Abstract
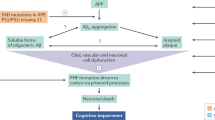
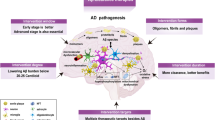
On 7 June 2021, aducanumab was granted accelerated approval for the treatment of Alzheimer disease (AD) by the FDA on the basis of amyloid-lowering effects considered reasonably likely to confer clinical benefit. This decision makes aducanumab the first new drug to be approved for the treatment of AD since 2003 and the first drug to ever be approved for modification of the course of AD. Many have questioned how scientific evidence, expert advice and the best interests of patients and families were considered in the approval decision. In this article, we argue that prior to approval, the FDA and Biogen’s shared interpretation of clinical trial data — that high-dose aducanumab was substantially clinically effective — avoided conventional scientific scrutiny, was prominently advanced by patient representative groups who had been major recipients of Biogen funds, and raised concerns that safeguards were insufficient to mitigate regulatory capture within the FDA. Here, we reflect on events leading to the FDA’s decision on 7 June 2021 and consider whether any lessons can be learned for the field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Alzheimer’s Disease International. Numbers of people with dementia around the world. https://www.alzint.org/u/numbers-people-with-dementia-2017.pdf (2020).
Rizzi, L., Rosset, I. & Roriz-Cruz, M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed. Res. Int. 2014, 908915 (2014).
Hyman, B. T. et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13 (2012).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Cavazzoni, P. FDA’s decision to approve new treatment for Alzheimer’s disease. FDA https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease (2021).
Howard, R. & Liu, K. Y. Questions EMERGE as Biogen claims aducanumab turnaround. Nat. Rev. Neurol. 16, 63–64 (2020).
Knopman, D. S., Jones, D. T. & Greicius, M. D. Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 17, 696–701 (2020).
Schneider, L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 19, 111–112 (2020).
Alexander, G. C., Emerson, S. & Kesselheim, A. S. Evaluation of aducanumab for Alzheimer disease: scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA 325, 1717–1718 (2021).
Liu, K. Y., Schneider, L. S. & Howard, R. The need to show minimum clinically important differences in Alzheimer’s disease trials. Lancet Psychiatry https://doi.org/10.1016/S2215-0366(21)00197-8 (2021).
Hollmann, P. & Lundebjerg, N. E. Letter. https://www.americangeriatrics.org/sites/default/files/inline-files/American%20Geriatrics%20Society_Letter%20to%20FDA%20Biogen%20Drug%20for%20Alzheimer%27s%20%28June%202021%29%20FINAL%20%281%29.pdf (Hollmann and Lundebjerg to Woodcock, 2 June 2021).
Mahase, E. Three FDA advisory panel members resign over approval of Alzheimer’s drug. BMJ 373, n1503 (2021).
US Food and Drug Administration Center for Drug Evaluation and Research. Final summary minutes of the Peripheral and Central Nervous System Drugs Advisory Committee meeting (aducanumab). https://www.fda.gov/media/145690/download (2020).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Neurimmune. Neurimmune receives major development milestone upon initiation of global phase 3 studies with aducanumab for early Alzheimer’s disease. Neurimmune https://www.neurimmune.com/news/neurimmune-receives-major-development-milestone-upon-initiation-of-global-phase-3-studies-with-aducanumab-for-early-alzheimers-disease (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01397539 (2015).
Ferrero, J. et al. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2, 169–176 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01677572 (2020).
Sperling, R. A. et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 7, 367–385 (2011).
Alzforum. Much ‘adu’ about a little: phase 1 data feeds the buzz at CTAD. Alzforum https://www.alzforum.org/news/conference-coverage/much-adu-about-little-phase-1-data-feeds-buzz-ctad (2016).
US Food and Drug Administration. Peripheral and Central Nervous System (PCNS) Drugs Advisory Committee Meeting November 6, 2020. NDA/BLA# 761178: Aducanumab. Combined FDA and Applicant PCNS Advisory Committee Briefing Document. https://www.fda.gov/media/143502/download (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02477800 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02484547 (2021).
Biogen. Biogen and Eisai to discontinue phase 3 ENGAGE and EMERGE trials of aducanumab in Alzheimer’s disease. Biogen https://investors.biogen.com/news-releases/news-release-details/biogen-and-eisai-discontinue-phase-3-engage-and-emerge-trials (2019).
Salloway, S. et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 370, 322–333 (2014).
Roche. Roche provides update on gantenerumab development programme. Roche https://www.roche.com/media/releases/med-cor-2014-12-19b.htm (2014).
Salloway, S. et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat. Med. 27, 1187–1196 (2021).
Honig, L. S. et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med. 378, 321–330 (2018).
Roche. Roche to discontinue phase III CREAD 1 and 2 clinical studies of crenezumab in early Alzheimer’s disease (AD) - other company programmes in AD continue. Roche https://www.roche.com/media/releases/med-cor-2019-01-30.htm (2019).
Panza, F., Lozupone, M., Logroscino, G. & Imbimbo, B. P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15, 73–88 (2019).
US Food and Drug Administration. Early Alzheimer’s disease: developing drugs for treatment guidance for industry. FDA https://www.fda.gov/media/110903/download (2018).
Biogen. Biogen plans regulatory filing for aducanumab in Alzheimer’s disease based on new analysis of larger dataset from phase 3 studies. Biogen https://investors.biogen.com/news-releases/news-release-details/biogen-plans-regulatory-filing-aducanumab-alzheimers-disease (2019).
US Food and Drug Administration Center for Drug Evaluation and Research. Peripheral and Central Nervous System Drugs Advisory Committee (PCNS) meeting transcript. FDA https://www.fda.gov/media/145691/download (2020).
Haeberlein. S. B. et al. EMERGE and ENGAGE topline results: two phase 3 studies to evaluate aducanumab in patients with early Alzheimer’s disease. investors.biogen.com https://investors.biogen.com/static-files/ddd45672-9c7e-4c99-8a06-3b557697c06f (2019).
Bulik, B. S. Celeb-backed Alzheimer’s Association campaign aims to build grassroots support for Biogen’s aducanumab ahead of FDA decision. Fierce Pharma https://www.fiercepharma.com/marketing/alzheimer-s-association-campaign-more-time-supports-biogen-s-aducanumab-awaiting-fda (2021).
Cummings, J. et al. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res. Ther. 13, 98 (2021).
Dunn, B., Stein, P. & Cavazzoni, P. Approval of aducanumab for Alzheimer disease–the FDA’s perspective. JAMA Intern. Med. https://doi.org/10.1001/jamainternmed.2021.4607 (2021).
Herman, B. Biogen pulled Aduhelm paper after JAMA demanded edits Axios https://www.axios.com/biogen-jama-aduhelm-clinical-trial-results-publish-fc7c2876-a684-4bfc-8462-4165f57d735a.htmlA (2021).
Andrews, J. S. et al. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimers Dement. 5, 354–363 (2019).
Birks, J. S. & Harvey, R. J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 6, CD001190 (2018).
Jutten, R. J. et al. Finding treatment effects in Alzheimer trials in the face of disease progression heterogeneity. Neurology 96, e2673–e2684 (2021).
Wasserstein, R. L. & Lazar, N. A. The ASA statement on p-values: context, process, and purpose. Am. Stat. 70, 129–133 (2016).
US Food and Drug Administration. Drug development & approval process: drugs. https://www.fda.gov/drugs/development-approval-process-drugs (2019).
Buracchio, T., Yasuda, S. J., Bastings, E. & Dunn, B. Summary memorandum. BLA# 761178: Aducanumab. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/Aducanumab_BLA761178_Dunn_2021_06_07.pdf (2021).
Stein, P. Concurrence memorandum. BLA# 761178: Aducanumab. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/Aducanumab_BLA761178_Stein_2021_06_07.pdf (2021).
US Department of Health and Human Services. Statistical Review and Evaluation: Clinical Studies. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761178Orig1s000StatR_Redacted.pdf (2021).
Ackley, S. F. et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: instrumental variable meta-analysis. BMJ 372, n156 (2021).
Sims, J. R. et al. Trailblazer-ALZ study: dynamics of amyloid reduction after donanemab treatment. https://assets.ctfassets.net/mpejy6umgthp/6cTd4wATIjtb9hpBdGXpMv/8db4866aca850ba8fd4ae146c3784c7b/105117___until_fixed_VV-DONPT3_AAIC2021_Sims_Dona_Program_Amyloid_Imaging.pdf (Eli Lilly, 2021).
Mintun, M. A. et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med. 384, 1691–1704 (2021).
Swanson, C. J. et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res. Ther. 13, 80 (2021).
Choi, S. R. et al. Correlation of amyloid PET ligand florbetapir F 18 binding with Aβ aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer Dis. Assoc. Disord. 26, 8–16 (2012).
Sabri, O. et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 11, 964–974 (2015).
Landau, S. M. et al. Measurement of longitudinal β-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J. Nucl. Med. 56, 567–574 (2015).
Lammertsma, A. A. Forward to the past: the case for quantitative PET imaging. J. Nucl. Med. 58, 1019–1024 (2017).
Mullard, A. Landmark Alzheimer’s drug approval confounds research community. Nature 594, 309–310 (2021).
Naci, H., Smalley, K. R. & Kesselheim, A. S. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA 318, 626–636 (2017).
Wallach, J. D., Luxkaranayagam, A. T., Dhruva, S. S., Miller, J. E. & Ross, J. S. Postmarketing commitments for novel drugs and biologics approved by the US Food and Drug Administration: a cross-sectional analysis. BMC Med. 17, 117 (2019).
US Food and Drug Administration. Full prescribing information for ADUHELM. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761178s000lbl.pdf (2021).
US Food and Drug Administration. Updated full prescribing information for ADUHELM. https://www.biogencdn.com/us/aduhelm-pi.pdf (2021).
Cummings, J. & Salloway, S. Aducanumab: appropriate use recommendations. Alzheimers Dement. https://doi.org/10.1002/alz.12444 (2021).
Chételat, G. et al. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin. 2, 356–365 (2013).
Alexopoulos, P. et al. Conflicting cerebrospinal fluid biomarkers and progression to dementia due to Alzheimer’s disease. Alzheimers Res. Ther. 8, 51 (2016).
Okello, A. et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology 73, 754–760 (2009).
Dubois, B. et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 20, 484–496 (2021).
US Food and Drug Administration. FDA: Guidance for Industry - Formal Meetings Between the FDA and Sponsors or Applicants. https://www.fda.gov/media/72253/download (2009).
Carome, M. A. Letter. https://mkus3lurbh3lbztg254fzode-wpengine.netdna-ssl.com/wp-content/uploads/2560.pdf (Carome to Grimm, 9 Dec 2020).
Feuerstein, A., Herper, M, & Garde D. How Biogen used an FDA back channel to win Alzheimer’s drug approval. STAT https://www.statnews.com/2021/06/29/biogen-fda-alzheimers-drug-approval-aduhelm-project-onyx/ (2021).
Woodcock, J. Letter. https://twitter.com/DrWoodcockFDA/status/1413540801934774283/photo/1 (Woodcock to Grimm, 9 Jul 2021).
Dal Bό, E. Regulatory capture: a review. Oxf. Rev. Econ. Policy 22, 203–225 (2006).
Carpenter, D. & Moss, D. (eds) Preventing Regulatory Capture: Special Interest Influence and how to Regulate it (Cambridge Univ. Press, 2013).
Darrow, J. J., Avorn, J. & Kesselheim, A. S. Speed, safety, and industry funding–from PDUFA I to PDUFA VI. N. Engl. J. Med. 377, 2278–2286 (2017).
Alzheimer’s Association. Re: Docket No. FDA-2018-N-0410: Peripheral and Central Nervous System Drugs Advisory Committee; Notice of Meeting; Establishment of a Public Docket; Request for Comments. https://www.regulations.gov/comment/FDA-2018-N-0410-0031 (2020).
Vradenburg, G. & Paulsen, R. Letter. https://www.usagainstalzheimers.org/sites/default/files/2021-01/UsA2-FDA%20re%20aducanumab%20review%201-19-21%20%28002%29.pdf (Vradenburg & Paulsen to Cavazzoni, Stein & Dunn, 2021).
Alzheimer’s Association. Alzheimer’s Association annual report: fiscal year 2017. Alzheimer’s Association https://www.alz.org/media/documents/annual-report-2017.pdf (2017).
Alzheimer’s Association. Alzheimer’s association annual report: fiscal year 2018. Alzheimer’s Association https://www.alz.org/media/documents/annual-report-2018.pdf (2018).
Alzheimer’s Association. Alzheimer’s Association annual report: fiscal year 2019. https://www.alz.org/media/Documents/annual-report-2019.pdf (2019).
Alzheimer’s Association. Pharmaceutical Industry Contributions: FY20. https://www.alz.org/media/Documents/Pharmaceutical-Industry-Contributions-FY20.pdf (2021).
UsAgainstAlzheimer’s 2020 National Alzheimer’s Summit. https://www.usa2summit.org/#sponsors (2020).
CFA Institute. Corrupt or collaborative? An Assessment of Regulatory Capture. https://www.cfainstitute.org/-/media/documents/article/position-paper/corrupt-or-collaborative-an-assessment.ashx (2016).
US Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Drug. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug (2021).
Biogen. EISAI and Biogen Inc. announce U.S. FDA grants breakthrough therapy designation for LECANEMAB (BAN2401), an anti-amyloid beta protofibril antibody for the treatment of Alzheimer’s disease. https://investors.biogen.com/news-releases/news-release-details/eisai-and-biogen-inc-announce-us-fda-grants-breakthrough-therapy (2021).
Lilly. Lilly’s donanemab receives U.S. FDA’s Breakthrough Therapy designation for treatment of Alzheimer’s disease. https://investor.lilly.com/news-releases/news-release-details/lillys-donanemab-receives-us-fdas-breakthrough-therapy (2021).
Golde, T. E. Alzheimer disease therapy: can the amyloid cascade be halted? J. Clin. Invest. 111, 11–18 (2003).
Kemp, R. & Prasad, V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 15, 134 (2017).
Gyawali, B., Hey, S. P. & Kesselheim, A. S. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine 21, 100332 (2020).
Gyawali, B., Hey, S. P. & Kesselheim, A. S. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern. Med. 179, 906–913 (2019).
Lovelace, B., Jr. Biogen faces tough questions over $56K-a-year price of newly approved Alzheimer’s drug. CNBC https://www.cnbc.com/2021/06/08/biogen-faces-tough-questions-over-56k-a-year-price-of-newly-approved-alzheimers-drug.html (2021).
Authenticated U.S. Government Information. Public Law 108-173-Dec. 8, 2003. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. https://truecostofhealthcare.org/wp-content/uploads/2018/07/PrescriptionDrugAct2003.pdf (2003).
Khera, N. Reporting and grading financial toxicity. J. Clin. Oncol. 32, 3337–3338 (2014).
Desai, A. & Gyawali, B. Financial toxicity of cancer treatment: moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine 20, 100269 (2020).
Cherla, A., Naci, H., Kesselheim, A. S., Gyawali, B. & Mossialos, E. Assessment of coverage in England of cancer drugs qualifying for US Food and Drug Administration accelerated approval. JAMA Intern. Med. 181, 490–498 (2021).
US Social Security Administration. Compilation of the Social Security Laws: Payment for covered outpatient drugs. https://www.ssa.gov/OP_Home/ssact/title19/1927.htm (2021).
Saltzman, J. ‘This is unprecedented’: Several private insurers won’t cover Biogen’s Alzheimer’s drug. The Boston Globe https://www.bostonglobe.com/2021/07/13/business/this-is-unprecedented-several-private-insurers-wont-cover-biogens-alzheimers-drug/ (2021).
Lin, G. A. et al. Aducanumab for Alzheimer’s disease: effectiveness and value; final evidence report and meeting summary. ICER https://icer.org/wp-content/uploads/2020/10/ICER_ALZ_Final_Report_080521.pdf (2021).
Belluck, P. Cleveland clinic and Mount Sinai won’t administer Aduhelm to patients. The New York Times https://www.nytimes.com/2021/07/14/health/cleveland-clinic-aduhelm.html (2021).
Zhang, A. D., Schwartz, J. L. & Ross, J. S. Association between Food and Drug Administration Advisory Committee recommendations and agency actions, 2008-2015. Milbank Q. 97, 796–819 (2019).
Author information
Authors and Affiliations
Contributions
K.Y.L. researched data for the article, made a substantial contribution to discussion of content, wrote the article and reviewed and edited the manuscript before submission. R.H. researched data for the article, made a substantial contribution to discussion of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks H. Fillit, T. Golde and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Accelerated approval
-
An FDA-instituted programme to allow drugs that treat serious conditions and that fill an unmet medical need to receive earlier approval on the basis of a surrogate end point considered reasonably likely to predict a clinical benefit.
- Breakthrough therapy designation
-
A process designed to expedite the development and review of drugs intended to treat a serious condition when preliminary clinical evidence indicates that the drug might represent a substantial improvement over available therapies on a clinically significant end point or end points, which can be a surrogate end point considered reasonably likely to predict a clinical benefit.
- Type C meeting
-
A formal meeting between the FDA and sponsor concerning the development and review of a product that does not fall within the scope of a Type A meeting (a meeting necessary for an otherwise stalled product development programme to proceed or to address an important safety issue) or a Type B meeting (a milestone meeting, such as an end-of-phase I or II meeting).
Rights and permissions
About this article
Cite this article
Liu, K.Y., Howard, R. Can we learn lessons from the FDA’s approval of aducanumab?. Nat Rev Neurol 17, 715–722 (2021). https://doi.org/10.1038/s41582-021-00557-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00557-x
This article is cited by
-
Mild Cognitive Impairment in Relation to Alzheimer’s Disease: An Investigation of Principles, Classifications, Ethics, and Problems
Neuroethics (2023)
-
Diagnostic value of serum versus plasma phospho-tau for Alzheimer’s disease
Alzheimer's Research & Therapy (2022)
-
Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility
Nature Reviews Neurology (2022)
-
CMS coverage decision on anti-amyloid monoclonal antibodies for Alzheimer disease
Nature Reviews Neurology (2022)
-
Immunogenicity of MultiTEP platform technology-based Tau vaccine in non-human primates
npj Vaccines (2022)