Abstract
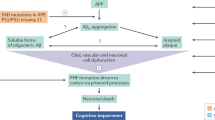
Frontotemporal dementia (FTD) encompasses a spectrum of clinical syndromes characterized by progressive executive, behavioural and language dysfunction. The various FTD spectrum disorders are associated with brain accumulation of different proteins: tau, the transactive response DNA binding protein of 43 kDa (TDP43), or fused in sarcoma (FUS) protein, Ewing sarcoma protein and TATA-binding protein-associated factor 15 (TAF15) (collectively known as FET proteins). Approximately 60% of patients with FTD have autosomal dominant mutations in C9orf72, GRN or MAPT genes. Currently available treatments are symptomatic and provide limited benefit. However, the increased understanding of FTD pathogenesis is driving the development of potential disease-modifying therapies. Most of these drugs target pathological tau — this category includes tau phosphorylation inhibitors, tau aggregation inhibitors, active and passive anti-tau immunotherapies, and MAPT-targeted antisense oligonucleotides. Some of these therapeutic approaches are being tested in phase II clinical trials. Pharmacological approaches that target the effects of GRN and C9orf72 mutations are also in development. Key results of large clinical trials will be available in a few years. However, clinical trials in FTD pose several challenges, and the development of specific brain imaging and molecular biomarkers could facilitate the recruitment of clinically homogenous groups to improve the chances of positive clinical trial results.
Key points
-
Frontotemporal dementia (FTD) refers to a group of progressive neurodegenerative syndromes with different clinical presentations, the most common of which is behavioural variant FTD.
-
FTD is caused by an accumulation of pathological tau, transactive response DNA binding protein of 43 kDa (TDP43), or fused in sarcoma protein, Ewing sarcoma protein and TATA-binding protein-associated factor 15 (FET proteins).
-
Symptomatic treatments have limited benefits and can exacerbate symptoms.
-
An increased understanding of FTD pathogenesis is helping the development of disease-modifying therapies.
-
Disease-modifying drugs for FTD mainly target tau, but therapies for genetic forms of FTD (caused by mutations in GRN, C9orf72 and MAPT) are also being pursued.
-
Several potential disease-modifying therapies have been or are being tested in phase II clinical trials.
-
Several challenges must be overcome to recruit homogeneous patient cohorts and ensure that appropriate biomarkers and clinical end points are used so that clinical trials produce meaningful results.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bang, J., Spina, S. & Miller, B. L. Frontotemporal dementia. Lancet 386, 1672–1682 (2015).
Coyle-Gilchrist, I. T. et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86, 1736–1743 (2016).
Tsai, R. M. & Boxer, A. L. Therapy and clinical trials in frontotemporal dementia: past, present, and future. J. Neurochem. 138, 211–221 (2016).
Young, J. J. et al. Frontotemporal dementia: latest evidence and clinical implications. Ther. Adv. Psychopharmacol. 8, 33–48 (2018).
Panza, F. et al. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15, 73–88 (2019).
Ihl, R. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J. Biol. Psychiatry 12, 2–32 (2011).
Huey, E. D., Putnam, K. T. & Grafman, J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 66, 17–22 (2006).
Li, Y. et al. Cholinesterase inhibitors for rarer dementias associated with neurological conditions. Cochrane Database Syst. Rev. 3, CD009444 (2015).
Buoli, M. et al. Pharmacological management of psychiatric symptoms in frontotemporal dementia: a systematic review. J. Geriatr. Psychiatry Neurol. 30, 162–169 (2017).
O’Brien, J. T. et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J. Psychopharmacol. 31, 147–166 (2017).
Moretti, R. et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging 21, 931–937 (2019).
Kertesz, A. et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement. Geriatr. Cogn. Disord. 25, 178–185 (2008).
Litvan, I. et al. Randomized placebo-controlled trial of donepezil in patients with progressive supranuclear palsy. Neurology 57, 467–473 (2001).
Kishi, T., Matsunaga, S. & Iwata, N. Memantine for the treatment of frontotemporal dementia: a meta-analysis. Neuropsychiatr. Dis. Treat. 11, 2883–2885 (2015).
Vercelletto, M. et al. Memantine in behavioral variant frontotemporal dementia: negative results. J. Alzheimer Dis. 23, 749–759 (2011).
Boxer, A. L. et al. Memantine in patients with frontotemporal lobar degeneration: a multicenter, randomized, double-blind, placebo-controlled trial. Lancet Neurol. 12, 149–156 (2013).
Mendez, M. F. et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am. J. Geriatr. Psychiatr. 15, 84–87 (2007).
Fabbrini, G. et al. Donepezil in the treatment of progressive supranuclear palsy. Acta Neurol. Scand. 103, 123–125 (2001).
Liepelt, I. et al. Rivastigmine for the treatment of dementia in patients with progressive supranuclear palsy: clinical observations as a basis for power calculations and safety analysis. Alzheimers Dement. 6, 70–74 (2010).
Lozupone, M. et al. Pharmacotherapy for the treatment of depression in patients with Alzheimer’s disease: a treatment-resistant depressive disorder. Expert Opin. Pharmacother. 19, 823–842 (2018).
Swartz, J. R. et al. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J. Clin. Psychiatry 58, 212–216 (1997).
Chow, T. W. & Mendez, M. F. Goals in symptomatic pharmacologic management of frontotemporal lobar degeneration. Am. J. Alzheimers Dis. Other Demen. 17, 267–272 (2002).
Moretti, R. et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. Eur. Neurol. 49, 13–19 (2003).
Deakin, J. B. et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology 172, 400–408 (2004).
Herrmann, N. et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am. J. Geriatr. Psychiatry 20, 789–797 (2012).
Hughes, L. E. et al. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain 138, 1961–1975 (2015).
Meyer, S. et al. Citalopram improves obsessive-compulsive crossword puzzling in frontotemporal dementia. Case Rep. Neurol. 11, 94–105 (2019).
Lebert, F. et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement. Geriatr. Cogn. Disord. 17, 355–359 (2004).
Mendez, M. F., Shapira, J. S. & Miller, B. L. Stereotypical movements and frontotemporal dementia. Mov. Disord. 20, 742–745 (2005).
Prodan, C. I., Monnon, M. & Ross, E. D. Behavioral abnormalities associated with rapid deterioration of language functions in semantic dementia respond to sertraline. J. Neurol. Neurosurg. Psychiatry 80, 1416–1417 (2009).
Anneser, J. M., Jox, R. J. & Borasio, G. D. Inappropriate sexual behavior in a case of ALS and FTD: successful treatment with sertraline. Amyotroph. Lateral Scler. 8, 189–190 (2007).
Ikeda, M. et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement. Geriatr. Cogn. Disord. 17, 117–121 (2004).
Anderson, I. M., Scott, K. & Harborne, G. Serotonine and depression in frontal lobe dementia. Am. J. Psychiatry 152, 645 (1995).
Panza, F. et al. Progresses in treating agitation: a major clinical challenge in Alzheimer’s disease. Expert Opin. Pharmacother. 16, 2581–2588 (2015).
Pijnenburg, Y. A. et al. Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. Int. J. Geriatr. Psychiatr. 18, 67–72 (2003).
Curtis, R. C. & Resch, D. S. Case of Pick´s central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J. Clin. Psychopharmacol. 20, 384–385 (2000).
Fellgiebel, A. et al. Clinical improvement in a case of frontotemporal dementia under aripiprazole treatment corresponds to partial recovery of disturbed frontal glucose metabolism. World J. Biol. Psychiatry 8, 123–126 (2007).
Moretti, R. et al. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: a 24-month follow-up of 68 patients. Am. J. Alzheimers Dis. Other Demen. 18, 205–214 (2003).
Jha, M. K. et al. A case of frontotemporal dementia presenting with treatment-refractory psychosis and extreme violence: Response to combination of clozapine, medroxyprogesterone, and sertraline. J. Clin. Psychopharmacol. 35, 732–733 (2015).
Riedl, L. et al. Frontotemporal lobar degeneration: current perspectives. Neuropsychiatr. Dis. Treat. 10, 297–310 (2014).
Hodges, J. R. et al. Clinicopathotogical correlates in frontotemporal dementia. Ann. Neurol. 56, 399–406 (2004).
Goldman, L. S. et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents, Council on Scientific Affairs, American Medical Association. JAMA 279, 1100–1107 (1998).
Huey, E. D. et al. Stimulant treatment of frontotemporal dementia in 8 patients. J. Clin. Psychiatry 69, 1981–1982 (2008).
Rahman, S. et al. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology 31, 651–658 (2006).
Reed, D. A. et al. A clinical trial of bromocriptine for treatment of primary progressive aphasia. Ann. Neurol. 56, 750 (2004).
Adler, G., Teufel, M. & Drach, L. M. Pharmacological treatment of frontotemporal dementia: treatment response to the MAO-A inhibitor moclobemide. Int. J. Geriatr. Psychiatry 18, 653–655 (2003).
Moretti, R. et al. Effects of selegiline on fronto-temporal dementia: a neuropsychological evaluation. Int. J. Geriatr. Psychiatry 17, 391–392 (2002).
Jesso, S. et al. The effects of oxytocin on social cognition and behavior in frontotemporal dementia. Brain 134, 2493–2501 (2011).
Finger, E. C. et al. Oxytocin for frontotemporal dementia: a randomized dose-finding study of safety and tolerability. Neurology 84, 174–181 (2015).
Callegari, I. et al. Agomelatine improves apathy in frontotemporal dementia. Neurodegener. Dis. 16, 352–356 (2016).
Solfrizzi, V. et al. Nutritional interventions and cognitive-related outcomes in patients with late-life cognitive disorders: a systematic review. Neurosci. Biobehav. Rev. 95, 480–498 (2018).
Pardini, M. et al. Souvenaid reduces behavioral deficits and improves social cognition skills in frontotemporal dementia: a proof-of-concept study. Neurodegener. Dis. 15, 58–62 (2015).
Augustin, K. et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 17, 84–93 (2018).
Nygaard, H. B. Pharmacokinetics and dynamics of a ketogenic intervention in Alzheimer’s disease and frontotemporal dementia. Alzheimers Dement. 13, p838 (2017).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Capozzo, R. et al. Clinical and genetic analyses of familial and sporadic frontotemporal dementia patients in Southern Italy. Alzheimers Dement. 13, 858–869 (2017).
Gorno-Tempini, M. L. et al. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014 (2011).
Höglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov. Disord. 32, 853–864 (2017).
Armstrong, M. J. et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503 (2013).
Strong, M. J. et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 153–174 (2017).
Josephs, K. A. Frontotemporal dementia and related disorders: deciphering the enigma. Ann. Neurol. 64, 4–14 (2008).
Josephs, K. A. et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 122, 137–153 (2011).
Hutton, M. et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Neumann, M. et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132, 2922–2931 (2009).
Kwiatkowski, T. J. Jr. et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009).
Vance, C. et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009).
Neumann, M. et al. FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain 134, 2595–2609 (2011).
Brelstaff, J. et al. Transportin1: a marker of FTLD-FUS. Acta Neuropathol. 122, 591–600 (2011).
Elahi, F. M. & Miller, B. L. A clinicopathological approach to the diagnosis of dementia. Nat. Rev. Neurol. 13, 457–476 (2017).
Rohrer, J. D. et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134, 2565–2581 (2011).
Spillantini, M. G. & Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol. 12, 609–622 (2013).
Perry, D. C. et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 140, 3329–3345 (2017).
Mailliot, C. et al. Phosphorylation of specific sets of tau isoforms explains different neurodegeneration processes. FEBS Lett. 433, 201–204 (1998).
Sergeant, N., Wattez, A. & Delacourte, A. Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms. J. Neurochem. 72, 1243–1249 (1999).
Sergeant, N. et al. Different distribution of phosphorylated tau protein isoforms in Alzheimer’s and Pick’s diseases. FEBS Lett. 412, 578–582 (1997).
Togo, T. et al. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J. Neuropathol. Exp. Neurol. 61, 547–556 (2002).
Bigio, E. H. et al. Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J. Neuropathol. Exp. Neurol. 60, 328–341 (2001).
Goedert, M. & Jakes, R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta 1739, 240–250 (2005).
Ahmed, Z. et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol. 122, 415–428 (2011).
Irwin, D. J. et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 129, 469–491 (2015).
Neumann, M. & Mackenzie, I. R. Review: neuropathology of non-tau frontotemporal lobar degeneration. Neuropathol. Appl. Neurobiol. 45, 19–40 (2019).
Arai, T. et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006).
Mackenzie, I. R. et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 122, 111–113 (2011).
Mackenzie, I. R. & Neumann, M. Reappraisal of TDP-43 pathology in FTLD-U subtypes. Acta Neuropathol. 134, 79–96 (2017).
Dobson-Stone, C. et al. C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts. Neurology 79, 995–1001 (2012).
Tang, W. et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 (2001).
Van Damme, P. et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J. Cell Biol. 181, 37–41 (2008).
Neumann, M. et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J. Neuropathol. Exp. Neurol. 66, 152–157 (2007).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9orf72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Ash, P. E. et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77, 639–646 (2013).
Gendron, T. F. et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 126, 829–844 (2013).
Mori, K. et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol. 126, 881–893 (2013).
Mori, K. et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339, 1335–1338 (2013).
Zu, T. et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl Acad. Sci. USA 110, E4968–E4977 (2013).
Davidson, Y. S. et al. Brain distribution of dipeptide repeat proteins in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol. Commun. 2, 70 (2014).
Mackenzie, I. R. et al. Quantitative analysis and clinico-pathological correlations of different dipeptide repeat protein pathologies in C9ORF72 mutation carriers. Acta Neuropathol. 130, 845–861 (2015).
Neumann, M. et al. Transportin 1 accumulates specifically with FET proteins but no other transportin cargos in FTLD-FUS and is absent in FUS inclusions in ALS with FUS mutations. Acta Neuropathol. 124, 705–716 (2012).
Mackenzie, I. R. et al. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 131, 1282–1293 (2008).
Cairns, N. J. et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 63, 1376–1384 (2004).
Munoz, D. G. et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 118, 617–627 (2009).
Kusaka, H., Matsumoto, S. & Imai, T. An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol. 80, 660–665 (1990).
Kawakami, I. et al. Chorea as a clinical feature of the basophilic inclusion body disease subtype of fused-in-sarcoma associated frontotemporal lobar degeneration. Acta Neuropathol. Commun. 4, 36 (2016).
Urwin, H. et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol. 120, 33–41 (2010).
Skibinski, G. et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 37, 806–808 (2005).
van der Zee, J. et al. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum. Mol. Genet. 17, 313–322 (2008).
Isaacs, A. M. et al. Frontotemporal dementia caused by CHMP2B mutations. Curr. Alzheimer Res. 8, 246–251 (2011).
Knopman, D. S. et al. Dementia lacking distinctive histologic features: a common non- Alzheimer degenerative dementia. Neurology 40, 251–256 (1990).
Mackenzie, I. R. et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 119, 1–4 (2010).
Goldman, J. S. et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 65, 1817–1819 (2005).
Rohrer, J. D. et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology 73, 1451–1456 (2009).
Wood, E. M. et al. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol. 70, 1411–1417 (2013).
Greaves, C. V. & Rohrer, J. D. An update on genetic frontotemporal dementia. J. Neurol. 266, 2075–2086 (2019).
Mackenzie, I. R. et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129, 3081–3090 (2006).
Masellis, M. et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain 129, 3115–3123 (2006).
Moreno, F. et al. “Frontotemporoparietal” dementia: clinical phenotype associated with the c.709–1G>A PGRN mutation. Neurology 73, 1367–1374 (2009).
Ghetti, B., Hutton, M. & Wszolek, Z. Neurodegeneration: the molecular pathology of dementia and movement disorders (ed. Dickson, D.) 86–102 (ISN Neuropath Press, 2003).
Pickering-Brown, S. M. et al. Inherited frontotemporal dementia in nine British families associated with intronic mutations in the tau gene. Brain 125, 732–751 (2002).
Watts, G. D. et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381 (2004).
Benajiba, L. et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann. Neurol. 65, 470–473 (2009).
Yan, J. et al. Frameshift and novel mutations in FUS in familial amyotrophic lateral sclerosis and ALS/dementia. Neurology 75, 807–814 (2010).
Synofzik, M. et al. Screening in ALS and FTD patients reveals 3 novel UBQLN2 mutations outside the PXX domain and a pure FTD phenotype. Neurobiol. Aging 33, 2949.e13-7 (2012).
Le Ber, I. et al. SQSTM1 mutations in French patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 70, 1403–1410 (2013).
Bannwarth, S. et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain 137, 2329–2345 (2014).
Pottier, C. et al. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 130, 77–92 (2015).
Williams, K. L. et al. CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat. Commun. 15, 11253 (2016).
Mackenzie, I. R. et al. TIA1 Mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95, 808–816 (2017).
Lattante, S., Rouleau, G. A. & Kabashi, E. TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update. Hum. Mutat. 34, 812–826 (2013).
de Majo, M. et al. ALS-associated missense and nonsense TBK1 mutations can both cause loss of kinase function. Neurobiol. Aging 71, 266.e1–266.e10 (2018).
Irwin, D. J. Tauopathies as clinicopathological entities. Parkinsonism Relat. Disord. 22, S29–S33 (2016).
Oeckl, P., Steinacker, P., Feneberg, E. & Otto, M. Neurochemical biomarkers in the diagnosis of frontotemporal lobar degeneration: an update. J. Neurochem. 138 (Suppl. 1), 184–192 (2016).
Liu, M. N., Lau, C. I. & Lin, C. P. Precision Medicine for frontotemporal dementia. Front. Psychiatry 10, 75 (2019).
Hedl, T. J. et al. Proteomics approaches for biomarker and drug target discovery in ALS and FTD. Front. Neurosci. 13, 548 (2019).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Boxer, A. L. et al. The advantages of frontotemporal degeneration drug development (part 2 of frontotemporal degeneration: the next therapeutic frontier). Alzheimers Dement. 9, 189–198 (2013).
Tang, W. et al. Assessment of CSF Ab42 as an aid to discriminating Alzheimer’s disease from other dementias and mild cognitive impairment: a meta-analysis of 50 studies. J. Neurol. Sci. 345, 26–36 (2014).
van Harten, A. C. et al. Tau and p-tau as CSF biomarkers in dementia: a meta-analysis. Clin. Chem. Lab. Med. 49, 353–366 (2011).
Tang, W. et al. Does CSF p-tau181 help to discriminate Alzheimer’s disease from other dementias and mild cognitive impairment? A meta-analysis of the literature. J. Neural. Transm. 121, 1541–1553 (2014).
Skillbäck, T. et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138, 2716–2731 (2015).
Baldeiras, I. et al. Cerebrospinal fluid Ab40 is similarly reduced in patients with Frontotemporal lobar degeneration and Alzheimer’s disease. J. Neurol. Sci. 358, 308–316 (2015).
Struyfs, H. et al. Cerebrospinal fluid P-Tau181P: biomarker for improved differential dementia diagnosis. Front. Neurol. 6, 138 (2015).
de Jong, D. et al. CSF neurofilament proteins in the differential diagnosis of dementia. J. Neurol. Neurosurg. Psychiatry 78, 936–938 (2007).
Zerr, I. et al. Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: Evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimers Dement. 14, 751–763 (2018).
Herbert, M. K. CSF neurofilament light chain but not FLT3 ligand discriminates Parkinsonian disorders. Front. Neurol. 6, 91 (2015).
Magdalinou, N. K. et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 86, 1240–1247 (2015).
Hu, W. T. et al. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurol. 81, 1945–1952 (2013).
Kortvelyessy, P. et al. CSF biomarkers of neurodegeneration in progressive non-fluent aphasia and other forms of frontotemporal dementia: clues for pathomechanisms? Front. Neurol. 9, 504 (2018).
Meeter, L. H. H. et al. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology 90, e1231–e1239 (2018).
Foiani, M. S. et al. Plasma tau is increased in frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 89, 804–807 (2018).
Janssens, J. & Van Broeckhoven, C. Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD-ALS spectrum disorders. Hum. Mol. Genet. 22, R77–R87 (2013).
Borroni, B. et al. Csf p-tau181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 86–91 (2015).
Ghidoni, R. et al. Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology 14, 1235–1239 (2008).
Finch, N. et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132, 583–591 (2009).
Bruun, M. et al. Detecting frontotemporal dementia syndromes using MRI biomarkers. Neuroimage Clin. 22, 101711 (2019).
D’Alton, S. & Lewis, J. Therapeutic and diagnostic challenges for frontotemporal dementia. Front. Aging Neurosci. 6, 204 (2014).
De Conti, L., Borroni, B. & Baralle, M. New routes in frontotemporal dementia drug discovery. Expert Opin. Drug Discov. 12, 659–671 (2017).
Panza, F. et al. Disease-modifying therapies for tauopathies: agents in the pipeline. Expert Rev. Neurother. 19, 397–408 (2019).
Drechsel, D. N. et al. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 3, 1141–1154 (1992).
Goedert, M. et al. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3, 519–526 (1989).
Andreadis, A., Brown, W. M. & Kosik, K. S. Structure and novel exons of the human tau gene. Biochemistry 3, 10626–10633 (1992).
Uversky, V. N. What does it mean to be natively unfolded? Eur. J. Biochem. 269, 2–12 (2002).
Jeganathan, S. et al. The natively unfolded character of tau and its aggregation to Alzheimer-like paired helical filaments. Biochemistry 47, 10526–10539 (2008).
Lindwall, G. & Cole, R. D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 259, 5301–5305 (1984).
Holmes, B. B. & Diamond, M. I. Prion-like properties of Tau protein: the importance of extracellular Tau as a therapeutic target. J. Biol. Chem. 18, 19855–19861 (2014).
Rohrer, J. D. & Warren, J. D. Phenotypic signatures of genetic frontotemporal dementia. Curr. Opin. Neurol. 24, 542–549 (2011).
Papazacharias, A. et al. Bipolar disorder and frontotemporal dementia: an intriguing association. J. Alzheimers Dis. 55, 973–979 (2017).
Hong, M. et al. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 272, 25326–25332 (1997).
Noble, W. et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl Acad. Sci. USA 102, 6990–6995 (2005).
Engel, T. et al. Chronic lithium administration to FTDP-17 tau and GSK-3β overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J. Neurochem. 99, 1445–1555 (2006).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00703677 (2015).
Höglinger, G. U. et al. Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial. Mov. Disord. 29, 479–487 (2014).
Tolosa, E. et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov. Disord. 29, 470–478 (2014).
Wischik, C. M. et al. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl Acad. Sci. USA 93, 11213–11218 (1996).
Seripa, D. et al. Tau-directed approaches for the treatment of Alzheimer’s disease: focus on leuco-methylthioninium. Expert Rev. Neurother. 16, 259–277 (2016).
Gauthier, S. et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388, 2873–2884 (2016).
TauRx Pharmaceuticals, Press Release at the 10th International Conference on Frontotemporal Dementias, Munich, August 31–September 2 https://taurx.com/trx-237-007-phase-3-clinical-trial-update.pdf (2016).
Min, S. W. et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21, 1154–1162 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02422485 (2018).
Paholikova, K. et al. N-terminal truncation of microtubule associated protein tau dysregulates its cellular localization. J. Alzheimers Dis. 43, 915–926 (2015).
Kontsekova, E. et al. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res. Ther. 6, 44 (2014).
Novak, P. et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 16, 123–134 (2017).
Panza, F. & Logroscino, G. Anti-tau vaccine in Alzheimer’s disease: a tentative step. Lancet Neurol. 16, 99–100 (2017).
Novak, P. et al. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res. Ther. 10, 108 (2018).
AXON Neuroscience SE. Axon Announces Positive Results From Phase II ADAMANT Trial for AADvac1 in Alzheimer’s Disease. CISION PR Newswire https://www.prnewswire.com/news-releases/axon-announces-positive-results-from-phase-ii-adamant-trial-for-aadvac1-in-alzheimers-disease-300914509.html (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03174886 (2019).
Dai, C. L. et al. Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies. J. Neural. Transm. 122, 607–617 (2015).
Panza, F. et al. Tau-based therapeutics for Alzheimer’s disease: active and passive immunotherapy. Immunotherapy 8, 1119–1134 (2016).
Bright, J. et al. Human secreted tau increases amyloid-β production. Neurobiol. Aging 36, 693–709 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02294851 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02460094 (2018).
Boxer, A. L. et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 18, 549–558 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03068468 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03658135 (2019).
Gosuranemab, Biogen’s Anti-Tau Immunotherapy, Does not fly for PSP. ALZFORUM https://www.alzforum.org/news/research-news/gosuranemab-biogens-anti-tau-immunotherapy-does-not-fly-psp (2019).
Braak, H. & Del Tredici, K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 121, 589–595 (2011).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02494024 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03413319 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02985879 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03391765 (2019).
Carroll, J. AbbVie scraps an anti-tau study, and that may foretell more big trouble for a beleaguered Biogen. ENDPOINTS NEWS. https://endpts.com/abbvie-scraps-an-anti-tau-study-and-that-may-foretell-more-big-trouble-for-a-beleaguered-biogen/ (2019).
To Block Tau’s Proteopathic Spread, Antibody Must Attack its Mid-Region. ALZFORUM. https://www.alzforum.org/news/conference-coverage/block-taus-proteopathic-spread-antibody-must-attack-its-mid-region (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03464227 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04185415 (2020).
Jarskog, L. F. et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology 38, 1245–1252 (2013).
Javitt, D. C. et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr. Res. 136, 25–31 (2012).
Vulih-Shultzman, I. et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J. Pharmacol. Exp. Ther. 323, 438–449 (2007).
Matsuoka, Y. et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 325, 146–153 (2008).
Jouroukhin, Y. et al. NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: protection against impairments in axonal transport. Neurobiol. Dis. 56, 79–94 (2013).
Morimoto, B. H. et al. A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 35, 325–326 (2013).
Boxer, A. L. et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 13, 676–685 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01056965 (2019).
Tsai, R. M. et al. Reactions to multiple ascending doses of the microtubule stabilizer TPI-287 in patients with Alzheimer disease, progressive supranuclear palsy, and corticobasal syndrome: a randomized clinical trial. JAMA Neurol. 77, 215–224 (2020).
Sud, R., Geller, E. T. & Schellenberg, G. D. Antisense-mediated exon skipping decreases tau protein expression: a potential therapy for tauopathies. Mol. Ther. Nucleic Acids 3, e180 (2014).
Kalbfuss, B., Mabon, S. A. & Misteli, T. Correction of alternative splicing of tau in frontotemporal dementia and parkinsonism linked to chromosome 17. J. Biol. Chem. 276, 42986–42993 (2001).
DeVos, S. L. et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med. 9, eaag0481 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03186989 (2020).
Wang, Y. & Mandelkow, E. Degradation of tau protein by autophagy and proteasomal pathways. Biochem. Soc. Trans. 40, 644–652 (2012).
Khanna, M. R. et al. Therapeutic strategies for the treatment of tauopathies: hopes and challenges. Alzheimers Dement. 12, 1051–1065 (2016).
Luo, W. et al. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc. Natl Acad. Sci. USA 104, 9511–9516 (2007).
Karagoz, G. E. et al. Hsp90-Tau complex reveals molecular basis for specificity in chaperone action. Cell 156, 963–974 (2014).
Soga, S., Akinaga, S. & Shiotsu, Y. Hsp90 inhibitors as anti-cancer agents, from basic discoveries to clinical development. Curr. Pharm. Des. 19, 366–376 (2013).
Dickey, C. A. et al. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. FASEB J. 20, 753–755 (2006).
Kamal, A., Boehm, M. F. & Burrows, F. J. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol. Med. 10, 283–290 (2004).
Bence, N. F., Sampat, R. M. & Kopito, R. R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Ozcelik, S. et al. Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS One 8, e62459 (2013).
Siman, R., Cocca, R. & Dong, Y. The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early-stage Alzheimer-type tauopathy. PLoS One 10, e0142340 (2015).
Orr, M. E., Sullivan, A. C. & Frost, B. A brief overview of tauopathy: causes, consequences, and therapeutic strategies. Trends Pharmacol. Sci. 38, 637–648 (2017).
Liu, F. et al. O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 101, 10804–10809 (2004).
Liu, F. et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain 132, 1820–1832 (2009).
Borghgraef, P. et al. Increasing brain protein O-GlcNAc-ylation mitigates breathing defects and mortality of Tau.P301L mice. PLoS One 8, e84442 (2013).
Hastings, N. B. et al. Inhibition of O-GlcNAcase leads to elevation of OGlcNAc tau and reduction of tauopathy and cerebrospinal fluid tau in rTg4510 mice. Mol. Neurodegener. 12, 39 (2017).
Pooler, A. M. et al. Propagation of tau pathology in Alzheimer’s disease: identification of novel therapeutic targets. Alzheimers Res. Ther. 5, 1–8 (2013).
Borroni, B. et al. Anti-AMPA GluA3 antibodies in frontotemporal dementia: a new molecular target. Sci. Rep. 7, 6723 (2017).
Alberici, A. et al. Autoimmunity and frontotemporal dementia. Curr. Alzheimer Res. 15, 602–609 (2018).
Benussi, A. et al. Toward a glutamate hypothesis of frontotemporal dementia. Front. Neurosci. 13, 304 (2019).
Zheng, Y. et al. C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PLoS One 6, e21023 (2011).
Nonaka, T. et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 4, 124–134 (2013).
Kraemer, B. C. et al. Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol. 119, 409–419 (2010).
Yamashita, M. et al. Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett. 583, 2419–2424 (2009).
Boyd, J. D. et al. A high-content screen identifies novel compounds that inhibit stress-induced TDP-43 cellular aggregation and associated cytotoxicity. J. Biomol. Screen. 19, 44–56 (2014).
Capell, A. et al. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J. Neurosci. 31, 1885–1894 (2011).
Alberici, A. et al. Results from a pilot study on amiodarone administration in monogenic frontotemporal dementia with granulin mutation. Neurol. Sci. 35, 1215–1219 (2014).
Cenik, B. et al. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. J. Biol. Chem. 286, 16101–16108 (2011).
Patzke, H. et al. Development of the novel histone deacetylase inhibitor EVP-0334 for CNS indications [Poster 831.21/I12]. 38th Annual Meeting for the Society of Neuroscience https://www.abstractsonline.com/plan/ViewAbstract.aspx?mID=1981&sKey=7608b1d6-24c8-4a0d-85e8-68357f77ef82&cKey=b58f0420-5bde-407b-afe3-948d274e708b&mKey=afea068d-d012-4520-8e42-10e4d1af7944 (2008).
Leventhal, L. et al. The histone deacetylase inhibitor EVP-0334 is pro-cognitive in mice [Poster 831.20/I11]. 38th Annual Meeting for the Society of Neuroscience https://www.abstractsonline.com/plan/ViewAbstract.aspx?mID=1981&sKey=7608b1d6-24c8-4a0d-85e8-68357f77ef82&cKey=4256d6f9-3c58-4903-83ed-8d46d22972dd&mKey=afea068d-d012-4520-8e42-10e4d1af7944 (2008).
Patzke, H. et al. The novel histone deacetylase inhibitor EVP-0334 is pro-cognitive in rats [Poster 886.4/FF106]. 39th Annual Meeting for the Society of Neuroscience https://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=74e6ee34-69bf-41db-a73b-fdf5d47df43e&cKey=cfc48c84-c049-4c83-930b-2343b96acb62&mKey=081f7976-e4cd-4f3d-a0af-e8387992a658 (2009).
De Muynck, L. & Van Damme, P. In Frontiers in Clinical Drug Research – Alzheimer Disorders (ed. Atta-ur-Rahman) 231–291 (Bentham Books, 2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02149160 (2016).
Sha, S. J. et al. An 8-week, open-label, dose-finding study of nimodipine for the treatment of progranulin insufficiency from GRN gene mutations. Alzheimers Dement. 3, 507–512 (2017).
Galimberti, D., Fenoglio, C. & Scarpini, E. Progranulin as a therapeutic target for dementia. Expert Opin. Ther. Targets 22, 579–585 (2018).
Hu, F. et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68, 654–667 (2010).
Lee, W. C. et al. Targeted manipulation of the sortilin-progranulin axis rescues progranulin haploinsufficiency. Hum. Mol. Genet. 23, 1467–1478 (2014).
Alector Announces Data from On-going Phase 1b Trial Demonstrating that AL001 Reverses Progranulin Deficiency in Frontotemporal Dementia Patients. BioSpace https://www.biospace.com/article/-alector-announces-data-from-on-going-phase-1b-trial-demonstrating-that-al001-reverses-progranulin-deficiency-in-frontotemporal-dementia-patients/ (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03987295 (2019).
Bright, F. et al. Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 15, 540–555 (2019).
Tang, W. et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 (2011).
Holler, C. J. et al. Trehalose upregulates progranulin expression in human and mouse models of GRN haploinsufficiency: a novel therapeutic lead to treat frontotemporal dementia. Mol. Neurodegener. 11, 46 (2016).
Minami, S. S. et al. Reducing inflammation and rescuing FTD-related behavioral deficits in progranulin-deficient mice with α7 nicotinic acetylcholine receptor agonists. Biochem. Pharmacol. 97, 454–462 (2015).
Prevail Therapeutics Announces FDA Orphan Drug Designation Granted to PR006 for the Treatment of Patients with Frontotemporal Dementia with a GRN Mutation. BioSpace https://www.biospace.com/article/releases/prevail-therapeutics-announces-fda-orphan-drug-designation-granted-to-pr006-for-the-treatment-of-patients-with-frontotemporal-dementia-with-a-grn-mutation/ (2019).
Van Der Zee, J. et al. A pan-European study of the C9orf72 repeat associated with FTLD: geographic prevalence, genomic instability, and intermediate repeats. Hum. Mutat. 34, 363–373 (2013).
Kramer, N. J. et al. Spt4 selectively regulates the expression of C9orf72 sense and antisense mutant transcripts. Science 353, 708–712 (2016).
Donnelly, C. J. et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80, 415–428 (2013).
Sareen, D. et al. Targeting RNA foci in iPSC derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci. Transl Med. 5, 208ra149 (2013).
Lagier-Tourenne, C. et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl Acad. Sci. USA 19, E4530–E4539 (2013).
Hu, J. et al. Engineering duplex RNAs for challenging targets: recognition of GGGGCC/CCCCGG repeats at the ALS/FTD C9orf72 locus. Chem. Biol. 22, 1505–1511 (2015).
Martier, R. et al. Targeting RNA-mediated toxicity in C9orf72 ALS and/or FTD by RNAi-based gene therapy. Mol. Ther. Nucleic Acids 16, 26–37 (2019).
Kapeli, K. et al. Distinct and shared functions of ALS-associated proteins TDP-43, FUS and TAF15 revealed by multisystem analyses. Nat. Commun. 7, 12143 (2016).
Fujii, S. et al. Treatment with a global methyltransferase inhibitor induces the intranuclear aggregation of ALS-linked FUS mutant in vitro. Neurochem. Res. 41, 826–835 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02372773 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02365922 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02590276 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02327845 (2019).
Desmarais, P. et al. Therapeutic trial design for frontotemporal dementia and related disorders. J. Neurol. Neurosurg. Psychiatry 90, 412–442 (2019).
Semler, E. et al. A language-based sum score for the course and therapeutic intervention in primary progressive aphasia. Alzheimers Res. Ther. 10, 41 (2018).
Staffaroni, A. M. et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain 142, 443–459 (2019).
Ljubenkov, P. A. et al. Cerebrospinal fluid biomarkers predict frontotemporal dementia trajectory. Ann. Clin. Transl Neurol. 5, 1250–1263 (2018).
Modrego, P. & Lobo, A. A good marker does not mean a good target for clinical trials in Alzheimer’s disease: the amyloid hypothesis questioned. Neurodegener. Dis. Manag. 9, 119–121 (2019).
Author information
Authors and Affiliations
Contributions
F.P., M.L., D.S., A.D. and B.P.I. researched data for the manuscript. F.P., M.L., M.W. and B.P.I. wrote the manuscript. F.P., M.L., G.G. and B.P.I. contributed substantially to discussions of the article content and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.P.I. is an employee at Chiesi Farmaceutici and has developed and is co-inventor of patents on anti-Alzheimer disease drugs; he does not hold stock options. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks D. Galimberti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
The Genetic Frontotemporal Dementia Initiative: http://genfi.org.uk/
Glossary
- O-GlcNAcylation
-
A post-translational modification that involves the addition of a single N-acetyl-glucosamine residue (GlcNAc) to specific serine or threonine residues in proteins.
- Taxane diterpenoid
-
A class of diterpenes, some of which are widely used as chemotherapy agents.
Rights and permissions
About this article
Cite this article
Panza, F., Lozupone, M., Seripa, D. et al. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat Rev Neurol 16, 213–228 (2020). https://doi.org/10.1038/s41582-020-0330-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-0330-x
This article is cited by
-
Progranulin haploinsufficiency mediates cytoplasmic TDP-43 aggregation with lysosomal abnormalities in human microglia
Journal of Neuroinflammation (2024)
-
Primary progressive aphasia: six questions in search of an answer
Journal of Neurology (2024)
-
Recombinant Antibody Fragments for Immunotherapy of Parkinson’s Disease
BioDrugs (2024)
-
Den Rauch riechen, bevor man das Feuer sieht – die oligosymptomatische Prodromalphase neurodegenerativer Erkrankungen
Der Nervenarzt (2024)
-
Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets
Signal Transduction and Targeted Therapy (2023)