory of ALS. Furthermore, we summarize the current status of therapies developed on the basis of genetic knowledge established for ALS over the past 30 years, and we discuss how developing treatments for ALS will advance our understanding of targeting other neurological diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rowland, L. P. & Shneider, N. A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1688–1700 (2001).
Kumar, D. R., Aslinia, F., Yale, S. H. & Mazza, J. J. Jean-Martin Charcot: the father of neurology. Clin. Med. Res. 9, 46–49 (2011).
Chio, A. et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology 41, 118–130 (2013).
Xu, L. et al. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. 267, 944–953 (2020).
U.S. Food and Drug Administration. Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting [online], https://www.youtube.com/watch?v=AVVZMMvDOUg (2022).
Arthur, K. C. et al. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 7, 12408 (2016).
Achtert, K. & Kerkemeyer, L. The economic burden of amyotrophic lateral sclerosis: a systematic review. Eur. J. Health Econ. 22, 1151–1166 (2021).
Chio, A., Gauthier, A., Calvo, A., Ghiglione, P. & Mutani, R. Caregiver burden and patients’ perception of being a burden in ALS. Neurology 64, 1780–1782 (2005).
Wicks, P. The ALS Ice Bucket Challenge – can a splash of water reinvigorate a field? Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 479–480 (2014).
Sohn, E. Fundraising: the Ice Bucket Challenge delivers. Nature 550, S113–S114 (2017).
Goutman, S. A. et al. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 21, 465–479 (2022).
Renton, A. E., Chio, A. & Traynor, B. J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23 (2014).
Keller, M. F. et al. Genome-wide analysis of the heritability of amyotrophic lateral sclerosis. JAMA Neurol. 71, 1123–1134 (2014).
Al-Chalabi, A. et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. J. Neurol. Neurosurg. Psychiatry 81, 1324–1326 (2010).
Fang, F. et al. Familial aggregation of amyotrophic lateral sclerosis. Ann. Neurol. 66, 94–99 (2009).
Chia, R., Chio, A. & Traynor, B. J. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 17, 94–102 (2018).
Lopez, E. R., Borschel, W. F. & Traynor, B. J. New antisense oligonucleotide therapies reach first base in ALS. Nat. Med. 28, 25–27 (2022).
Siddique, T. et al. Linkage of a gene causing familial amyotrophic lateral sclerosis to chromosome 21 and evidence of genetic-locus heterogeneity. N. Engl. J. Med. 324, 1381–1384 (1991).
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993). This is the first study to identify SOD1 mutations as the genetic cause of familial ALS.
Abel, O., Powell, J. F., Andersen, P. M. & Al-Chalabi, A. ALSoD: a user-friendly online bioinformatics tool for amyotrophic lateral sclerosis genetics. Hum. Mutat. 33, 1345–1351 (2012).
McCann, E. P. et al. Evidence for polygenic and oligogenic basis of Australian sporadic amyotrophic lateral sclerosis. J. Med. Genet. 58, 87–95 (2021).
Andersen, P. M. et al. Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutation. A clinical and genealogical study of 36 patients. Brain 119, 1153–1172 (1996).
Parton, M. J. et al. D90A-SOD1 mediated amyotrophic lateral sclerosis: a single founder for all cases with evidence for a Cis-acting disease modifier in the recessive haplotype. Hum. Mutat. 20, 473 (2002).
Feneberg, E., Turner, M. R., Ansorge, O. & Talbot, K. Amyotrophic lateral sclerosis with a heterozygous D91A SOD1 variant and classical ALS-TDP neuropathology. Neurology 95, 595–596 (2020).
Cudkowicz, M. E. et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann. Neurol. 41, 210–221 (1997).
Berdynski, M. et al. Recurrent G41S mutation in Cu/Zn superoxide dismutase gene (SOD1) causing familial amyotrophic lateral sclerosis in a large Polish family. Amyotroph. Lateral Scler. 13, 132–136 (2012).
Berdynski, M. et al. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 12, 103 (2022).
Kuzma-Kozakiewicz, M. et al. Putative founder effect in the Polish, Iranian and United States populations for the L144S SOD1 mutation associated with slowly uniform phenotype of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 80–85 (2021).
Bernard, E. et al. Clinical and molecular landscape of ALS patients with SOD1 mutations: novel pathogenic variants and novel phenotypes. A single ALS center study. Int. J. Mol. Sci. 21, 6807 (2020).
Gamez, J. et al. Mutational analysis of the Cu/Zn superoxide dismutase gene in a Catalan ALS population: should all sporadic ALS cases also be screened for SOD1. J. Neurol. Sci. 247, 21–28 (2006).
Hou, L. et al. Screening of SOD1, FUS and TARDBP genes in patients with amyotrophic lateral sclerosis in central-southern China. Sci. Rep. 6, 32478 (2016).
Hayashi, Y., Homma, K. & Ichijo, H. SOD1 in neurotoxicity and its controversial roles in SOD1 mutation-negative ALS. Adv. Biol. Regul. 60, 95–104 (2016).
Bosco, D. A. et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 13, 1396–1403 (2010).
Raoul, C. et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat. Med. 11, 423–428 (2005).
Ralph, G. S. et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat. Med. 11, 429–433 (2005). The first study to show the therapeutic efficacy of RNAi in ALS.
Smith, R. A. et al. Antisense oligonucleotide therapy for neurodegenerative disease. J. Clin. Invest. 116, 2290–2296 (2006).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). This study was the first to identify cytoplasmic accumulation and nuclear depletion of TDP43 in motor neurons of patients with ALS.
Kabashi, E. et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 40, 572–574 (2008).
Sreedharan, J. et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 (2008). Kabashi et al. (2008) and Sreedharan et al. (2008) were the first reports of pathogenic TARDBP mutations in ALS.
Turner, M. R. et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 12, 310–322 (2013).
Besser, L. M., Teylan, M. A. & Nelson, P. T. Limbic predominant age-related TDP-43 encephalopathy (LATE): clinical and neuropathological associations. J. Neuropathol. Exp. Neurol. 79, 305–313 (2020).
Suk, T. R. & Rousseaux, M. W. C. The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol. Neurodegener. 15, 45 (2020).
Nonaka, T. et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 4, 124–134 (2013).
Nomura, T. et al. Intranuclear aggregation of mutant FUS/TLS as a molecular pathomechanism of amyotrophic lateral sclerosis. J. Biol. Chem. 289, 1192–1202 (2014).
Chia, R. et al. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS ONE 5, e10627 (2010).
Kwiatkowski, T. J. Jr et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009).
Vance, C. et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009). Kwiatkowski et al. (2009) and Vance et al. (2009) were the first reports of pathogenic FUS mutations in ALS.
Chio, A. et al. A de novo missense mutation of the FUS gene in a “true” sporadic ALS case. Neurobiol. Aging 32, 553 e523–553 e556 (2011).
Suzuki, N. et al. FALS with FUS mutation in Japan, with early onset, rapid progress and basophilic inclusion. J. Hum. Genet. 55, 252–254 (2010).
Lattante, S., Rouleau, G. A. & Kabashi, E. TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update. Hum. Mutat. 34, 812–826 (2013).
Hubers, A. et al. De novo FUS mutations are the most frequent genetic cause in early-onset German ALS patients. Neurobiol. Aging 36, 3117 e3111–3117 e3116 (2015).
Kim, S. H., Shanware, N. P., Bowler, M. J. & Tibbetts, R. S. Amyotrophic lateral sclerosis-associated proteins TDP-43 and FUS/TLS function in a common biochemical complex to co-regulate HDAC6 mRNA. J. Biol. Chem. 285, 34097–34105 (2010).
Khalfallah, Y. et al. TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Sci. Rep. 8, 7551 (2018).
Bosco, D. A. et al. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet. 19, 4160–4175 (2010).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011). Renton et al. (2011) and DeJesus-Hernandez et al. (2011) were the first studies that identified GGGGCC repeat expansion in C9orf72 in ALS and FTD.
Majounie, E. et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 11, 323–330 (2012). This study was among the first studies to evaluate the genetics of ALS and FTD globally and across populations of different ancestries.
Dols-Icardo, O. et al. Characterization of the repeat expansion size in C9orf72 in amyotrophic lateral sclerosis and frontotemporal dementia. Hum. Mol. Genet. 23, 749–754 (2014).
Ebbert, M. T. W. et al. Long-read sequencing across the C9orf72 ‘GGGGCC’ repeat expansion: implications for clinical use and genetic discovery efforts in human disease. Mol. Neurodegener. 13, 46 (2018).
van der Ende, E. L. et al. Unravelling the clinical spectrum and the role of repeat length in C9ORF72 repeat expansions. J. Neurol. Neurosurg. Psychiatry 92, 502–509 (2021).
Gijselinck, I. et al. The C9orf72 repeat size correlates with onset age of disease, DNA methylation and transcriptional downregulation of the promoter. Mol. Psychiatry 21, 1112–1124 (2016).
Nordin, A. et al. Extensive size variability of the GGGGCC expansion in C9orf72 in both neuronal and non-neuronal tissues in 18 patients with ALS or FTD. Hum. Mol. Genet. 24, 3133–3142 (2015).
Beck, J. et al. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am. J. Hum. Genet. 92, 345–353 (2013).
Xu, Z. et al. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc. Natl Acad. Sci. USA 110, 7778–7783 (2013).
Ash, P. E. et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77, 639–646 (2013).
Balendra, R. & Isaacs, A. M. C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat. Rev. Neurol. 14, 544–558 (2018).
Fumagalli, L. et al. C9orf72-derived arginine-containing dipeptide repeats associate with axonal transport machinery and impede microtubule-based motility. Sci. Adv. 7, eabg3013 (2021).
Shi, Y. et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 24, 313–325 (2018).
Abramzon, Y. A., Fratta, P., Traynor, B. J. & Chia, R. The overlapping genetics of amyotrophic lateral sclerosis and frontotemporal dementia. Front. Neurosci. 14, 42 (2020).
Majounie, E. et al. Repeat expansion in C9ORF72 in Alzheimer’s disease. N. Engl. J. Med. 366, 283–284 (2012).
Kenna, K. P. et al. NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 48, 1037–1042 (2016).
Nicolas, A. et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron 97, 1268–1283 e1266 (2018).
Brenner, D. et al. Hot-spot KIF5A mutations cause familial ALS. Brain 141, 688–697 (2018). Nicolas et al. (2018) and Brenner et al. (2018) were the first reports of pathogenic KIF5A mutations in ALS.
Saez-Atienzar, S. et al. Identification of a pathogenic intronic KIF5A mutation in an ALS–FTD kindred. Neurology 95, 1015–1018 (2020).
Baron, D. M. et al. ALS-associated KIF5A mutations abolish autoinhibition resulting in a toxic gain of function. Cell Rep. 39, 110598 (2022).
Castellanos-Montiel, M. J., Chaineau, M. & Durcan, T. M. The neglected genes of ALS: cytoskeletal dynamics impact synaptic degeneration in ALS. Front. Cell Neurosci. 14, 594975 (2020).
Wenting, G., Laura, F. & Ludo Van Den, B. in Amyotrophic Lateral Sclerosis (ed. Hegde, L. M.) (IntechOpen, 2020).
Tunca, C. et al. ERLIN1 mutations cause teenage-onset slowly progressive ALS in a large Turkish pedigree. Eur. J. Hum. Genet. 26, 745–748 (2018).
Zhu, Z. Y. et al. A novel homozygous mutation in ERLIN1 gene causing spastic paraplegia 62 and literature review. Eur. J. Med. Genet. 65, 104608 (2022).
Novarino, G. et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science 343, 506–511 (2014).
Egorova, P. A. & Bezprozvanny, I. B. Inositol 1,4,5-trisphosphate receptors and neurodegenerative disorders. FEBS J. 285, 3547–3565 (2018).
Higo, T. et al. Mechanism of ER stress-induced brain damage by IP3 receptor. Neuron 68, 865–878 (2010).
Casey, J. P. et al. A novel gain-of-function mutation in the ITPR1 suppressor domain causes spinocerebellar ataxia with altered Ca2+ signal patterns. J. Neurol. 264, 1444–1453 (2017).
Farhan, S. M. K. et al. Exome sequencing in amyotrophic lateral sclerosis implicates a novel gene, DNAJC7, encoding a heat-shock protein. Nat. Neurosci. 22, 1966–1974 (2019).
Jih, K. Y., Tsai, P. C., Tsai, Y. S., Liao, Y. C. & Lee, Y. C. Rapid progressive ALS in a patient with a DNAJC7 loss-of-function mutation. Neurol. Genet. 6, e503 (2020).
He, J. et al. Validation of the pathogenic role of rare DNAJC7 variants in Chinese patients with amyotrophic lateral sclerosis. Neurobiol. Aging 106, 314 e311–314 e316 (2021).
Wang, M. et al. A novel potentially pathogenic rare variant in the DNAJC7 gene identified in amyotrophic lateral sclerosis patients from mainland China. Front. Genet. 11, 821 (2020).
Tohnai, G. et al. Mutation screening of the DNAJC7 gene in Japanese patients with sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 113, 131–136 (2022).
Dilliott, A. A. et al. DnaJC7 in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 23, 4076 (2022).
Jinwal, U. K. et al. Cdc37/Hsp90 protein complex disruption triggers an autophagic clearance cascade for TDP-43 protein. J. Biol. Chem. 287, 24814–24820 (2012).
Dewan, R. et al. Pathogenic huntingtin repeat expansions in patients with frontotemporal dementia and amyotrophic lateral sclerosis. Neuron 109, 448–460 e444 (2021).
Hickman, R. A. et al. Amyotrophic lateral sclerosis is over-represented in two Huntington’s disease brain bank cohorts: further evidence to support genetic pleiotropy of pathogenic HTT gene expansion. Acta Neuropathol. 143, 105–108 (2022).
Dewan, R. et al. CAG somatic instability in a Huntington disease expansion carrier presenting with a progressive supranuclear palsy-like phenotype. Mov. Disord. 37, 1555–1557 (2022).
Grima, J. C. et al. Mutant huntingtin disrupts the nuclear pore complex. Neuron 94, 93–107 e106 (2017).
Freibaum, B. D. et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 525, 129–133 (2015).
Tabrizi, S. J. et al. Targeting huntingtin expression in patients with Huntington’s disease. N. Engl. J. Med. 380, 2307–2316 (2019).
Johnson, J. O. et al. Association of variants in the SPTLC1 gene with juvenile amyotrophic lateral sclerosis. JAMA Neurol. 78, 1236–1248 (2021).
Mohassel, P. et al. Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat. Med. 27, 1197–1204 (2021).
Bejaoui, K. et al. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat. Genet. 27, 261–262 (2001).
Penno, A. et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285, 11178–11187 (2010).
Garofalo, K. et al. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Invest. 121, 4735–4745 (2011).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47, D1005–D1012 (2019).
Schymick, J. C. et al. Genome-wide genotyping in amyotrophic lateral sclerosis and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 6, 322–328 (2007).
van Es, M. A. et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat. Genet. 41, 1083–1087 (2009).
Laaksovirta, H. et al. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol. 9, 978–985 (2010).
Shatunov, A. et al. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 9, 986–994 (2010).
van Rheenen, W. et al. Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 48, 1043–1048 (2016).
Benyamin, B. et al. Cross-ethnic meta-analysis identifies association of the GPX3–TNIP1 locus with amyotrophic lateral sclerosis. Nat. Commun. 8, 611 (2017).
van Rheenen, W. et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat. Genet. 53, 1636–1648 (2021).
Fogh, I. et al. A genome-wide association meta-analysis identifies a novel locus at 17q11.2 associated with sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 23, 2220–2231 (2014).
Nakamura, R. et al. A multi-ethnic meta-analysis identifies novel genes, including ACSL5, associated with amyotrophic lateral sclerosis. Commun. Biol. 3, 526 (2020).
Cirulli, E. T. et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347, 1436–1441 (2015).
Freischmidt, A. et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631–636 (2015).
Brenner, D. et al. NEK1 mutations in familial amyotrophic lateral sclerosis. Brain 139, e28 (2016).
Saez-Atienzar, S. et al. Genetic analysis of amyotrophic lateral sclerosis identifies contributing pathways and cell types. Sci. Adv. 7, eabd9036 (2021).
Ho, W. Y. et al. The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy. Autophagy 15, 827–842 (2019).
Yang, M. et al. A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci. Adv. 2, e1601167 (2016).
Maekawa, S. et al. Cortical selective vulnerability in motor neuron disease: a morphometric study. Brain 127, 1237–1251 (2004).
Maniatis, S. et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364, 89–93 (2019).
Humphrey, J. et al. Integrative transcriptomic analysis of the amyotrophic lateral sclerosis spinal cord implicates glial activation and suggests new risk genes. Nat. Neurosci. 26, 150–162 (2023).
Lee, H. et al. Multi-omic analysis of selectively vulnerable motor neuron subtypes implicates altered lipid metabolism in ALS. Nat. Neurosci. 24, 1673–1685 (2021).
Tazelaar, G. H. P. et al. ATXN1 repeat expansions confer risk for amyotrophic lateral sclerosis and contribute to TDP-43 mislocalization. Brain Commun. 2, fcaa064 (2020).
Elden, A. C. et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466, 1069–1075 (2010).
Daoud, H. et al. Association of long ATXN2 CAG repeat sizes with increased risk of amyotrophic lateral sclerosis. Arch. Neurol. 68, 739–742 (2011).
Glass, J. D. et al. ATXN2 intermediate expansions in amyotrophic lateral sclerosis. Brain 145, 2671–2676 (2022).
Blauw, H. M. et al. NIPA1 polyalanine repeat expansions are associated with amyotrophic lateral sclerosis. Hum. Mol. Genet. 21, 2497–2502 (2012).
Al Khleifat, A. et al. Structural variation analysis of 6,500 whole genome sequences in amyotrophic lateral sclerosis. NPJ Genom. Med. 7, 8 (2022).
Ross, J. P., Dion, P. A. & Rouleau, G. A. in Spectrums of Amyotrophic Lateral Sclerosis (eds Shaw, C. A. & Morrice, J. R.) 17–34 (Wiley, 2021).
Valdmanis, P. N. et al. A mutation that creates a pseudoexon in SOD1 causes familial ALS. Ann. Hum. Genet. 73, 652–657 (2009).
Morgan, S. et al. A comprehensive analysis of rare genetic variation in amyotrophic lateral sclerosis in the UK. Brain 140, 1611–1618 (2017).
Eitan, C. et al. Whole-genome sequencing reveals that variants in the Interleukin 18 Receptor Accessory Protein 3’UTR protect against ALS. Nat. Neurosci. 25, 433–445 (2022).
Birve, A. et al. A novel SOD1 splice site mutation associated with familial ALS revealed by SOD activity analysis. Hum. Mol. Genet. 19, 4201–4206 (2010).
Kawata, A. et al. Aberrant splicing of human Cu/Zn superoxide dismutase (SOD1) RNA transcripts. Neuroreport 11, 2649–2653 (2000).
Jeong, Y. H. et al. Tdp-43 cryptic exons are highly variable between cell types. Mol. Neurodegener. 12, 13 (2017).
Ling, J. P., Pletnikova, O., Troncoso, J. C. & Wong, P. C. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 349, 650–655 (2015).
Brown, A. L. et al. TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature 603, 131–137 (2022).
Alexander, M. D. et al. “True” sporadic ALS associated with a novel SOD-1 mutation. Ann. Neurol. 52, 680–683 (2002).
Muller, K. et al. De novo mutations in SOD1 are a cause of ALS. J. Neurol. Neurosurg. Psychiatry 93, 201–206 (2022).
Garcia-Montojo, M., Doucet-O’Hare, T., Henderson, L. & Nath, A. Human endogenous retrovirus-K (HML-2): a comprehensive review. Crit. Rev. Microbiol. 44, 715–738 (2018).
MacGowan, D. J. et al. A controlled study of reverse transcriptase in serum and CSF of HIV-negative patients with ALS. Neurology 68, 1944–1946 (2007).
Li, W. et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci. Transl. Med. 7, 307ra153 (2015).
Garcia-Montojo, M. et al. Inhibition of HERV-K (HML-2) in amyotrophic lateral sclerosis patients on antiretroviral therapy. J. Neurol. Sci. 423, 117358 (2021).
Watts, G. D. et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381 (2004).
Johnson, J. O. et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68, 857–864 (2010).
Chio, A. et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 93, e984–e994 (2019).
Dupuis, L. et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70, 1004–1009 (2008).
Abdel-Khalik, J. et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J. Lipid Res. 58, 267–278 (2017).
Dodge, J. C. et al. Neutral lipid cacostasis contributes to disease pathogenesis in amyotrophic lateral sclerosis. J. Neurosci. 40, 9137–9147 (2020).
Andres-Benito, P. et al. Lipid alterations in human frontal cortex in ALS-FTLD-TDP43 proteinopathy spectrum are partly related to peroxisome impairment. Neuropathol. Appl. Neurobiol. 47, 544–563 (2021).
Ikeda, K., Hirayama, T., Takazawa, T., Kawabe, K. & Iwasaki, Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern. Med. 51, 1501–1508 (2012).
Thompson, A. G., Talbot, K. & Turner, M. R. Higher blood high density lipoprotein and apolipoprotein A1 levels are associated with reduced risk of developing amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 93, 75–81 (2022).
Cutler, R. G., Pedersen, W. A., Camandola, S., Rothstein, J. D. & Mattson, M. P. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann. Neurol. 52, 448–457 (2002).
Theofilopoulos, S. et al. Cholestenoic acids regulate motor neuron survival via liver X receptors. J. Clin. Invest. 124, 4829–4842 (2014).
Dodge, J. C. et al. Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 112, 8100–8105 (2015).
D’Amico, E. et al. Metabolic abnormalities, dietary risk factors and nutritional management in amyotrophic lateral sclerosis. Nutrients 13, 2273 (2021).
Chio, A. et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology 73, 1681–1685 (2009).
Yang, J. W. et al. Hypolipidemia in patients with amyotrophic lateral sclerosis: a possible gender difference? J. Clin. Neurol. 9, 125–129 (2013).
Sutedja, N. A. et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 82, 638–642 (2011).
Hartmann, H., Ho, W. Y., Chang, J. C. & Ling, S. C. Cholesterol dyshomeostasis in amyotrophic lateral sclerosis: cause, consequence, or epiphenomenon? FEBS J. 289, 7688–7709 (2022).
Vasta, R., Chia, R., Traynor, B. J. & Chio, A. Unraveling the complex interplay between genes, environment, and climate in ALS. EBioMedicine 75, 103795 (2022).
Bandres-Ciga, S. et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann. Neurol. 85, 470–481 (2019).
Goutman, S. A. et al. Metabolomics identifies shared lipid pathways in independent amyotrophic lateral sclerosis cohorts. Brain 145, 4425–4439 (2022).
Zeng, P., Wang, T., Zheng, J. & Zhou, X. Causal association of type 2 diabetes with amyotrophic lateral sclerosis: new evidence from Mendelian randomization using GWAS summary statistics. BMC Med. 17, 225 (2019).
Zhang, L., Tang, L., Huang, T. & Fan, D. Association between type 2 diabetes and amyotrophic lateral sclerosis. Sci. Rep. 12, 2544 (2022).
Chen, H. et al. Type 2 diabetes mellitus and amyotrophic lateral sclerosis: genetic overlap, causality, and mediation. J. Clin. Endocrinol. Metab. 106, e4497–e4508 (2021).
Julian, T. H. et al. A review of Mendelian randomization in amyotrophic lateral sclerosis. Brain 145, 832–842 (2022).
D’Ovidio, F. et al. The role of pre-morbid diabetes on developing amyotrophic lateral sclerosis. Eur. J. Neurol. 25, 164–170 (2018).
Nakken, O., Meyer, H. E., Stigum, H. & Holmoy, T. High BMI is associated with low ALS risk: a population-based study. Neurology 93, e424–e432 (2019).
Vasta, R., D’Ovidio, F., Logroscino, G. & Chio, A. The links between diabetes mellitus and amyotrophic lateral sclerosis. Neurol. Sci. 42, 1377–1387 (2021).
Reid, E. et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am. J. Hum. Genet. 71, 1189–1194 (2002).
Crimella, C. et al. Mutations in the motor and stalk domains of KIF5A in spastic paraplegia type 10 and in axonal Charcot–Marie-Tooth type 2. Clin. Genet. 82, 157–164 (2012).
Cronin, S., Hardiman, O. & Traynor, B. J. Ethnic variation in the incidence of ALS: a systematic review. Neurology 68, 1002–1007 (2007).
Zou, Z. Y. et al. Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 88, 540–549 (2017).
Pliner, H. A., Mann, D. M. & Traynor, B. J. Searching for Grendel: origin and global spread of the C9ORF72 repeat expansion. Acta Neuropathol. 127, 391–396 (2014).
Suzuki, N., Nishiyama, A., Warita, H. & Aoki, M. Genetics of amyotrophic lateral sclerosis: seeking therapeutic targets in the era of gene therapy. J. Hum. Genet. 68, 131–152 (2023).
Dombroski, B. A. et al. C9orf72 hexanucleotide repeat expansion and Guam amyotrophic lateral sclerosis-Parkinsonism-dementia complex. JAMA Neurol. 70, 742–745 (2013).
Logroscino, G. & Piccininni, M. Amyotrophic lateral sclerosis descriptive epidemiology: the origin of geographic difference. Neuroepidemiology 52, 93–103 (2019).
Chio, A. et al. Large proportion of amyotrophic lateral sclerosis cases in Sardinia due to a single founder mutation of the TARDBP gene. Arch. Neurol. 68, 594–598 (2011).
Chadi, G. et al. Genetic analysis of patients with familial and sporadic amyotrophic lateral sclerosis in a Brazilian Research Center. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 249–255 (2017).
Johnson, J. O. et al. Exome sequencing reveals riboflavin transporter mutations as a cause of motor neuron disease. Brain 135, 2875–2882 (2012).
Green, P. et al. Brown–Vialetto–Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in c20orf54. Am. J. Hum. Genet. 86, 485–489 (2010).
Bandres-Ciga, S., Noyce, A. J. & Traynor, B. J. Mendelian randomization — a journey from obscurity to center stage with a few potholes along the way. JAMA Neurol. 77, 7–8 (2020).
Pingault, J. B. et al. Using genetic data to strengthen causal inference in observational research. Nat. Rev. Genet. 19, 566–580 (2018).
Ebbert, M. T. W., Lank, R. J. & Belzil, V. V. An epigenetic spin ALS FTD. Adv. Neurobiol. 20, 1–29 (2018).
Bennett, S. A., Tanaz, R., Cobos, S. N. & Torrente, M. P. Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease. Transl. Res. 204, 19–30 (2019).
Dolinar, A., Ravnik-Glavac, M. & Glavac, D. Epigenetic mechanisms in amyotrophic lateral sclerosis: a short review. Mech. Ageing Dev. 174, 103–110 (2018).
Hop, P. J. et al. Genome-wide study of DNA methylation shows alterations in metabolic, inflammatory, and cholesterol pathways in ALS. Sci. Transl. Med. 14, eabj0264 (2022).
McMillan, C. T. et al. C9orf72 promoter hypermethylation is neuroprotective: neuroimaging and neuropathologic evidence. Neurology 84, 1622–1630 (2015).
Butovsky, O. et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 77, 75–99 (2015).
Rothman, K. J. Causes. Am. J. Epidemiol. 104, 587–592 (1976).
Alfahad, T. & Nath, A. Retroviruses and amyotrophic lateral sclerosis. Antivir. Res. 99, 180–187 (2013).
Van Damme, P., Robberecht, W. & Van Den Bosch, L. Modelling amyotrophic lateral sclerosis: progress and possibilities. Dis. Model. Mech. 10, 537–549 (2017).
Al-Chalabi, A. et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 13, 1108–1113 (2014).
Traynor, B. J. & Singleton, A. B. Nature versus nurture: death of a dogma, and the road ahead. Neuron 68, 196–200 (2010).
Boyle, E. A., Li, Y. I. & Pritchard, J. K. An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177–1186 (2017).
Bensimon, G., Lacomblez, L. & Meininger, V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 330, 585–591 (1994).
Writing, G. & Edaravone, A. L. S. S. G. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Sufit, R. L., Ajroud-Driss, S., Casey, P. & Kessler, J. A. Open label study to assess the safety of VM202 in subjects with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 269–278 (2017).
Korobeynikov, V. A., Lyashchenko, A. K., Blanco-Redondo, B., Jafar-Nejad, P. & Shneider, N. A. Antisense oligonucleotide silencing of FUS expression as a therapeutic approach in amyotrophic lateral sclerosis. Nat. Med. 28, 104–116 (2022).
Mullard, A. ALS antisense drug falters in phase III. Nat. Rev. Drug Discov. 20, 883–885 (2021).
Biogen Investor Relations. New 12-Month Tofersen Data Presented at ENCALS Meeting Show Clinically Meaningful Benefit in People With SOD1-ALS [Online], https://investors.biogen.com/news-releases/news-release-details/new-12-month-tofersen-data-presented-encals-meeting-show (2022).
Tabrizi, S. J. et al. Potential disease-modifying therapies for Huntington’s disease: lessons learned and future opportunities. Lancet Neurol. 21, 645–658 (2022).
Benatar, M. et al. Design of a randomized, placebo-controlled, phase 3 trial of tofersen initiated in clinically presymptomatic SOD1 variant carriers: the ATLAS study. Neurotherapeutics 19, 1248–1258 (2022).
Tran, H. et al. Suppression of mutant C9orf72 expression by a potent mixed backbone antisense oligonucleotide. Nat. Med. 28, 117–124 (2022).
Becker, L. A. et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 544, 367–371 (2017).
Sullivan, J. M. et al. Convective forces increase rostral delivery of intrathecal radiotracers and antisense oligonucleotides in the cynomolgus monkey nervous system. J. Transl. Med. 18, 309 (2020).
Belov, V. et al. Large-volume intrathecal administrations: impact on CSF pressure and safety implications. Front. Neurosci. 15, 604197 (2021).
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Nagata, T. et al. Cholesterol-functionalized DNA/RNA heteroduplexes cross the blood–brain barrier and knock down genes in the rodent CNS. Nat. Biotechnol. 39, 1529–1536 (2021).
Gennemark, P. et al. An oral antisense oligonucleotide for PCSK9 inhibition. Sci. Transl. Med. 13, eabe9117 (2021).
Figlewicz, D. A. et al. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum. Mol. Genet. 3, 1757–1761 (1994).
Yang, Y. et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat. Genet. 29, 160–165 (2001).
Puls, I. et al. Mutant dynactin in motor neuron disease. Nat. Genet. 33, 455–456 (2003).
Nishimura, A. L. et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 75, 822–831 (2004).
Chen, Y. Z. et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am. J. Hum. Genet. 74, 1128–1135 (2004).
Parkinson, N. et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67, 1074–1077 (2006).
Skibinski, G. et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 37, 806–808 (2005).
Orlacchio, A. et al. SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain 133, 591–598 (2010).
Maruyama, H. et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223–226 (2010).
Deng, H. X. et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477, 211–215 (2011).
Fecto, F. et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 68, 1440–1446 (2011).
Wu, C. H. et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature 488, 499–503 (2012).
Kim, H. J. et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 (2013).
Smith, B. N. et al. Exome-wide rare variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron 84, 324–331 (2014).
Johnson, J. O. et al. Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat. Neurosci. 17, 664–666 (2014).
Bannwarth, S. et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain 137, 2329–2345 (2014).
Williams, K. L. et al. CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat. Commun. 7, 11253 (2016).
Smith, B. N. et al. Mutations in the vesicular trafficking protein annexin A11 are associated with amyotrophic lateral sclerosis. Sci. Transl. Med. 9, eaad9157 (2017).
Cooper-Knock, J. et al. Mutations in the glycosyltransferase domain of GLT8D1 are associated with familial amyotrophic lateral sclerosis. Cell Rep. 26, 2298–2306 e2295 (2019).
Grassano, M. et al. Systematic evaluation of genetic mutations in ALS: a population-based study. J. Neurol. Neurosurg. Psychiatry 93, 1190–1193 (2022).
McCann, E. P. et al. The genotype-phenotype landscape of familial amyotrophic lateral sclerosis in Australia. Clin. Genet. 92, 259–266 (2017).
Smith, B. N. et al. The C9ORF72 expansion mutation is a common cause of ALS+/− FTD in Europe and has a single founder. Eur. J. Hum. Genet. 21, 102–108 (2013).
Wittrup, A. & Lieberman, J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 16, 543–552 (2015).
Shahryari, A. et al. Development and clinical translation of approved gene therapy products for genetic disorders. Front. Genet. 10, 868 (2019).
Kwok, A., Raulf, N. & Habib, N. Developing small activating RNA as a therapeutic: current challenges and promises. Ther. Deliv. 10, 151–164 (2019).
Wengert, E. R. et al. Targeted augmentation of nuclear gene output (TANGO) of Scn1a rescues parvalbumin interneuron excitability and reduces seizures in a mouse model of Dravet Syndrome. Brain Res. 1775, 147743 (2022).
Sanders, R. FDA approves first test of CRISPR to correct genetic defect causing sickle cell disease [online], https://news.berkeley.edu/2021/03/30/fda-approves-first-test-of-crispr-to-correct-genetic-defect-causing-sickle-cell-disease/ (2021).
Frangoul, H. et al. CRISPR–Cas9 gene editing for sickle cell disease and beta-thalassemia. N. Engl. J. Med. 384, 252–260 (2021).
No authors listed. FDA approves hereditary blindness gene therapy. Nat. Biotechnol. 36, 6 (2018).
Acknowledgements
This work was supported by the NIH Intramural Research Program, the US National Institute on Aging (NIA) grant Z01-AG000949-02, the US National Institute of Neurological Disorders and Stroke (NINDS) grant NS03130 and the US National Center for Advancing Translational Sciences (NCATS).
Author information
Authors and Affiliations
Contributions
All authors wrote the manuscript, researched content for the article, substantially contributed to discussion of content, and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
B.J.T. holds a patent on the diagnostic and therapeutic applications of the pathogenic repeat expansion in C9orf72. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Karen Morrison, who co-reviewed with Gavin McCluskey, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Answer ALS: https://www.answerals.org/
Clinical trial information for Engensis (VM202): https://alsnewstoday.com/vm202/
dbGaP: https://www.ncbi.nlm.nih.gov/gap/
N=1 Collaborative network: https://www.n1collaborative.org/
New York Genome Center ALS Consortium: https://www.nygenome.org/als-consortium/
Online Mendelian Inheritance in Man: https://www.omim.org/
Project MinE: https://www.projectmine.com/
Safety study of VM202 to treat amyotrophic lateral sclerosis: https://clinicaltrials.gov/ct2/show/NCT02039401
Glossary
- De novo mutations
-
Mutations not inherited from either parent and present for the first time in a family.
- End point
-
The targeted outcome of a clinical trial to determine the efficacy and safety of the therapy. The amyotrophic lateral sclerosis functional rating — revised (ALSFRS-R) score is the commonly used primary end point for ALS clinical trials.
- Enrichment analysis
-
A statistical approach to identify over-represented or differentially expressed genes or biological pathways contributing to a phenotype.
- Familial aggregation studies
-
Methods to assess the relative risk of a trait in affected family members compared with unaffected family members. Familial aggregation is the tendency for a trait to occur or cluster in multiple family members beyond what would be expected by chance.
- Founder effect
-
The reduction in genetic diversity when a newly established population is descendent from a small number of ancestors.
- Genome-wide association studies
-
(GWAS). Studies in which genetic variants across the genome are genotyped and tested for association with a trait.
- Haploinsufficiency
-
A situation in which a diploid organism has only one copy of a gene when both copies are required for proper function.
- Hydrocephalus
-
Abnormal cerebrospinal fluid in the ventricles of the brain.
- Linkage analysis
-
An approach that examines the co-segregation of the chromosomal region (or regions) with traits of interest, usually designed to localize disease-causing genes in co-segregating segments.
- Mendelian randomization
-
The use of genetic variation to address the causal effect of one trait on another, minimizing the issues of confounding and reverse causality.
- Mimic syndromes
-
A diverse group of conditions, the clinical features of which may resemble amyotrophic lateral sclerosis.
- Missing heritability
-
The discrepancy between the heritability explained by trait-associated genetic variants from genome-wide association studies and the amount of total heritability from familial aggregation or twin studies.
- Prion-like motifs
-
Unstructured protein domains that can undergo conformational switches in response to environmental stress. Their phase transition activity renders RNA-binding proteins prone to misfolding in neurodegenerative diseases.
- Segregation analysis
-
A study that examines the carrier status of a genetic variant in the affected and unaffected members of a family. It is used to validate whether a specific genetic variant is causal for the disease in a family and the established mode of inheritance based on the pedigree structure.
- Twin studies
-
Studies that compare the resemblance of monozygotic (identical) and dizygotic (non-identical) twins. An increased concordance rate in monozygotic twins compared with dizygotic twins provides evidence of a genetic component.
- Whole-exome sequencing
-
Targeted sequencing method that selectively determines exome sequences, which are highly enriched for protein-coding regions.
- Whole-genome sequencing
-
Sequencing method that examines the entire genome of an individual.
Rights and permissions
About this article
Cite this article
Akçimen, F., Lopez, E.R., Landers, J.E. et al. Amyotrophic lateral sclerosis: translating genetic discoveries into therapies. Nat Rev Genet 24, 642–658 (2023). https://doi.org/10.1038/s41576-023-00592-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-023-00592-y
This article is cited by
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Translating the ALS Genetic Revolution into Therapies: A Review
Current Treatment Options in Neurology (2024)
-
Molecular hallmarks of ageing in amyotrophic lateral sclerosis
Cellular and Molecular Life Sciences (2024)
-
High serum uric acid levels are protective against cognitive impairment in amyotrophic lateral sclerosis
Journal of Neurology (2024)