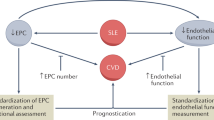
Abstract
Vascular disease is a major cause of morbidity and mortality in patients with systemic autoimmune diseases, particularly systemic lupus erythematosus (SLE). Although comorbid cardiovascular risk factors are frequently present in patients with SLE, they do not explain the high burden of premature vascular disease. Profound innate and adaptive immune dysregulation seems to be the primary driver of accelerated vascular damage in SLE. In particular, evidence suggests that dysregulation of type 1 interferon (IFN-I) and aberrant neutrophils have key roles in the pathogenesis of vascular damage. IFN-I promotes endothelial dysfunction directly via effects on endothelial cells and indirectly via priming of immune cells that contribute to vascular damage. SLE neutrophils are vasculopathic in part because of their increased ability to form immunostimulatory neutrophil extracellular traps. Despite improvements in clinical care, cardiovascular disease remains the leading cause of mortality among patients with SLE, and treatments that improve vascular outcomes are urgently needed. Improved understanding of the mechanisms of vascular injury in inflammatory conditions such as SLE could also have implications for common cardiovascular diseases, such as atherosclerosis and hypertension, and may ultimately lead to personalized therapeutic approaches to the prevention and treatment of this potentially fatal complication.
Key points
-
Systemic lupus erythematosus (SLE) is associated with an increased risk of developing cardiovascular disease (CVD), which is a leading cause of morbidity and mortality.
-
Traditional risk factors, such as older age, male sex, smoking, hypertension, total cholesterol levels and diabetes mellitus, do not fully account for the increased risk of CVD among patients with SLE.
-
Type 1 interferon and neutrophils may have important pathogenic roles in vascular damage in SLE.
-
SLE remains a leading cause of premature death in women, mainly as a result of CVD; therefore, treatments that improve vascular outcomes are urgently needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Del Rincón, I., Williams, K., Stern, M. P., Freeman, G. L. & Escalante, A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 44, 2737–2745 (2001).
Solomon, D. H. et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann. Rheum. Dis. 69, 1920–1925 (2010).
Roman, M. J. et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349, 2399–2406 (2003).
Weber, B. & Liao, K. P. Evidence for biologic drug modifying anti-rheumatoid drugs and association with cardiovascular disease risk mitigation in inflammatory arthritis. Rheum. Dis. Clin. North. Am. 49, 165–178 (2023).
Myasoedova, E., Davis, J. M., Roger, V. L., Achenbach, S. J. & Crowson, C. S. Improved incidence of cardiovascular disease in patients with incident rheumatoid arthritis in the 2000s: a population-based cohort study. J. Rheumatol. 48, 1379–1387 (2021).
Yen, E. Y. & Singh, R. R. Brief report: lupus-an unrecognized leading cause of death in young females: a population-based study using nationwide death certificates, 2000-2015. Arthritis Rheumatol. 70, 1251–1255 (2018).
Moghaddam, B., Marozoff, S., Li, L., Sayre, E. C. & Zubieta, J. A. A.- All-cause and cause-specific mortality in systemic lupus erythematosus: a population-based study. Rheumatology 61, 367–376 (2021).
Asanuma, Y. et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349, 2407–2415 (2003).
Mendoza-Pinto, C. et al. Endothelial dysfunction and arterial stiffness in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Atherosclerosis 297, 55–63 (2020).
Bulkley, B. H. & Roberts, W. C. The heart in systemic lupus erythematosus and the changes induced in it by corticosteroid therapy: a study of 36 necropsy patients. Am. J. Med. 58, 243–264 (1975).
Urowitz, M. B. et al. The bimodal mortality pattern of systemic lupus erythematosus. Am. J. Med. 60, 221–225 (1976).
Esdaile, J. M. et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 44, 2331–2337 (2001).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am. J. Epidemiol. 145, 408–415 (1997).
Bruce, I. N. ‘Not only…but also’: factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology 44, 1492–1502 (2005).
Schanberg, L. E. et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 64, 285–296 (2012).
Barbhaiya, M. et al. Comparative risks of cardiovascular disease in patients with systemic lupus erythematosus, diabetes mellitus, and in general Medicaid recipients. Arthritis Care Res. 72, 1431–1439 (2020).
Mak, A., Liu, Y. & Ho, R. C. Endothelium-dependent but not endothelium-independent flow-mediated dilation is significantly reduced in patients with systemic lupus erythematosus without vascular events: a metaanalysis and metaregression. J. Rheumatol. 38, 1296–1303 (2011).
Weber, B. N. et al. Coronary microvascular dysfunction in systemic lupus erythematosus. J. Am. Heart Assoc. 10, e018555 (2021).
Koletsos, N. et al. Skin microvascular dysfunction in systemic lupus erythematosus patients with and without cardiovascular risk factors. Rheumatology 60, 2834–2841 (2021).
Munguia-Realpozo, P. et al. Systemic lupus erythematosus and hypertension. Autoimmun. Rev. 18, 102371 (2019).
Thacker, S. G. et al. The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J. Immunol. 185, 4457–4469 (2010).
Clowse, M. E., Jamison, M., Myers, E. & James, A. H. A national study of the complications of lupus in pregnancy. Am. J. Obstet. Gynecol. 199, 127.e121–126 (2008).
Tselios, K., Koumaras, C., Urowitz, M. B. & Gladman, D. D. Do current arterial hypertension treatment guidelines apply to systemic lupus erythematosus patients? A critical appraisal. Semin. Arthritis Rheum. 43, 521–525 (2014).
Schoenfeld, S. R., Kasturi, S. & Costenbader, K. H. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin. Arthritis Rheum. 43, 77–95 (2013).
Gandelman, J. S. et al. Increased incidence of resistant hypertension in patients with systemic lupus erythematosus: a retrospective cohort study. Arthritis Care Res. 72, 534–543 (2020).
Whelton, P. K. et al. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines: comparisons, reflections, and recommendations. Circulation 146, 868–877 (2022).
Urowitz, M. B. et al. Accumulation of coronary artery disease risk factors over three years: data from an international inception cohort. Arthritis Care Res. 59, 176–180 (2008).
Tselios, K., Koumaras, C., Gladman, D. D. & Urowitz, M. B. Dyslipidemia in systemic lupus erythematosus: just another comorbidity? Semin. Arthritis Rheum. 45, 604–610 (2016).
Sun, C. et al. Prevalence and risk of metabolic syndrome in patients with systemic lupus erythematosus: a meta-analysis. Int. J. Rheum. Dis. 20, 917–928 (2017).
Apostolopoulos, D., Vincent, F., Hoi, A. & Morand, E. Associations of metabolic syndrome in SLE. Lupus Sci. Med. 7, e000436 (2020).
Ronda, N. et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 73, 609–615 (2014).
Tyrrell, P. N., Beyene, J., Benseler, S. M., Sarkissian, T. & Silverman, E. D. Predictors of lipid abnormalities in children with new-onset systemic lupus erythematosus. J. Rheumatol. 34, 2112–2119 (2007).
Ockene, I. S. & Miller, N. H. Cigarette smoking, cardiovascular disease, and stroke. Circulation 96, 3243–3247 (1997).
Lopez-Neyman, S. M. et al. Racial disparities and prevalence of cardiovascular disease risk factors, cardiometabolic risk factors, and cardiovascular health metrics among US adults: NHANES 2011–2018. Sci. Rep. 12, 19475 (2022).
Costenbader, K. H. et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 50, 849–857 (2004).
Barbhaiya, M. et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Ann. Rheum. Dis. 77, 196–202 (2018).
Montes, R. A. et al. Smoking and its association with morbidity in systemic lupus erythematosus evaluated by the systemic lupus International Collaborating Clinics/American College of Rheumatology Damage Index: preliminary data and systematic review. Arthritis Rheumatol. 68, 441–448 (2016).
Raymond, W. D., Hamdorf, M., Furfaro, M., Eilertsen, G. O. & Nossent, J. C. Smoking associates with increased BAFF and decreased interferon-γ levels in patients with systemic lupus erythematosus. Lupus Sci. Med. 8, e000537 (2021).
Bruce, I. N., Urowitz, M. B., Gladman, D. D., Ibañez, D. & Steiner, G. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto risk factor study. Arthritis Rheum. 48, 3159–3167 (2003).
Legge, A., Blanchard, C. & Hanly, J. G. Physical activity, sedentary behaviour and their associations with cardiovascular risk in systemic lupus erythematosus. Rheumatology 59, 1128–1136 (2019).
Matthews, C. E. et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 167, 875–881 (2008).
Barbhaiya, M. et al. Race/ethnicity and cardiovascular events among patients with systemic lupus erythematosus. Arthritis Rheumatol. 69, 1823–1831 (2017).
Scalzi, L. V., Hollenbeak, C. S. & Wang, L. Racial disparities in age at time of cardiovascular events and cardiovascular-related death in patients with systemic lupus erythematosus. Arthritis Rheum. 62, 2767–2775 (2010).
Pryor, K. P., Barbhaiya, M., Costenbader, K. H. & Feldman, C. H. Disparities in lupus and lupus nephritis care and outcomes among US Medicaid beneficiaries. Rheum. Dis. Clin. North. Am. 47, 41–53 (2021).
Eneanya, N. D. et al. Health inequities and the inappropriate use of race in nephrology. Nat. Rev. Nephrol. 18, 84–94 (2022).
Larsen, C. P., Beggs, M. L., Saeed, M. & Walker, P. D. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J. Am. Soc. Nephrol. 24, 722–725 (2013).
Blazer, A. et al. Apolipoprotein L1 risk genotypes in Ghanaian patients with systemic lupus erythematosus: a prospective cohort study. Lupus Sci. Med. 8, e000460 (2021).
Freedman, B. I. et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 66, 390–396 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137–144 (2020).
David, C. et al. Clonal haematopoiesis of indeterminate potential and cardiovascular events in systemic lupus erythematosus (HEMATOPLUS study).Rheumatology 61, 4355–4363 (2022).
Magder, L. S. & Petri, M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am. J. Epidemiol. 176, 708–719 (2012).
Nikpour, M., Urowitz, M. B., Ibanez, D., Harvey, P. J. & Gladman, D. D. Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective proof-of-concept cohort study. Arthritis Res. Ther. 13, R156 (2011).
Kandane-Rathnayake, R. et al. Lupus low disease activity state and remission and risk of mortality in patients with systemic lupus erythematosus: a prospective, multinational, longitudinal cohort study. Lancet Rheumatol. 4, e822–e830 (2022).
Ajeganova, S., Hafström, I. & Frostegård, J. Patients with SLE have higher risk of cardiovascular events and mortality in comparison with controls with the same levels of traditional risk factors and intima-media measures, which is related to accumulated disease damage and antiphospholipid syndrome: a case-control study over 10 years. Lupus Sci. Med. 8, e000454 (2021).
Li, D. et al. Initial disease severity, cardiovascular events and all-cause mortality among patients with systemic lupus erythematosus. Rheumatology 59, 495–504 (2020).
Ugarte-Gil, M. F. et al. Impact of glucocorticoids on the incidence of lupus-related major organ damage: a systematic literature review and meta-regression analysis of longitudinal observational studies. Lupus Sci. Med. 8, e000590 (2021).
Hoover, P. J. & Costenbader, K. H. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int. 90, 487–492 (2016).
Hermansen, M.-L., Lindhardsen, J., Torp-Pedersen, C., Faurschou, M. & Jacobsen, S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology 56, 709–715 (2017).
Garg, S. et al. High burden of premature arteriosclerosis on renal biopsy results in incident lupus nephritis. Arthritis Care Res. 73, 394–401 (2021).
Ünlü, O., Zuily, S. & Erkan, D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur. J. Rheumatol. 3, 75–84 (2016).
Crow, M. K., Kirou, K. A. & Wohlgemuth, J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity 36, 481–490 (2003).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Borden, E. C. et al. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug. Discov. 6, 975–990 (2007).
Feinstein, M. J. et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 140, e98–e124 (2019).
Petta, S. et al. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational studies. Gastroenterology 150, 145–155.e4 (2016).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
RAMESH, V. et al. Intracerebral large artery disease in Aicardi–Goutières syndrome implicates SAMHD1 in vascular homeostasis. Dev. Med. Child. Neurol. 52, 725–732 (2010).
Zhang, M., Malik, A. B. & Rehman, J. Endothelial progenitor cells and vascular repair. Curr. Opin. Hematol. 21, 224–228 (2014).
Hill, J. M. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 348, 593–600 (2003).
Rajagopalan, S. et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood 103, 3677–3683 (2004).
Denny, M. F. et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110, 2907–2915 (2007).
Thacker, S. G. et al. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum. 64, 2975–2985 (2012).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019).
Kahlenberg, J. M. et al. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J. Immunol. 187, 6143–6156 (2011).
Kahlenberg, J. M. et al. An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheumatol. 66, 152–162 (2014).
Elhage, R., Ljunggren, H.-G. & Hansson, G. K. Proatherogenic role of interleukin-18: effects on inflammation and action on vascular cells: authors’ retrospective. Cardiovasc. Res. 96, 176–180 (2012).
Rabkin, S. W. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat. Clin. Pract. Cardiovasc. Med. 6, 192–199 (2009).
Thomas, J. M. et al. IL-18 (Interleukin-18) produced by renal tubular epithelial cells promotes renal inflammation and injury during deoxycorticosterone/salt-induced hypertension in mice. Hypertension 78, 1296–1309 (2021).
Buie, J. J., Renaud, L. L., Muise-Helmericks, R. & Oates, J. C. IFN-α negatively regulates the expression of endothelial nitric oxide synthase and nitric oxide production: implications for systemic lupus erythematosus. J. Immunol. 199, 1979–1988 (2017).
Daneshpajouhnejad, P., Kopp, J. B., Winkler, C. A. & Rosenberg, A. Z. The evolving story of apolipoprotein L1 nephropathy: the end of the beginning. Nat. Rev. Nephrol. 18, 307–320 (2022).
Blazer, A. et al. APOL1 variant-expressing endothelial cells exhibit autophagic dysfunction and mitochondrial stress. Front. Genet. 13, 769936 (2022).
Shah, S. S. et al. APOL1 kidney risk variants induce cell death via mitochondrial translocation and opening of the mitochondrial permeability transition pore. J. Am. Soc. Nephrol. 30, 2355–2368 (2019).
Lin, J.-D. et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4, e124574 (2019).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Nehar-Belaid, D. et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 21, 1094–1106 (2020).
Billi, A. C. et al. Nonlesional lupus skin contributes to inflammatory education of myeloid cells and primes for cutaneous inflammation. Sci. Transl. Med. 14, eabn2263 (2022).
Perez, R. K. et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science 376, eabf1970 (2022).
Li, J. et al. Interferon-α priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-α and atherosclerosis in lupus. Arthritis Rheumatism 63, 492–502 (2011).
Palmer, H. & Libby, P. Interferon-β. A potential autocrine regulator of human vascular smooth muscle cell growth. Lab. Invest. 66, 715–721 (1992).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Lood, C. et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood 116, 1951–1957 (2010).
Duffau, P. et al. Platelet CD154 potentiates interferon-α secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2, 47ra63–47ra63 (2010).
Nagahama, M. et al. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity 33, 85–94 (2001).
Nhek, S. et al. Activated platelets induce endothelial cell activation via an interleukin-1β pathway in systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 37, 707–716 (2017).
Weckerle, C. E. et al. Brief report: large-scale analysis of tumor necrosis factor α levels in systemic lupus erythematosus. Arthritis Rheum. 64, 2947–2952 (2012).
Cates, A. M. et al. Interleukin 10 hampers endothelial cell differentiation and enhances the effects of interferon α on lupus endothelial cell progenitors. Rheumatology 54, 1114–1123 (2015).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Marczynski, P. et al. Vascular inflammation and dysfunction in lupus-prone mice-IL-6 as mediator of disease initiation. Int. J. Mol. Sci. 22, 2291 (2021).
Wigerblad, G. & Kaplan, M. J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 23, 274–288 (2022).
Wang, Y. et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184, 205–213 (2009).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Denny, M. F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 (2010).
Carmona-Rivera, C. & Kaplan, M. J. Low-density granulocytes in systemic autoimmunity and autoinflammation. Immunol. Rev. 314, 313–325 (2023).
Mistry, P. et al. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 116, 25222–25228 (2019).
Bashant, K. R. et al. Proteomic, biomechanical and functional analyses define neutrophil heterogeneity in systemic lupus erythematosus. Ann. Rheum. Dis. 80, 209–218 (2021).
Kahlenberg, J. M., Carmona-Rivera, C., Smith, C. K. & Kaplan, M. J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 190, 1217–1226 (2013).
Carlucci, P. M. et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 3, e99276 (2018).
Hartl, J. et al. Autoantibody-mediated impairment of DNASE1L3 activity in sporadic systemic lupus erythematosus. J. Exp. Med. 218, e20201138 (2021).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19–73ra19 (2011).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Gehrke, N. et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39, 482–495 (2013).
Blanco, L. P. et al. RNA externalized by neutrophil extracellular traps promotes inflammatory pathways in endothelial cells. Arthritis Rheumatol. 73, 2282–2292 (2021).
Carmona-Rivera, C., Zhao, W., Yalavarthi, S. & Kaplan, M. J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74, 1417–1424 (2015).
Smith, C. K. et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 66, 2532–2544 (2014).
Knight, J. S. et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 74, 2199–2206 (2015).
Liu, Y. et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front. Immunol. 9, 1680 (2018).
Moore, S. et al. Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J. Rheumatol. 47, 1652–1660 (2020).
Walport, M. J. Complement and systemic lupus erythematosus. Arthritis Res. 4, S279–293 (2002).
Watanabe, H. et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J. Immunol. 164, 786–794 (2000).
Bao, L., Haas, M. & Quigg, R. J. Complement factor H deficiency accelerates development of lupus nephritis. J. Am. Soc. Nephrol. 22, 285–295 (2011).
Svenungsson, E. et al. Complement deposition, C4d, on platelets is associated with vascular events in systemic lupus erythematosus. Rheumatology 59, 3264–3274 (2020).
Holers, V. M. et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 195, 211–220 (2002).
Kim, M. Y. et al. Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies. Ann. Rheum. Dis. 77, 549–555 (2018).
Kello, N. et al. Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: case series and review of literature. Semin. Arthritis Rheum. 49, 74–83 (2019).
Steinmetz, O. M. et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis1. J. Immunol. 183, 4693–4704 (2009).
Suárez-Fueyo, A., Bradley, S. J. & Tsokos, G. C. T cells in systemic lupus erythematosus. Curr. Opin. Immunol. 43, 32–38 (2016).
Wilhelm, A. J., Rhoads, J. P., Wade, N. S. & Major, A. S. Dysregulated CD4+ T cells from SLE-susceptible mice are sufficient to accelerate atherosclerosis in LDLr−/− mice. Ann. Rheum. Dis. 74, 778–785 (2015).
Crispín, J. C. et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181, 8761–8766 (2008).
Shin, M. S., Lee, N. & Kang, I. Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr. Opin. Rheumatol. 23, 444–448 (2011).
Li, W. et al. T cells expressing the lupus susceptibility allele Pbx1d enhance autoimmunity and atherosclerosis in dyslipidemic mice. JCI Insight 5, e138274 (2020).
Choi, S. C. et al. The lupus susceptibility gene Pbx1 regulates the balance between follicular helper T cell and regulatory T cell differentiation. J. Immunol. 197, 458–469 (2016).
Tellides, G. et al. Interferon-γ elicits arteriosclerosis in the absence of leukocytes. Nature 403, 207–211 (2000).
Dunlap, G. S. et al. Single-cell transcriptomics reveals distinct effector profiles of infiltrating T cells in lupus skin and kidney. JCI Insight 7, e156341 (2022).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Baumgarth, N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46 (2011).
Gruber, S. et al. Sialic acid-binding immunoglobulin-like lectin g promotes atherosclerosis and liver inflammation by suppressing the protective functions of b-1 cells. Cell Rep. 14, 2348–2361 (2016).
Enghard, P. et al. Class switching and consecutive loss of dsDNA-reactive B1a B cells from the peritoneal cavity during murine lupus development. Eur. J. Immunol. 40, 1809–1818 (2010).
Anania, C. et al. Increased prevalence of vulnerable atherosclerotic plaques and low levels of natural IgM antibodies against phosphorylcholine in patients with systemic lupus erythematosus. Arthritis Res. Ther. 12, R214 (2010).
Svenungsson, E. et al. Decreased levels of autoantibodies against apolipoprotein B-100 antigens are associated with cardiovascular disease in systemic lupus erythematosus. Clin. Exp. Immunol. 181, 417–426 (2015).
Knight, J. S. & Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin. Immunopathol. 44, 347–362 (2022).
Frostegård, J. et al. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheumatism 52, 192–200 (2005).
Purmalek, M. M. et al. Association of lipoprotein subfractions and glycoprotein acetylation with coronary plaque burden in SLE. Lupus Sci. Med. 6, e000332 (2019).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364, 127–135 (2011).
Connelly, M. A., Otvos, J. D., Shalaurova, I., Playford, M. P. & Mehta, N. N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 15, 219 (2017).
De Nardo, D. et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 15, 152–160 (2014).
Smith, C. K. et al. Lupus high-density lipoprotein induces proinflammatory responses in macrophages by binding lectin-like oxidised low-density lipoprotein receptor 1 and failing to promote activating transcription factor 3 activity. Ann. Rheum. Dis. 76, 602–611 (2017).
Ghazarian, M. et al. Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci. Immunol. 2, eaai7616 (2017).
Norlander, A. E., Madhur, M. S. & Harrison, D. G. The immunology of hypertension. J. Exp. Med. 215, 21–33 (2017).
Venegas-Pont, M. et al. Tumor necrosis factor-α antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56, 643–649 (2010).
Ripley, B. J. M., Goncalves, B., Isenberg, D. A., Latchman, D. S. & Rahman, A. Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann. Rheum. Dis. 64, 849–853 (2005).
Li, K. et al. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R590–595 (2010).
Nguyen, H. et al. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc. Res. 97, 696–704 (2012).
Kamat, N. V. et al. Renal transporter activation during angiotensin-ii hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension 65, 569–576 (2015).
Rodríguez-Iturbe, B. et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 59, 2222–2232 (2001).
Urowitz, M. B., Ibañez, D., Su, J. & Gladman, D. D. Modified Framingham risk factor score for systemic lupus erythematosus. J. Rheumatol. 43, 875–879 (2016).
Hippisley-Cox, J., Coupland, C. & Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 357, j2099 (2017).
Petri, M. A., Barr, E. & Magder, L. S. Development of a systemic lupus erythematosus cardiovascular risk equation. Lupus Sci. Med. 6, e000346 (2019).
McMahon, M. et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 66, 130–139 (2014).
Skaggs, B. J. et al. A panel of biomarkers associates with increased risk for cardiovascular events in women with systemic lupus erythematosus. ACR Open. Rheumatol. 3, 209–220 (2021).
Zhu, L. et al. Assessing the validity of QRISK3 in predicting cardiovascular events in systemic lupus erythematosus. Lupus Sci. Med. 9, e000564 (2022).
Sivakumaran, J. et al. Assessment of cardiovascular risk tools as predictors of cardiovascular disease events in systemic lupus erythematosus. Lupus Sci. Med. 8, e000448 (2021).
Enocsson, H. et al. Interferon-α mediates suppression of C-reactive protein: explanation for muted C-reactive protein response in lupus flares? Arthritis Rheum. 60, 3755–3760 (2009).
Chang, J. C. et al. Nocturnal blood pressure dipping as a marker of endothelial function and subclinical atherosclerosis in pediatric-onset systemic lupus erythematosus. Arthritis Res. Ther. 22, 129 (2020).
Stojan, G., Li, J., Budoff, M., Arbab-Zadeh, A. & Petri, M. A. High-risk coronary plaque in SLE: low-attenuation non-calcified coronary plaque and positive remodelling index. Lupus Sci. Med. 7, e000409 (2020).
Kiani, A. N. et al. Semiquantified noncalcified coronary plaque in systemic lupus erythematosus. J. Rheumatol. 39, 2286–2293 (2012).
Williams, M. C. et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 141, 1452–1462 (2020).
Frerix, M., Stegbauer, J., Kreuter, A. & Weiner, S. M. Atherosclerotic plaques occur in absence of intima-media thickening in both systemic sclerosis and systemic lupus erythematosus: a duplexsonography study of carotid and femoral arteries and follow-up for cardiovascular events. Arthritis Res. Ther. 16, R54 (2014).
Bakshi, J. et al. Extent of vascular plaque predicts future cardiovascular events in patients with systemic lupus erythematosus. Rheumatology 62, 225–233 (2022).
Drosos, G. C. et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 81, 768–779 (2022).
Ugarte-Gil, M. F. et al. Remission and low disease activity (LDA) prevent damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann. Rheum. Dis. 81, 1541–1548 (2022).
Petri, M. & Magder, L. S. Comparison of remission and lupus low disease activity state in damage prevention in a United States systemic lupus erythematosus cohort. Arthritis Rheumatol. 70, 1790–1795 (2018).
Ruiz-Irastorza, G., Ramos-Casals, M., Brito-Zeron, P. & Khamashta, M. A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann. Rheum. Dis. 69, 20–28 (2010).
Yang, D. H., Leong, P. Y., Sia, S. K., Wang, Y. H. & Wei, J. C. Long-term hydroxychloroquine therapy and risk of coronary artery disease in patients with systemic lupus erythematosus. J. Clin. Med. 8, 796 (2019).
Liu, D. et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: a systematic review and meta-analysis. Drug. Des. Devel Ther. 12, 1685–1695 (2018).
Schrezenmeier, E. & Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16, 155–166 (2020).
Virdis, A. et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Res. Ther. 17, 277 (2015).
Richez, C. et al. The effect of mycophenolate mofetil on disease development in the gld.apoE−/− mouse model of accelerated atherosclerosis and systemic lupus erythematosus. PLoS One 8, e61042 (2013).
van Leuven, S. I. et al. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis 211, 231–236 (2010).
Kaczmarek, I. et al. Preventing cardiac allograft vasculopathy: long-term beneficial effects of mycophenolate mofetil. J. Heart Lung Transpl. 25, 550–556 (2006).
Ait-Oufella, H. et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 207, 1579–1587 (2010).
Kridin, K., Mruwat, N. & Ludwig, R. J. Association of rituximab with risk of long-term cardiovascular and metabolic outcomes in patients with pemphigus. JAMA Dermatol. 159, 56–61 (2023).
Furie, R. et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N. Engl. J. Med. 383, 1117–1128 (2020).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Sage, A. P. et al. BAFF receptor deficiency reduces the development of atherosclerosis in mice — brief report. Arterioscler. Thromb. Vasc. Biol. 32, 1573–1576 (2012).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2019).
Casey, K. A. et al. Modulation of cardiometabolic disease markers by type I interferon inhibition in systemic lupus erythematosus. Arthritis Rheumatol. 73, 459–471 (2021).
Werth, V. P. et al. Trial of anti-BDCA2 antibody litifilimab for cutaneous lupus erythematosus. N. Engl. J. Med. 387, 321–331 (2022).
Furie, R. A. et al. Trial of anti-BDCA2 antibody litifilimab for systemic lupus erythematosus. N. Engl. J. Med. 387, 894–904 (2022).
Hasni, S. A. et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 12, 3391 (2021).
Winthrop, K. L. & Cohen, S. B. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat. Rev. Rheumatol. 18, 301–304 (2022).
Morand, E. et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 75, 242–252 (2023).
Knight, J. S. et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J. Clin. Invest. 123, 2981–2993 (2013).
Knight, J. S. et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 114, 947–956 (2014).
MACANOVIC, M. et al. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin. Exp. Immunol. 106, 243–252 (1996).
Davis Jr, J. C. et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 8, 68–76 (1999).
Hasni, S. et al. Peroxisome proliferator activated receptor-γ agonist pioglitazone improves vascular and metabolic dysfunction in systemic lupus erythematosus. Ann. Rheum. Dis. 81, 1576–1584 (2022).
Wang, H., Li, T., Chen, S., Gu, Y. & Ye, S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 67, 3190–3200 (2015).
Sun, F. et al. Safety and efficacy of metformin in systemic lupus erythematosus: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2, e210–e216 (2020).
Sun, F. et al. Effects of metformin on disease flares in patients with systemic lupus erythematosus: post hoc analyses from two randomised trials. Lupus Sci. Med. 7, e000429 (2020).
Marx, N., Husain, M., Lehrke, M., Verma, S. & Sattar, N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation 146, 1882–1894 (2022).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Petri, M. A., Kiani, A. N., Post, W., Christopher-Stine, L. & Magder, L. S. Lupus atherosclerosis prevention study (LAPS). Ann. Rheum. Dis. 70, 760 (2011).
van Rosendael, A. R. et al. Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA Cardiol. 6, 1257–1266 (2021).
Yu, H.-H. et al. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: a nationwide population-based cohort study. Atherosclerosis 243, 11–18 (2015).
Gibson, C. M. et al. Rationale and design of ApoA-I event reducing in ischemic syndromes II (AEGIS-II): a phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to investigate the efficacy and safety of CSL112 in subjects after acute myocardial infarction. Am. Heart J. 231, 121–127 (2021).
Woo, J. M. et al. Treatment with apolipoprotein A-1 mimetic peptide reduces lupus-like manifestations in a murine lupus model of accelerated atherosclerosis. Arthritis Res. Ther. 12, R93 (2010).
Arnett, D. K. et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140, e596–e646 (2019).
Iudici, M. et al. Low-dose aspirin as primary prophylaxis for cardiovascular events in systemic lupus erythematosus: a long-term retrospective cohort study. Rheumatology 55, 1623–1630 (2016).
Fanouriakis, A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745 (2019).
Dai, J. et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell 176, 1447–1460.e1414 (2019).
Rovin, B. H. et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 397, 2070–2080 (2021).
Korbet, S. M. & Lewis, E. J., Collaborative Study Group. Severe lupus nephritis: the predictive value of a ≥ 50% reduction in proteinuria at 6 months. Nephrol. Dial. Transplant. 28, 2313–2318 (2013).
Agca, R. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 76, 17–28 (2017).
Herrington, W., Lacey, B., Sherliker, P., Armitage, J. & Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 118, 535–546 (2016).
Borén, J. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 41, 2313–2330 (2020).
Edfeldt, K., Swedenborg, J., Hansson, G. K. & Yan, Z. Q. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105, 1158–1161 (2002).
Stemme, S. et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA 92, 3893–3897 (1995).
Hermansson, A. et al. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 207, 1081–1093 (2010).
Spitz, C. et al. Regulatory T cells in atherosclerosis: critical immune regulatory function and therapeutic potential. Cell Mol. Life Sci. 73, 901–922 (2016).
Gisterå, A. & Hansson, G. K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 13, 368–380 (2017).
Libby, P., Pasterkamp, G., Crea, F. & Jang, I. K. Reassessing the mechanisms of acute coronary syndromes. Circ. Res. 124, 150–160 (2019).
Quillard, T. et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 36, 1394–1404 (2015).
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127, 1437–1455 (2020).
Durcan, L., Clarke, W. A., Magder, L. S. & Petri, M. Hydroxychloroquine blood levels in systemic lupus erythematosus: clarifying dosing controversies and improving adherence. J. Rheumatol. 42, 2092–2097 (2015).
Acknowledgements
The authors’ work was supported by the Intramural Research Program at NIAMS (ZIA AR041199).
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, discussed the content, wrote the text and edited or reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
NIAMS has collaborative research agreements with Astra Zeneca and Bristol Myers Squibb that relate to mechanisms of vascular damage in autoimmunity. M.J.K. is a member of the scientific advisory boards of Neutrolis and Cytrill.
Peer review
Peer review information
Nature Reviews Nephrology thanks Anne Davidson, who co-reviewed with YoungMin Cho, Keisa Mathis, Anisur Rahman and Alan Salama for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Emergency granulopoiesis
-
A rapid increase in neutrophil generation in the bone marrow with a subsequent increase in neutrophil output in the circulation that occurs in response to an increase in neutrophil growth factors such as granulocyte colony-stimulating factor owing to an immune response, often to an infection.
- Foam cells
-
Macrophages with a specialized phenotype that are present within atherosclerotic plaques. These cells have a high lipid content in their cytoplasm, which results in a foamy appearance.
- Resistant hypertension
-
Hypertension that persists despite treatment with three concurrent antihypertensive agents at the maximally tolerated doses.
Rights and permissions
About this article
Cite this article
Ambler, W.G., Kaplan, M.J. Vascular damage in systemic lupus erythematosus. Nat Rev Nephrol 20, 251–265 (2024). https://doi.org/10.1038/s41581-023-00797-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00797-8