Abstract
Sirtuins (SIRTs) are putative regulators of lifespan in model organisms. Since the initial discovery that SIRTs could promote longevity in nematodes and flies, the identification of additional properties of these proteins has led to understanding of their roles as exquisite sensors that link metabolic activity to oxidative states. SIRTs have major roles in biological processes that are important in kidney development and physiological functions, including mitochondrial metabolism, oxidative stress, autophagy, DNA repair and inflammation. Furthermore, altered SIRT activity has been implicated in the pathophysiology and progression of acute and chronic kidney diseases, including acute kidney injury, diabetic kidney disease, chronic kidney disease, polycystic kidney disease, autoimmune diseases and renal ageing. The renoprotective roles of SIRTs in these diseases make them attractive therapeutic targets. A number of SIRT-activating compounds have shown beneficial effects in kidney disease models; however, further research is needed to identify novel SIRT-targeting strategies with the potential to treat and/or prevent the progression of kidney diseases and increase the average human healthspan.
Key points
-
Sirtuins (SIRTs) were initially of great scientific interest owing to their putative roles in modulating lifespan in experimental models; however, they are now generally understood to be drivers of healthy ageing.
-
SIRTs regulate key processes in cellular homeostasis, including metabolism, mitochondrial functions, oxidative stress, DNA repair, apoptosis, senescence and inflammation.
-
SIRTs have essential roles in kidney development and physiology owing to their broad cytoprotective effects in highly specialized and differentiated renal cell types, including podocytes and tubular cells.
-
SIRT alterations have been identified as drivers of various kidney diseases in humans and experimental models, including acute kidney injury, chronic kidney disease and diabetic kidney disease.
-
The identification of sirtuin-activating compounds is a major goal in the field; some sirtuin-activating compounds have been shown to boost SIRT activity and confer renoprotection in experimental models but few have reached clinical trials.
-
Novel SIRT-targeting compounds with improved potency and bioavailability are needed to realize the potential of these interventions to treat and prevent kidney diseases and increase the human healthspan.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Guarente, L. & Franklin, H. Epstein lecture: sirtuins, aging, and medicine. N. Engl. J. Med. 364, 2235–2244 (2011).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Rogina, B. & Helfand, S. L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA 101, 15998–16003 (2004).
Kanfi, Y. et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature 483, 218–221 (2012).
Burnett, C. et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477, 482–485 (2011).
Satoh, A. et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 18, 416–430 (2013).
Benigni, A. et al. Sirt3 deficiency shortens life span and impairs cardiac mitochondrial function rescued by Opa1 gene transfer. Antioxid. Redox Signal. 31, 1255–1271 (2019).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Yuan, H. & Marmorstein, R. Structural basis for sirtuin activity and inhibition. J. Biol. Chem. 287, 42428–42435 (2012).
Kiran, S. et al. Intracellular distribution of human SIRT7 and mapping of the nuclear/nucleolar localization signal. FEBS J. 280, 3451–3466 (2013).
Tanner, K. G., Landry, J., Sternglanz, R. & Denu, J. M. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc. Natl Acad. Sci. USA 97, 14178–14182 (2000).
Vaquero, A. et al. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell 16, 93–105 (2004).
Jin, Q. et al. Cytoplasm-localized SIRT1 enhances apoptosis. J. Cell Physiol. 213, 88–97 (2007).
Tanno, M., Sakamoto, J., Miura, T., Shimamoto, K. & Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 282, 6823–6832 (2007).
Li, Y., Xu, W., McBurney, M. W. & Longo, V. D. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 8, 38–48 (2008).
Hisahara, S. et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc. Natl Acad. Sci. USA 105, 15599–15604 (2008).
Tennen, R. I. & Chua, K. F. Chromatin regulation and genome maintenance by mammalian SIRT6. Trends Biochem. Sci. 36, 39–46 (2011).
Tsai, Y.-C., Greco, T. M., Boonmee, A., Miteva, Y. & Cristea, I. M. Functional proteomics establishes the interaction of SIRT7 with chromatin remodeling complexes and expands its role in regulation of RNA polymerase I transcription. Mol. Cell. Proteom. 11, 60–76 (2012).
Dryden, S. C., Nahhas, F. A., Nowak, J. E., Goustin, A.-S. & Tainsky, M. A. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol. Cell. Biol. 23, 3173–3185 (2003).
Nekooki-Machida, Y. & Hagiwara, H. Role of tubulin acetylation in cellular functions and diseases. Med. Mol. Morphol. 53, 191–197 (2020).
Serrano, L. et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes. Dev. 27, 639–653 (2013).
North, B. J. et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 33, 1438–1453 (2014).
Baeza, J., Smallegan, M. J. & Denu, J. M. Mechanisms and dynamics of protein acetylation in mitochondria. Trends Biochem. Sci. 41, 231–244 (2016).
Lombard, D. B. et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27, 8807–8814 (2007).
Iwahara, T., Bonasio, R., Narendra, V. & Reinberg, D. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol. Cell. Biol. 32, 5022–5034 (2012).
Zhang, X. et al. Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov. 5, 35 (2019).
Scher, M. B., Vaquero, A. & Reinberg, D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes. Dev. 21, 920–928 (2007).
Guarente, L. Calorie restriction and sirtuins revisited. Genes. Dev. 27, 2072–2085 (2013).
Nakagawa, T., Lomb, D. J., Haigis, M. C. & Guarente, L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 (2009).
Du, J. et al. Sirt5 is an NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011).
Houtkooper, R. H., Pirinen, E. & Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 (2012).
Ahn, B.-H. et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl Acad. Sci. USA 105, 14447–14452 (2008).
Finley, L. W. S. et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One 6, e23295 (2011).
Rahman, M. et al. Drosophila Sirt2/mammalian SIRT3 deacetylates ATP synthase β and regulates complex V activity. J. Cell Biol. 206, 289–305 (2014).
Yang, Y. et al. NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10. J. Biol. Chem. 285, 7417–7429 (2010).
Dittenhafer-Reed, K. E. et al. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 21, 637–646 (2015).
Hirschey, M. D. et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 (2010).
Shimazu, T. et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 12, 654–661 (2010).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Frescas, D., Valenti, L. & Accili, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 (2005).
Zhong, L. et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell 140, 280–293 (2010).
Lee, Y. et al. Myeloid sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype. Diabetes 66, 2659–2668 (2017).
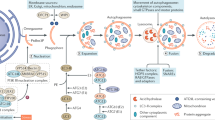
Zhan, M., Brooks, C., Liu, F., Sun, L. & Dong, Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 83, 568–581 (2013).
Zhang, Q. et al. Resveratrol activation of SIRT1/MFN2 can improve mitochondria function, alleviating doxorubicin-induced myocardial injury. Cancer Innov. 2, 253–264 (2023).
Samant, S. A. et al. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol. Cell. Biol. 34, 807–819 (2014).
Morigi, M. et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Invest. 125, 715–726 (2015).
Chen, Z. et al. Sirt6 deficiency contributes to mitochondrial fission and oxidative damage in podocytes via ROCK1-Drp1 signalling pathway. Cell Prolif. 55, e13296 (2022).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Dominy, J. E. et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol. Cell 48, 900–913 (2012).
Xin, T. & Lu, C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging 12, 16224–16237 (2020).
Wan, W. et al. Regulation of mitophagy by sirtuin family proteins: a vital role in aging and age-related diseases. Front. Aging Neurosci. 14, 845330 (2022).
Forrester, S. J., Kikuchi, D. S., Hernandes, M. S., Xu, Q. & Griendling, K. K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122, 877–902 (2018).
Ogura, Y., Kitada, M. & Koya, D. Sirtuins and renal oxidative stress. Antioxidants 10, 1198 (2021).
Wu, Q.-J. et al. The sirtuin family in health and disease. Signal. Transduct. Target. Ther. 7, 402 (2022).
He, F., Ru, X. & Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 21, 4777 (2020).
Chen, Y. et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 12, 534–541 (2011).
Xu, Y. et al. Studies on the regulatory mechanism of isocitrate dehydrogenase 2 using acetylation mimics. Sci. Rep. 7, 9785 (2017).
Alves-Fernandes, D. K. & Jasiulionis, M. G. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int. J. Mol. Sci. 20, 3153 (2019).
Meng, F. et al. Synergy between SIRT1 and SIRT6 helps recognize DNA breaks and potentiates the DNA damage response and repair in humans and mice. eLife 9, e55828 (2020).
Jeong, J. et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp. Mol. Med. 39, 8–13 (2007).
Kume, S. et al. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free. Radic. Biol. Med. 40, 2175–2182 (2006).
Kume, S. et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J. Biol. Chem. 282, 151–158 (2007).
Chuang, P. Y. et al. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One 6, e23566 (2011).
Jiao, X., Li, Y., Zhang, T., Liu, M. & Chi, Y. Role of Sirtuin3 in high glucose-induced apoptosis in renal tubular epithelial cells. Biochem. Biophys. Res. Commun. 480, 387–393 (2016).
Liu, M. et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat. Commun. 8, 413 (2017).
Guo, H. & Bechtel-Walz, W. The interplay of autophagy and oxidative stress in the kidney: what do we know? Nephron 147, 627–642 (2023).
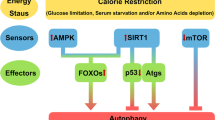
Lee, I. H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Exp. Mol. Med. 51, 1–11 (2019).
Lee, I. H. et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl Acad. Sci. USA 105, 3374–3379 (2008).
Chun, S. K. et al. Loss of sirtuin 1 and mitofusin 2 contributes to enhanced ischemia/reperfusion injury in aged livers. Aging Cell 17, e12761 (2018).
Wang, Y., Chang, J., Wang, Z.-Q. & Li, Y. Sirt3 promotes the autophagy of HK-2 human proximal tubular epithelial cells via the inhibition of Notch-1/Hes-1 signaling. Mol. Med. Rep. 24, 634 (2021).
Li, R. et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 18, 229–243 (2018).
Takasaka, N. et al. Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. J. Immunol. 192, 958–968 (2014).
Xu, C. et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 22, 1170–1179 (2020).
Lee, S.-H., Lee, J.-H., Lee, H.-Y. & Min, K.-J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 52, 24–34 (2019).
Hayakawa, T. et al. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS One 10, e0116480 (2015).
Huang, J. et al. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS One 3, e1710 (2008).
Zu, Y. et al. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ. Res. 106, 1384–1393 (2010).
Grootaert, M. O. J., Finigan, A., Figg, N. L., Uryga, A. K. & Bennett, M. R. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ. Res. 128, 474–491 (2021).
Zhao, G. et al. SIRT6 delays cellular senescence by promoting p27Kip1 ubiquitin-proteasome degradation. Aging 8, 2308–2323 (2016).
Diao, Z. et al. SIRT3 consolidates heterochromatin and counteracts senescence. Nucleic Acids Res. 49, 4203–4219 (2021).
Ma, C. et al. Sirt3 attenuates oxidative stress damage and rescues cellular senescence in rat bone marrow mesenchymal stem cells by targeting superoxide dismutase 2. Front. Cell Dev. Biol. 8, 599376 (2020).
Fan, X. et al. Sirt3 activates autophagy to prevent DOX-induced senescence by inactivating PI3K/AKT/mTOR pathway in A549 cells. Biochim. Biophys. Acta Mol. Cell Res. 1870, 119411 (2023).
Gómez, H. Reprogramming metabolism to enhance kidney tolerance during sepsis: the role of fatty acid oxidation, aerobic glycolysis, and epithelial de-differentiation. Nephron 147, 31–34 (2023).
Vachharajani, V. T. et al. Sirtuins link inflammation and metabolism. J. Immunol. Res. 2016, 8167273 (2016).
Kauppinen, A., Suuronen, T., Ojala, J., Kaarniranta, K. & Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 25, 1939–1948 (2013).
Rabadi, M. M. et al. High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int. 87, 95–108 (2015).
Labiner, H. E., Sas, K. M., Baur, J. A. & Sims, C. A. Sirtuin 1 deletion increases inflammation and mortality in sepsis. J. Trauma. Acute Care Surg. 93, 672–678 (2022).
Zhao, W.-Y., Zhang, L., Sui, M.-X., Zhu, Y.-H. & Zeng, L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 6, 33201 (2016).
Zhang, W. et al. An immune atlas of nephrolithiasis: single-cell mass cytometry on SIRT3 knockout and calcium oxalate-induced renal injury. J. Immunol. Res. 2021, 1260140 (2021).
Doke, T. et al. NAD+ precursor supplementation prevents mtRNA/RIG-I-dependent inflammation during kidney injury. Nat. Metab. 5, 414–430 (2023).
Pantaleon, M. & Kaye, P. L. Glucose transporters in preimplantation development. Rev. Reprod. 3, 77–81 (1998).
Ochocki, J. D. & Simon, M. C. Nutrient-sensing pathways and metabolic regulation in stem cells. J. Cell Biol. 203, 23–33 (2013).
Chevalier, R. L. Bioenergetic evolution explains prevalence of low nephron number at birth: risk factor for CKD. Kidney360 1, 863–879 (2020).
Mandal, S., Lindgren, A. G., Srivastava, A. S., Clark, A. T. & Banerjee, U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cell 29, 486–495 (2011).
Chung, S. et al. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 4, S60–S67 (2007).
Folmes, C. D. L. & Terzic, A. Metabolic determinants of embryonic development and stem cell fate. Reprod. Fertil. Dev. 27, 82–88 (2014).
Liu, J. et al. Regulation of nephron progenitor cell self-renewal by intermediary metabolism. J. Am. Soc. Nephrol. 28, 3323–3335 (2017).
Perico, L. et al. Post-translational modifications by SIRT3 de-2-hydroxyisobutyrylase activity regulate glycolysis and enable nephrogenesis. Sci. Rep. 11, 23580 (2021).
Pezzotta, A. et al. Low nephron number induced by maternal protein restriction is prevented by nicotinamide riboside supplementation depending on sirtuin 3 activation. Cells 11, 3316 (2022).
Luyckx, V. A. & Brenner, B. M. Birth weight, malnutrition and kidney-associated outcomes — a global concern. Nat. Rev. Nephrol. 11, 135–149 (2015).
Erichsen, L. & Adjaye, J. Crosstalk between age accumulated DNA-damage and the SIRT1-AKT-GSK3ß axis in urine derived renal progenitor cells. Aging 14, 8179–8204 (2022).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 3, 629–646 (2017).
Haschler, T. N. et al. Sirtuin 5 depletion impairs mitochondrial function in human proximal tubular epithelial cells. Sci. Rep. 11, 15510 (2021).
Chiba, T. et al. Sirtuin 5 regulates proximal tubule fatty acid oxidation to protect against AKI. J. Am. Soc. Nephrol. 30, 2384–2398 (2019).
Ozawa, S. et al. Glycolysis, but not mitochondria, responsible for intracellular ATP distribution in cortical area of podocytes. Sci. Rep. 5, 18575 (2015).
Perico, L., Conti, S., Benigni, A. & Remuzzi, G. Podocyte-actin dynamics in health and disease. Nat. Rev. Nephrol. 12, 692–710 (2016).
Tsai, Y.-C. et al. Upregulating sirtuin 6 ameliorates glycolysis, EMT and distant metastasis of pancreatic adenocarcinoma with krüppel-like factor 10 deficiency. Exp. Mol. Med. 53, 1623–1635 (2021).
Huang, W. et al. Sirt6 deficiency results in progression of glomerular injury in the kidney. Aging 9, 1069–1083 (2017).
Zhang, D., Li, S., Cruz, P. & Kone, B. C. Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription collecting duct. J. Biol. Chem. 284, 20917–20926 (2009).
Yang, S.-Y. et al. A low-salt diet increases the expression of renal sirtuin 1 through activation of the ghrelin receptor in rats. Sci. Rep. 6, 32787 (2016).
Noriega, L. G. et al. SIRT7 modulates the stability and activity of the renal K-Cl cotransporter KCC4 through deacetylation. EMBO Rep. 22, e50766 (2021).
Mercado, A. et al. The K+:Cl− cotransporter KCC4 is activated by deacetylation induced by the sirtuin7 (SIRT7). FASEB J. 29, 666.24 (2015).
Mattagajasingh, I. et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 104, 14855–14860 (2007).
Dioum, E. M. et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324, 1289–1293 (2009).
Potente, M. et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes. Dev. 21, 2644–2658 (2007).
Pezzotta, A. et al. Sirt3 deficiency promotes endothelial dysfunction and aggravates renal injury. PLoS One 18, e0291909 (2023).
Ho, K. M. & Morgan, D. J. R. The proximal tubule as the pathogenic and therapeutic target in acute kidney injury. Nephron 146, 494–502 (2022).
Koehler, F. C., Späth, M. R., Hoyer-Allo, K. J. R. & Müller, R.-U. Mechanisms of caloric restriction-mediated stress-resistance in acute kidney injury. Nephron 146, 234–238 (2022).
Hasegawa, K. et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 285, 13045–13056 (2010).
Funk, J. A. & Schnellmann, R. G. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol. Appl. Pharmacol. 273, 345–354 (2013).
Fan, H. et al. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 83, 404–413 (2013).
Han, S. et al. miR-132-3p promotes the cisplatin-induced apoptosis and inflammatory response of renal tubular epithelial cells by targeting SIRT1 via the NF-κB pathway. Int. Immunopharmacol. 99, 108022 (2021).
He, S. et al. NAD+ ameliorates endotoxin-induced acute kidney injury in a sirtuin1-dependent manner via GSK-3β/Nrf2 signalling pathway. J. Cell. Mol. Med. 26, 1979–1993 (2022).
Zhang, J., Yang, S., Chen, F., Li, H. & Chen, B. Ginkgetin aglycone ameliorates LPS-induced acute kidney injury by activating SIRT1 via inhibiting the NF-κB signaling pathway. Cell Biosci. 7, 44 (2017).
Lempiäinen, J., Finckenberg, P., Levijoki, J. & Mervaala, E. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br. J. Pharmacol. 166, 1905–1915 (2012).
Gan, Y., Tao, S., Cao, D., Xie, H. & Zeng, Q. Protection of resveratrol on acute kidney injury in septic rats. Hum. Exp. Toxicol. 36, 1015–1022 (2017).
Li, Z. et al. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int. 93, 881–892 (2018).
Miao, J. et al. Sirtuin 6 is a key contributor to gender differences in acute kidney injury. Cell Death Discov. 9, 134 (2023).
Jian, Y. et al. Sirt3 mitigates LPS-induced mitochondrial damage in renal tubular epithelial cells by deacetylating YME1L1. Cell Prolif. 56, e13362 (2023).
Shen, L., Zhang, Q., Tu, S. & Qin, W. SIRT3 mediates mitofusin 2 ubiquitination and degradation to suppress ischemia reperfusion-induced acute kidney injury. Exp. Cell Res. 408, 112861 (2021).
Brooks, C., Wei, Q., Cho, S.-G. & Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 119, 1275–1285 (2009).
Ugur, S. et al. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren. Fail. 37, 332–336 (2015).
Zhao, L. et al. Protective effect of the total flavonoids from Rosa laevigata Michx fruit on renal ischemia-reperfusion injury through suppression of oxidative stress and inflammation. Molecules 21, 952 (2016).
Li, Y. et al. Activation of sirtuin 3 by silybin attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Front. Pharmacol. 8, 178 (2017).
Xu, S. et al. SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model. Oxid. Med. Cell. Longev. 2016, 7296092 (2016).
Pan, J. S.-C. et al. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J. Am. Soc. Nephrol. 26, 364–378 (2015).
Perico, L. et al. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat. Commun. 8, 983 (2017).
Miyasato, Y. et al. Sirtuin 7 deficiency ameliorates cisplatin-induced acute kidney injury through regulation of the inflammatory response. Sci. Rep. 8, 5927 (2018).
Sánchez-Navarro, A. et al. Sirtuin 7 deficiency reduces inflammation and tubular damage induced by an episode of acute kidney injury. Int. J. Mol. Sci. 23, 2573 (2022).
Poyan Mehr, A. et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 24, 1351–1359 (2018).
Katsyuba, E. et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563, 354–359 (2018).
Cortinovis, M., Perico, N., Ruggenenti, P., Remuzzi, A. & Remuzzi, G. Glomerular hyperfiltration. Nat. Rev. Nephrol. 18, 435–451 (2022).
Kitada, M., Kume, S., Takeda-Watanabe, A., Kanasaki, K. & Koya, D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin. Sci. 124, 153–164 (2013).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Baur, J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006).
Zoja, C., Xinaris, C. & Macconi, D. Diabetic nephropathy: novel molecular mechanisms and therapeutic targets. Front. Pharmacol. 11, 586892 (2020).
Yasuda, I. et al. Pre-emptive short-term nicotinamide mononucleotide treatment in a mouse model of diabetic nephropathy. J. Am. Soc. Nephrol. 32, 1355–1370 (2021).
Hasegawa, K. et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 19, 1496–1504 (2013).
Yubero-Serrano, E. M. et al. Effects of sevelamer carbonate on advanced glycation end products and antioxidant/pro-oxidant status in patients with diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 10, 759–766 (2015).
Fan, Y. et al. Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through AMPK activation. Int. J. Biol. Sci. 15, 701–713 (2019).
Wang, X., Ji, T., Li, X., Qu, X. & Bai, S. FOXO3a protects against kidney injury in type II diabetic nephropathy by promoting Sirt6 expression and inhibiting Smad3 acetylation. Oxid. Med. Cell. Longev. 2021, 5565761 (2021).
Liu, J. et al. CircRNA circ-ITCH improves renal inflammation and fibrosis in streptozotocin-induced diabetic mice by regulating the miR-33a-5p/SIRT6 axis. Inflamm. Res. 70, 835–846 (2021).
Muraoka, H. et al. Role of Nampt-Sirt6 axis in renal proximal tubules in extracellular matrix deposition in diabetic nephropathy. Cell Rep. 27, 199–212.e5 (2019).
Ji, L. et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int. J. Oncol. 55, 103–115 (2019).
Locatelli, M. et al. Manipulating Sirtuin 3 pathway ameliorates renal damage in experimental diabetes. Sci. Rep. 10, 8418 (2020).
Wang, X. X. et al. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J. Am. Soc. Nephrol. 27, 1362–1378 (2016).
Locatelli, M. et al. Sirtuin 3 deficiency aggravates kidney disease in response to high-fat diet through lipotoxicity-induced mitochondrial damage. Int. J. Mol. Sci. 23, 8345 (2022).
Zhou, Y. et al. Metrnl alleviates lipid accumulation by modulating mitochondrial homeostasis in diabetic nephropathy. Diabetes 72, 611–626 (2023).
Macconi, D., Remuzzi, G. & Benigni, A. Key fibrogenic mediators: old players. Renin-angiotensin system. Kidney Int. Suppl. 4, 58–64 (2014).
Liu, X. et al. Impaired nicotinamide adenine dinucleotide biosynthesis in the kidney of chronic kidney disease. Front. Physiol. 12, 723690 (2021).
Zhang, Y. et al. Sirtuin 1 activation reduces transforming growth factor-β1-induced fibrogenesis and affords organ protection in a model of progressive, experimental kidney and associated cardiac disease. Am. J. Pathol. 187, 80–90 (2017).
He, W. et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Invest. 120, 1056–1068 (2010).
Simic, P. et al. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep. 3, 1175–1186 (2013).
Huang, X.-Z. et al. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J. Cell. Biochem. 115, 996–1005 (2014).
Vasko, R. et al. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J. Am. Soc. Nephrol. 25, 276–291 (2014).
Kida, Y., Zullo, J. A. & Goligorsky, M. S. Endothelial sirtuin 1 inactivation enhances capillary rarefaction and fibrosis following kidney injury through Notch activation. Biochem. Biophys. Res. Commun. 478, 1074–1079 (2016).
Li, P. et al. SIRT1 attenuates renal fibrosis by repressing HIF-2α. Cell Death Discov. 7, 59 (2021).
Lipphardt, M., Dihazi, H., Müller, G. A. & Goligorsky, M. S. Fibrogenic secretome of sirtuin 1-deficient endothelial cells: Wnt, notch and glycocalyx rheostat. Front. Physiol. 9, 1325 (2018).
Cai, J. et al. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 97, 106–118 (2020).
Li, X., Li, W., Zhang, Z., Wang, W. & Huang, H. SIRT6 overexpression retards renal interstitial fibrosis through targeting HIPK2 in chronic kidney disease. Front. Pharmacol. 13, 1007168 (2022).
Cai, J. et al. Phosphorylation by GSK-3β increases the stability of SIRT6 to alleviate TGF-β-induced fibrotic response in renal tubular cells. Life Sci. 308, 120914 (2022).
Benigni, A., Perico, L. & Macconi, D. Mitochondrial dynamics is linked to longevity and protects from end-organ injury: the emerging role of sirtuin 3. Antioxid. Redox Signal. 25, 185–199 (2016).
Sundaresan, N. R. et al. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol. Cell. Biol. 36, 678–692 (2015).
Cheng, L. et al. SIRT3 deficiency exacerbates early-stage fibrosis after ischaemia-reperfusion-induced AKI. Cell. Signal. 93, 110284 (2022).
Zhang, Y. et al. Sirtuin 3 regulates mitochondrial protein acetylation and metabolism in tubular epithelial cells during renal fibrosis. Cell Death Dis. 12, 847 (2021).
Quan, Y. et al. Sirtuin 3 activation by honokiol decreases unilateral ureteral obstruction-induced renal inflammation and fibrosis via regulation of mitochondrial dynamics and the renal NF-κB-TGF-β1/Smad signaling pathway. Int. J. Mol. Sci. 21, 402 (2020).
Li, N. et al. SIRT3-KLF15 signaling ameliorates kidney injury induced by hypertension. Oncotarget 8, 39592–39604 (2017).
Srivastava, S. P. et al. Endothelial SIRT3 regulates myofibroblast metabolic shifts in diabetic kidneys. iScience 24, 102390 (2021).
The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388, 117–127 (2023).
Wang, Z. et al. Canagliflozin ameliorates epithelial-mesenchymal transition in high-salt diet-induced hypertensive renal injury through restoration of sirtuin 3 expression and the reduction of oxidative stress. Biochem. Biophys. Res. Commun. 653, 53–61 (2023).
Packer, M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation 146, 1383–1405 (2022).
Li, W. et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 chronic kidney disease. J. Clin. Invest. 132, e150051 (2022).
Badi, I. et al. miR-34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (Sirtuin 1) and Axl (AXL receptor tyrosine kinase). Arterioscler. Thromb. Vasc. Biol. 38, 2079–2090 (2018).
Liu, X. et al. Spermidine inhibits vascular calcification in chronic kidney disease through modulation of SIRT1 signaling pathway. Aging Cell 20, e13377 (2021).
Liu, S.-M. et al. Intermedin alleviates vascular calcification in CKD through sirtuin 3-mediated inhibition of mitochondrial oxidative stress. Pharmaceuticals 15, 1224 (2022).
Ong, A. C. M., Devuyst, O., Knebelmann, B. & Walz, G. & ERA-EDTA Working Group for Inherited Kidney Diseases. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385, 1993–2002 (2015).
Zhou, X. et al. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J. Clin. Invest. 123, 3084–3098 (2013).
El Ters, M. et al. Biological efficacy and safety of niacinamide in patients with ADPKD. Kidney Int. Rep. 5, 1271–1279 (2020).
Shen, P. et al. SIRT1: a potential therapeutic target in autoimmune diseases. Front. Immunol. 12, 779177 (2021).
Olivares, D. et al. Urinary levels of sirtuin-1 associated with disease activity in lupus nephritis. Clin. Sci. 132, 569–579 (2018).
Consiglio, C. R. et al. SIRT1 promoter polymorphisms as clinical modifiers on systemic lupus erythematosus. Mol. Biol. Rep. 41, 4233–4239 (2014).
Hu, N., Long, H., Zhao, M., Yin, H. & Lu, Q. Aberrant expression pattern of histone acetylation modifiers and mitigation of lupus by SIRT1-siRNA in MRL/lpr mice. Scand. J. Rheumatol. 38, 464–471 (2009).
Gan, H. et al. B cell Sirt1 deacetylates histone and non-histone proteins for epigenetic modulation of AID expression and the antibody response. Sci. Adv. 6, eaay2793 (2020).
Guan, Y. et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352 (2017).
Kume, S. et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 120, 1043–1055 (2010).
Chuang, P. Y. et al. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am. J. Physiol. Renal Physiol. 313, F621–F628 (2017).
Chen, J. et al. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am. J. Pathol. 180, 973–983 (2012).
Mitchell, S. J. et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 6, 836–843 (2014).
Zhang, N. et al. Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-κB signaling. Cell Cycle 15, 1009–1018 (2016).
Benigni, A. et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 119, 524–530 (2009).
Dai, H., Sinclair, D. A., Ellis, J. L. & Steegborn, C. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol. Ther. 188, 140–154 (2018).
Dang, W. The controversial world of sirtuins. Drug. Discov. Today Technol. 12, e9–e17 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/search?intr=Resveratrol&limit=100&page=1&viewType=Table (accessed 2 February 2024).
Baksi, A. et al. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br. J. Clin. Pharmacol. 78, 69–77 (2014).
Curry, A. M., White, D. S., Donu, D. & Cen, Y. Human sirtuin regulators: the “Success” stories. Front. Physiol. 12, 752117 (2021).
Radenkovic, D. R. & Verdin, E. Clinical evidence for targeting NAD therapeutically. Pharmaceuticals 13, 247 (2020).
Camacho-Pereira, J. et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 (2016).
Ugamraj, H. S. et al. TNB-738, a biparatopic antibody, boosts intracellular NAD+ by inhibiting CD38 ecto-enzyme activity. mAbs 14, 2095949 (2022).
Loboda, A., Sobczak, M., Jozkowicz, A. & Dulak, J. TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016, 8319283 (2016).
Zhuo, L. et al. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell. Physiol. Biochem. 27, 681–690 (2011).
Ren, Y. et al. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 39, 1317–1324 (2017).
Mercken, E. M. et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 13, 787–796 (2014).
Davenport, A. M., Huber, F. M. & Hoelz, A. Structural and functional analysis of human SIRT1. J. Mol. Biol. 426, 526–541 (2014).
Author information
Authors and Affiliations
Contributions
L.P. and A.B. researched the data for the article and wrote the manuscript. All authors contributed to discussions of the content and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Michael Goligorsky and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perico, L., Remuzzi, G. & Benigni, A. Sirtuins in kidney health and disease. Nat Rev Nephrol 20, 313–329 (2024). https://doi.org/10.1038/s41581-024-00806-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-024-00806-4
This article is cited by
-
Ability of NAD and Sirt1 to epigenetically suppress albuminuria
Clinical and Experimental Nephrology (2024)