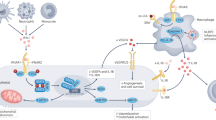
Abstract
The observations that traditional cardiovascular disease (CVD) risk factors fail to fully account for the excessive cardiovascular mortality in patients with systemic lupus erythematosus (SLE) compared with the general population have prompted in-depth investigations of non-traditional, SLE-related risk factors that contribute to cardiovascular complications in patients with SLE. Of the various perturbations of vascular physiology, endothelial dysfunction, which is believed to occur in the earliest step of atherosclerosis, has been extensively investigated for its contribution to CVD risk in SLE. Endothelial progenitor cells (EPCs), which play a crucial part in vascular repair, neovascularization and maintenance of endothelial function, are quantitatively and functionally reduced in patients with SLE. Yet, the lack of a unified definition of EPCs, standardization of the quantity and functional assessment of EPCs as well as endothelial function measurement pose challenges to the translation of endothelial function measurements and EPC levels into prognostic markers for CVD in patients with SLE. This Review discusses factors that contribute to CVD in SLE, with particular focus on how endothelial function and EPCs are evaluated currently, and how EPCs are quantitatively and functionally altered in patients with SLE. Potential strategies for the use of endothelial function measurements and EPC quantification as prognostic markers of CVD in patients with SLE, and the limitations of their prognostication potential, are also discussed.
Key points
-
Traditional cardiovascular disease (CVD) risk factors are prevalent in patients with systemic lupus erythematosus (SLE), but this observation cannot fully explain the excess of cardiovascular mortality and morbidity in these patients.
-
Endothelium-dependent flow-mediated dilation of the brachial artery, a common biophysical measure of endothelial function, is impaired in patients with SLE, even if they do not present with CVD.
-
Impaired endothelial function is associated with increased diastolic blood pressure, inflammation and vertebral bone loss in patients with SLE.
-
Endothelial progenitor cells (EPCs) are reduced quantitatively and functionally in patients with SLE, compared with healthy individuals.
-
Antimalarial drug use might be associated with elevation of levels of circulating angiogenic cells in patients with SLE.
-
Standardization of the definition and functional characterization of EPCs is paramount before EPCs can be used as prognostic biomarkers for CVD in patients with SLE.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barbhaiya, M. et al. Comparative risks of cardiovascular disease in patients with systemic lupus erythematosus, diabetes mellitus, and in general Medicaid recipients. Arthritis Care Res. 72, 1431–1439 (2020).
Jonsson, H., Nived, O. & Sturfelt, G. Outcome in systemic lupus erythematosus: a prospective study of patients from a defined population. Medicine 68, 141–150 (1989).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Bernatsky, S. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 54, 2550–2557 (2006).
Yurkovich, M., Vostretsova, K., Chen, W. & Aviña-Zubieta, J. A. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res. 66, 608–616 (2014).
Goldberg, R. J., Urowitz, M. B., Ibañez, D., Nikpour, M. & Gladman, D. D. Risk factors for development of coronary artery disease in women with systemic lupus erythematosus. J. Rheumatol. 36, 2454–2461 (2009).
Colombo, B. M. et al. Traditional and non traditional risk factors in accelerated atherosclerosis in systemic lupus erythematosus: role of vascular endothelial growth factor (VEGATS Study). Autoimmun. Rev. 8, 309–315 (2009).
Moghaddam, B., Marozoff, S., Li, L., Sayre, E. C. & Zubieta, J. A. A. All-cause and cause-specific mortality in systemic lupus erythematosus: a population-based study. Rheumatology https://doi.org/10.1093/rheumatology/keab362 (2021).
Leonard, D. et al. Coronary heart disease in systemic lupus erythematosus is associated with interferon regulatory factor-8 gene variants. Circ. Cardiovasc. Genet. 6, 255–263 (2013).
Bahrehmand, F. et al. Matrix metalloproteinase-2 functional promoter polymorphism G1575A is associated with elevated circulatory MMP-2 levels and increased risk of cardiovascular disease in systemic lupus erythematosus patients. Lupus 21, 616–624 (2012).
Bicakcigil, M., Tasan, D., Tasdelen, N., Mutlu, N. & Yavuz, S. Role of fibrinolytic parameters and plasminogen activator inhibitor 1 (PAI-1) promoter polymorphism on premature atherosclerosis in SLE patients. Lupus 20, 1063–1071 (2011).
Troelsen, L. N., Garred, P., Christiansen, B., Torp-Pedersen, C. & Jacobsen, S. Genetically determined serum levels of mannose-binding lectin correlate negatively with common carotid intima-media thickness in systemic lupus erythematosus. J. Rheumatol. 37, 1815–1821 (2010).
Marasini, B. et al. Genetic contribution to carotid vascular disease in patients with systemic lupus erythematosus. J. Clin. Immunol. 28, 131–133 (2008).
Szalai, A. J. et al. Systemic lupus erythematosus in a multiethnic US Cohort (LUMINA). XXX: association between C-reactive protein (CRP) gene polymorphisms and vascular events. Rheumatology 44, 864–868 (2005).
Frieri, M. Accelerated atherosclerosis in systemic lupus erythematosus: role of proinflammatory cytokines and therapeutic approaches. Curr. Allergy Asthma Rep. 12, 25–32 (2012).
Medeiros, P. B. S. et al. Disease activity index is associated with subclinical atherosclerosis in childhood-onset systemic lupus erythematosus. Pediatr. Rheumatol. Online J. 19, 35 (2021).
Faurschou, M. et al. High risk of ischemic heart disease in patients with lupus nephritis. J. Rheumatol. 38, 2400–2405 (2011).
Ajeganova, S., Hafström, I. & Frostegård, J. Patients with SLE have higher risk of cardiovascular events and mortality in comparison with controls with the same levels of traditional risk factors and intima-media measures, which is related to accumulated disease damage and antiphospholipid syndrome: a case-control study over 10 years. Lupus Sci. Med. 8, e000454 (2021).
Montarello, N. J., Nguyen, M. T., Wong, D. T. L., Nicholls, S. J. & Psaltis, P. J. Inflammation in coronary atherosclerosis and its therapeutic implications. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-020-07106-6 (2020).
Oikonomou, E. et al. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: clinical and therapeutic implications. Atherosclerosis 309, 16–26 (2020).
Miller, A. M. & McInnes, I. B. Cytokines as therapeutic targets to reduce cardiovascular risk in chronic inflammation. Curr. Pharm. Des. 17, 1–8 (2011).
Steyers, C. M. 3rd & Miller, F. J. Jr Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 15, 11324–11349 (2014).
Sitia, S. et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 9, 830–834 (2010).
Reynolds, J. A. et al. Brief report: vitamin D deficiency is associated with endothelial dysfunction and increases type I interferon gene expression in a murine model of systemic lupus erythematosus. Arthritis Rheumatol. 68, 2929–2935 (2016).
Stalc, M., Tomsic, M., Jezovnik, M. K. & Poredos, P. Endothelium-dependent and independent dilation capability of peripheral arteries in patients with systemic lupus erythematosus and antiphospholipid syndrome. Clin. Exp. Rheumatol. 29, 616–623 (2011).
Johnson, S. R. et al. Impaired brachial artery endothelium dependent flow mediated dilation in systemic lupus erythematosus: preliminary observations. Lupus 13, 590–593 (2004).
Kiss, E. et al. Reduced flow-mediated vasodilation as a marker for cardiovascular complications in lupus patients. J. Autoimmun. 27, 211–217 (2006).
Ghosh, P. et al. Subclinical atherosclerosis and endothelial dysfunction in young South-Asian patients with systemic lupus erythematosus. Clin. Rheumatol. 28, 1259–1265 (2009).
Go, E. & Yoder, M. C. Identification of endothelial cells and their progenitors. Methods Mol. Biol. 2206, 27–37 (2021).
Mao, S. Z., Ye, X., Liu, G., Song, D. & Liu, S. F. Resident endothelial cells and endothelial progenitor cells restore endothelial barrier function after inflammatory lung injury. Arterioscler. Thromb. Vasc. Biol. 35, 1635–1644 (2015).
Baker, J. F. et al. Circulating endothelial progenitor cells are reduced in SLE in the absence of coronary artery calcification. Rheumatol. Int. 32, 997–1002 (2012).
Westerweel, P. E. et al. Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Ann. Rheum. Dis. 66, 865–870 (2007).
Denny, M. F. et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110, 2907–2915 (2007).
Lee, P. Y. et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 56, 3759–3769 (2007).
Moonen, J. R. et al. Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 9, R84 (2007).
Huang, J., Kow, N. Y., Lee, H. Y., Fairhurst, A. M. & Mak, A. CD34+CD133+CD309+ circulating angiogenic cell level is reduced but positively related to hydroxychloroquine use in SLE patients — a case-control study and meta-regression analysis. Rheumatology 60, 3936–3944 (2021).
Kim, J. Y. et al. Osteoprotegerin causes apoptosis of endothelial progenitor cells by induction of oxidative stress. Arthritis Rheum. 65, 2172–2182 (2013).
Mak, A. & Kow, N. Y. Imbalance between endothelial damage and repair: a gateway to cardiovascular disease in systemic lupus erythematosus. Biomed. Res. Int. 2014, 178721 (2014).
Ding, X., Xiang, W. & He, X. IFN-I mediates dysfunction of endothelial progenitor cells in atherosclerosis of systemic lupus erythematosus. Front. Immunol. 11, 581385 (2020).
Komici, K. et al. Systemic lupus erythematosus, endothelial progenitor cells and intracellular Ca2+ signaling: a novel approach for an old disease. J. Autoimmun. 112, 102486 (2020).
Sánchez-Pérez, H. et al. Impaired HDL cholesterol efflux capacity in systemic lupus erythematosus patients is related to subclinical carotid atherosclerosis. Rheumatology 59, 2847–2856 (2020).
Quevedo-Abeledo, J. C. et al. Differences in HDL-cholesterol efflux capacity between patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res. https://doi.org/10.1002/acr.24407 (2020).
Li, J. et al. Novel insights: dynamic foam cells derived from the macrophage in atherosclerosis. J. Cell Physiol. 236, 6154–6167 (2021).
Smith, C. K. et al. Lupus high-density lipoprotein induces proinflammatory responses in macrophages by binding lectin-like oxidised low-density lipoprotein receptor 1 and failing to promote activating transcription factor 3 activity. Ann. Rheum. Dis. 76, 602–611 (2017).
McMahon, M. et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 66, 130–139 (2014).
Parikh, S. V., Almaani, S., Brodsky, S. & Rovin, B. H. Update on lupus nephritis: core curriculum 2020. Am. J. Kidney Dis. 76, 265–281 (2020).
Mak, A., Cheung, M. W., Chiew, H. J., Liu, Y. & Ho, R. C. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin. Arthritis Rheum. 41, 830–839 (2012).
Hermansen, M. L., Lindhardsen, J., Torp-Pedersen, C., Faurschou, M. & Jacobsen, S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology 56, 709–715 (2017).
Liu, Y. & Kaplan, M. J. Cardiovascular disease in systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 30, 441–448 (2018).
Sethna, C. B., Merchant, K. & Reyes, A. Cardiovascular disease risk in children with kidney disease. Semin. Nephrol. 38, 298–313 (2018).
Kahn, M. R., Robbins, M. J., Kim, M. C. & Fuster, V. Management of cardiovascular disease in patients with kidney disease. Nat. Rev. Cardiol. 10, 261–273 (2013).
Sun, E. Y., Alvarez, C. & Sheikh, S. Z. Association of lupus nephritis with coronary artery disease by ISN/RPS classification: results from a large real-world lupus population. ACR Open Rheumatol. 1, 244–250 (2019).
Frangou, E., Georgakis, S. & Bertsias, G. Update on the cellular and molecular aspects of lupus nephritis. Clin. Immunol. 216, 108445 (2020).
Dimou, P. et al. The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis. Sci. Rep. 9, 8348 (2019).
Deng, W. et al. CD8+CD103+ iTregs inhibit the progression of lupus nephritis by attenuating glomerular endothelial cell injury. Rheumatology 58, 2039–2050 (2019).
Choe, J. Y., Lee, S. S., Kwak, S. G. & Kim, S. K. Anti-Sm antibody, damage index, and corticosteroid use are associated with cardiac involvement in systemic lupus erythematosus: data from a prospective registry study. J. Korean Med. Sci. 35, e139 (2020).
Urowitz, M. B. et al. Accrual of atherosclerotic vascular events in a multicenter inception systemic lupus erythematosus cohort. Arthritis Rheumatol. 72, 1734–1740 (2020).
Fasano, S. et al. Prolonged remission is associated with a reduced risk of cardiovascular disease in patients with systemic lupus erythematosus: a GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Clin. Rheumatol. 38, 457–463 (2019).
Asanuma, Y. et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349, 2407–2415 (2003).
Rho, Y. H. et al. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J. Rheumatol. 35, 1789–1794 (2008).
Yiu, K. H. et al. Pattern of arterial calcification in patients with systemic lupus erythematosus. J. Rheumatol. 36, 2212–2217 (2019).
Kiani, A. N. et al. Coronary calcification in SLE: comparison with the multi-ethnic study of atherosclerosis. Rheumatology 54, 1976–1981 (2015).
Khan, A., Arbab-Zadeh, A., Kiani, A. N., Magder, L. S. & Petri, M. Progression of noncalcified and calcified coronary plaque by CT angiography in SLE. Rheumatol. Int. 37, 59–65 (2017).
Kiani, A. N., Magder, L. S. & Petri, M. Mycophenolate mofetil (MMF) does not slow the progression of subclinical atherosclerosis in SLE over 2 years. Rheumatol. Int. 32, 2701–2705 (2012).
Evans, C. E., Iruela-Arispe, M. L. & Zhao, Y. Y. Mechanisms of endothelial regeneration and vascular repair and their application to regenerative medicine. Am. J. Pathol. 191, 52–65 (2021).
Konukoglu, D. & Uzun, H. Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 956, 511–540 (2017).
Bai, B. et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 11, 776 (2020).
Tabrizi, R. et al. The effects of vitamin D supplementation on markers related to endothelial function among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of clinical trials. Horm. Metab. Res. 50, 587–596 (2018).
Simsek, E. C. et al. Endothelial dysfunction in patients with myocardial ischemia or infarction and nonobstructive coronary arteries. J. Clin. Ultrasound 49, 334–340 (2021).
Koo, B. K., Chung, W. Y. & Moon, M. K. Peripheral arterial endothelial dysfunction predicts future cardiovascular events in diabetic patients with albuminuria: a prospective cohort study. Cardiovasc. Diabetol. 19, 82 (2020).
Migliacci, R. et al. Walking-induced endothelial dysfunction predicts ischemic cardiovascular events in patients with intermittent claudication. Vasc. Med. 26, 394–400 (2021).
Schächinger, V., Britten, M. B. & Zeiher, A. M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101, 1899–1906 (2000).
Halcox, J. P. et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106, 653–658 (2002).
Barsalou, J. et al. Impact of disease duration on vascular surrogates of early atherosclerosis in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. 68, 237–246 (2016).
Taraborelli, M. et al. Endothelial dysfunction in early systemic lupus erythematosus patients and controls without previous cardiovascular events. Arthritis Care Res. 70, 1277–1283 (2018).
Wennmalm, A. Endothelial nitric oxide and cardiovascular disease. J. Intern. Med. 235, 317–327 (1994).
Furchgott, R. F. & Zawadzki, J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376 (1980).
Soga, J. et al. Relationship between augmentation index and flow-mediated vasodilation in the brachial artery. Hypertens. Res. 31, 1293–1298 (2008).
Gokce, N. et al. Effects of race and hypertension on flow-mediated and nitroglycerin-mediated dilation of the brachial artery. Hypertension 38, 1349–1354 (2001).
Mak, A., Liu, Y. & Ho, R. C. Endothelium-dependent but not endothelium-independent flow-mediated dilation is significantly reduced in patients with systemic lupus erythematosus without vascular events: a metaanalysis and metaregression. J. Rheumatol. 38, 1296–1303 (2011).
Mak, A. et al. Endothelial dysfunction in systemic lupus erythematosus — a case-control study and an updated meta-analysis and meta-regression. Sci. Rep. 7, 7320 (2017).
Riancho-Zarrabeitia, L. et al. Antiphospholipid syndrome (APS) in patients with systemic lupus erythematosus (SLE) implies a more severe disease with more damage accrual and higher mortality. Lupus 29, 1556–1565 (2020).
Ramsey-Goldman, R. & Manzi, S. Association of osteoporosis and cardiovascular disease in women with systemic lupus erythematosus. Arthritis Rheum. 44, 2338–2341 (2001).
Mak, A. et al. Lumbar spine bone mineral density predicts endothelial reactivity in patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 29, 261–268 (2011).
Zhu, J. et al. The association of endothelial nitric oxide synthase gene single nucleotide polymorphisms with paediatric systemic lupus erythematosus. Clin. Exp. Rheumatol. 36, 508–512 (2018).
Katkam, S. K., Indumathi, B., Tasneem, F. S. D., Rajasekhar, L. & Kutala, V. K. Impact of eNOS 27-bp VNTR (4b/a) gene polymorphism with the risk of Systemic Lupus Erythematosus in south Indian subjects. Gene 658, 105–112 (2018).
Hasni, S. A. et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 12, 3391 (2021).
Santos, M. J. et al. Early vascular alterations in SLE and RA patients — a step towards understanding the associated cardiovascular risk. PLoS One 7, e44668 (2012).
Tydén, H. et al. Low plasma concentrations of apolipoprotein M are associated with disease activity and endothelial dysfunction in systemic lupus erythematosus. Arthritis Res. Ther. 21, 110 (2019).
Fusco, E. et al. Preclinical vascular alterations in obese adolescents detected by Laser-Doppler Flowmetry technique. Nutr. Metab. Cardiovasc. Dis. 30, 306–312 (2020).
Tydén, H. et al. Endothelial dysfunction is associated with activation of the type I interferon system and platelets in patients with systemic lupus erythematosus. RMD Open 3, e000508 (2017).
Indraccolo, S. et al. Identification of genes selectively regulated by IFNs in endothelial cells. J. Immunol. 178, 1122–1135 (2007).
Hsu, K. S. et al. Dual regulation of Stat1 and Stat3 by the tumor suppressor protein PML contributes to interferon α-mediated inhibition of angiogenesis. J. Biol. Chem. 292, 10048–10060 (2017).
Niessner, A. & Weyand, C. M. Dendritic cells in atherosclerotic disease. Clin. Immunol. 134, 25–32 (2010).
Higashiyama, M. et al. Interferon-α increases monocyte migration via platelet-monocyte interaction in murine intestinal microvessels. Clin. Exp. Immunol. 162, 156–162 (2010).
Patiño-Trives, A. M. et al. Anti-dsDNA antibodies increase the cardiovascular risk in systemic lupus erythematosus promoting a distinctive immune and vascular activation. Arterioscler. Thromb. Vasc. Biol. 41, 2417–2430 (2021).
Campos-López, B. et al. Association of cardiometabolic risk status with clinical activity and damage in systemic lupus erythematosus patients: a cross-sectional study. Clin. Immunol. 222, 108637 (2021).
Rodríguez-Calvo, R. et al. Low-density lipoprotein from active SLE patients is more atherogenic to endothelial cells than low-density lipoprotein from the same patients during remission. Rheumatology 60, 866–871 (2021).
Theofilis, P. et al. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 9, 781 (2021).
Hernandez, N. M., Casselbrant, A., Joshi, M., Johansson, B. R. & Sumitran-Holgersson, S. Antibodies to kidney endothelial cells contribute to a “leaky” glomerular barrier in patients with chronic kidney diseases. Am. J. Physiol. Renal Physiol. 302, F884–F894 (2012).
Velásquez, M., Rojas, M., Abrahams, V. M., Escudero, C. & Cadavid, Á. P. Mechanisms of endothelial dysfunction in antiphospholipid syndrome: association with clinical manifestations. Front. Physiol. 9, 1840 (2018).
Mancardi, D. et al. Endothelial dysfunction and cardiovascular risk in lupus nephritis: new roles for old players? Eur. J. Clin. Invest. 51, e13441 (2021).
Valer, P., Paul, B., Eugenia, B. & Camelia, B. Annexin A5 as independent predictive biomarker for subclinical atherosclerosis and endothelial dysfunction in systemic lupus erythematosus patients. Clin. Lab. 59, 359–367 (2013).
Mobarrez, F. et al. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci. Rep. 6, 36025 (2016).
Martínez, M. C., Tesse, A., Zobairi, F. & Andriantsitohaina, R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am. J. Physiol. Heart Circ. Physiol. 288, H1004–H1009 (2005).
Shao, W. H. The role of microparticles in rheumatic diseases and their potentials as therapeutic tools. J. Mol. Immunol. 1, 101 (2016).
Puddu, P., Puddu, G. M., Cravero, E., Muscari, S. & Muscari, A. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can. J. Cardiol. 26, 140–145 (2010).
Markiewicz, M., Richard, E., Marks, N. & Ludwicka-Bradley, A. Impact of endothelial microparticles on coagulation, inflammation, and angiogenesis in age-related vascular diseases. J. Aging Res. 2013, 734509 (2013).
Mobarrez, F. et al. Microparticles in the blood of patients with SLE: size, content of mitochondria and role in circulating immune complexes. J. Autoimmun. 102, 142–149 (2019).
Combes, V. et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Invest. 104, 93–102 (1999).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Parker, B. et al. Suppression of inflammation reduces endothelial microparticles in active systemic lupus erythematosus. Ann. Rheum. Dis. 73, 1144–1150 (2014).
Atehortúa, L. et al. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 21, 34 (2019).
López, P., Rodríguez-Carrio, J., Martínez-Zapico, A., Caminal-Montero, L. & Suárez, A. Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity. Int. J. Cardiol. 236, 138–144 (2017).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Gupta, A. K. et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 584, 3193–3197 (2010).
Carmona-Rivera, C., Zhao, W., Yalavarthi, S. & Kaplan, M. J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74, 1417–1424 (2015).
von Brühl, M. L. et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835 (2012).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Pieterse, E. et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler. Thromb. Vasc. Biol. 37, 1371–1379 (2017).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Döring, Y. et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125, 1673–1683 (2012).
Knight, J. S. et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 74, 2199–2206 (2015).
Pardanaud, L., Altmann, C., Kitos, P., Dieterlen-Lievre, F. & Buck, C. A. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100, 339–349 (1987).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Chong, M. S., Ng, W. K. & Chan, J. K. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cell Transl. Med. 5, 530–538 (2016).
Purhonen, S. et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc. Natl Acad. Sci. USA 105, 6620–6625 (2008).
Barber, C. L. & Iruela-Arispe, M. L. The ever-elusive endothelial progenitor cell: identities, functions and clinical implications. Pediatr. Res. 59, 26R–32R (2006).
Basile, D. P. & Yoder, M. C. Circulating and tissue resident endothelial progenitor cells. J. Cell Physiol. 229, 10–16 (2014).
Schmeisser, A. & Strasser, R. H. Phenotypic overlap between hematopoietic cells with suggested angioblastic potential and vascular endothelial cells. J. Hematother. Stem Cell Res. 11, 69–79 (2002).
Schmeisser, A., Graffy, C., Daniel, W. G. & Strasser, R. H. Phenotypic overlap between monocytes and vascular endothelial cells. Adv. Exp. Med. Biol. 522, 59–74 (2003).
Desai, A. et al. Microarray-based characterization of a colony assay used to investigate endothelial progenitor cells and relevance to endothelial function in humans. Arterioscler. Thromb. Vasc. Biol. 29, 121–127 (2009).
Medina, R. J. et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol. Med. 17, 1045–1055 (2011).
Zhao, H. & He, Y. The inhibitory effect of lysophosphatidylcholine on proangiogenesis of human CD34+ cells derived endothelial progenitor cells. Front. Mol. Biosci. 8, 682367 (2021).
Liu, Y. et al. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng. A 19, 893–904 (2013).
Ingram, D. A. et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752–2760 (2004).
Yoder, M. C. et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109, 1801–1809 (2007).
Medina, R. J. et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cell Transl. Med. 6, 1316–1320 (2017).
Spinelli, F. R. et al. B lymphocyte stimulator modulates number and function of endothelial progenitor cells in systemic lupus erythematosus. Arthritis Res. Ther. 21, 245 (2019).
Deng, X. L., Li, X. X., Liu, X. Y., Sun, L. & Liu, R. Comparative study on circulating endothelial progenitor cells in systemic lupus erythematosus patients at active stage. Rheumatol. Int. 30, 1429–1436 (2010).
Kahlenberg, J. M. et al. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J. Immunol. 187, 6143–6156 (2011).
Thacker, S. G. et al. The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J. Immunol. 185, 4457–4469 (2010).
Ablin, J. N. et al. Enhanced adhesive properties of endothelial progenitor cells (EPCs) in patients with SLE. Rheumatol. Int. 31, 773–778 (2011).
Grisar, J. et al. Systemic lupus erythematosus patients exhibit functional deficiencies of endothelial progenitor cells. Rheumatology 47, 1476–1483 (2008).
Distler, J. H. et al. EULAR Scleroderma Trials and Research group statement and recommendations on endothelial precursor cells. Ann. Rheum. Dis. 68, 163–168 (2009).
Kuwana, M. & Okazaki, Y. Quantification of circulating endothelial progenitor cells in systemic sclerosis: a direct comparison of protocols. Ann. Rheum. Dis. 71, 617–620 (2012).
Williamson, K. A. et al. Age-related impairment of endothelial progenitor cell migration correlates with structural alterations of heparan sulfate proteoglycans. Aging Cell 12, 139–147 (2013).
Ebner, P. et al. Accumulation of VEGFR-2+/CD133+ cells and decreased number and impaired functionality of CD34+/VEGFR-2+ cells in patients with SLE. Rheumatology 49, 63–72 (2010).
Ding, X. et al. Neutralizing interferon-α blocks inflammation-mediated vascular injury via PI3K and AMPK in systemic lupus erythematosus. Immunology https://doi.org/10.1111/imm.13379 (2021).
Thacker, S. G. et al. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum. 64, 2975–2985 (2012).
Denny, M. F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 (2010).
Möckel, T., Basta, F., Weinmann-Menke, J. & Schwarting, A. B cell activating factor (BAFF): structure, functions, autoimmunity and clinical implications in Systemic Lupus Erythematosus (SLE). Autoimmun. Rev. 20, 102736 (2021).
Naserian, S. et al. The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell Commun. Signal. 18, 94 (2020).
Mak, A., Tang, C. S. & Ho, R. C. Serum tumour necrosis factor-alpha is associated with poor health-related quality of life and depressive symptoms in patients with systemic lupus erythematosus. Lupus 22, 254–261 (2013).
Hur, J. et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation 116, 1671–1682 (2007).
Miao, J. et al. Circulating angiogenic T cells and their subpopulations in patients with systemic lupus erythematosus. Mediators Inflamm. 2016, 2842143 (2016).
Zhao, P. et al. Circulating angiogenic T cells are increased in lupus nephritis patients. Med. Sci. Monit. 24, 5384–5390 (2018).
López, P., Rodríguez-Carrio, J., Martínez-Zapico, A., Caminal-Montero, L. & Suarez, A. Senescent profile of angiogenic T cells from systemic lupus erythematosus patients. J. Leukoc. Biol. 99, 405–412 (2016).
Xu, M. G. et al. The functions of endothelial progenitor cells were significantly improved after treatment with intravenous immunoglobulin and aspirin in children with Kawasaki disease. Pediatr. Cardiol. 32, 455–460 (2011).
Pérez-Sánchez, C. et al. Early restoration of immune and vascular phenotypes in systemic lupus erythematosus and rheumatoid arthritis patients after B cell depletion. J. Cell Mol. Med. 23, 6308–6318 (2019).
Hsue, P. Y. et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J. Am. Heart Assoc. 3, e001267 (2014).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Furumoto, Y. et al. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol. 69, 148–160 (2017).
Kaul, A. et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2, 16039 (2016).
Svenungsson, E. et al. A STAT4 risk allele is associated with ischaemic cerebrovascular events and anti-phospholipid antibodies in systemic lupus erythematosus. Ann. Rheum. Dis. 69, 834–840 (2010).
Huang, Z. et al. Vitamin D (1,25-(OH)2D3) improves endothelial progenitor cells function via enhanced no secretion in systemic lupus erythematosus. Cardiol. Res. Pract. 2020, 6802562 (2020).
Reynolds, J. A. et al. Vitamin D improves endothelial dysfunction and restores myeloid angiogenic cell function via reduced CXCL-10 expression in systemic lupus erythematosus. Sci. Rep. 6, 22341 (2016).
Sobrino, T., Blanco, M., Pérez-Mato, M., Rodríguez-Yáñez, M. & Castillo, J. Increased levels of circulating endothelial progenitor cells in patients with ischaemic stroke treated with statins during acute phase. Eur. J. Neurol. 19, 1539–1546 (2012).
Baran, Ç. et al. Effects of preoperative short term use of atorvastatin on endothelial progenitor cells after coronary surgery: a randomized, controlled trial. Stem Cell Rev. Rep. 8, 963–971 (2012).
Bahlmann, F. H. et al. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension 45, 526–529 (2005).
Cangiano, E. et al. ACE inhibition modulates endothelial apoptosis and renewal via endothelial progenitor cells in patients with acute coronary syndromes. Am. J. Cardiovasc. Drugs 11, 189–198 (2011).
França, C. N. et al. Endothelial progenitor cell mobilization and platelet microparticle release are influenced by clopidogrel plasma levels in stable coronary artery disease. Circ. J. 76, 729–736 (2012).
Chen, L. L. et al. Effects of gliclazide on endothelial function in patients with newly diagnosed type 2 diabetes. Eur. J. Pharmacol. 659, 296–301 (2011).
Fadini, G. P. et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care 33, 1607–1609 (2010).
Esposito, K. et al. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes — a randomized controlled trial. Diabetes Obes. Metab. 13, 439–445 (2011).
Mok, C. C. et al. Long-term outcome of a randomised controlled trial comparing tacrolimus with mycophenolate mofetil as induction therapy for active lupus nephritis. Ann. Rheum. Dis. 79, 1070–1076 (2020).
Mok, C. C. et al. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: a randomised controlled trial and long-term follow-up. Ann. Rheum. Dis. 75, 30–36 (2016).
Yang, L. et al. Cyclosporin A suppresses proliferation of endothelial progenitor cells: involvement of nitric oxide synthase inhibition. Intern. Med. 47, 1457–1464 (2008).
Meyer, N., Brodowski, L., von Kaisenberg, C., Schröder-Heurich, B. & von Versen-Höynck, F. Cyclosporine A and tacrolimus induce functional impairment and inflammatory reactions in endothelial progenitor cells. Int. J. Mol. Sci. 22, 9696 (2021).
Ingram, D. A. et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105, 2783–2786 (2005).
Yoon, C. H. et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 112, 1618–1627 (2005).
Schwartz, S. M. & Benditt, E. P. Clustering of replicating cells in aortic endothelium. Proc. Natl Acad. Sci. USA 73, 651–653 (1976).
Yoder, M. C. Is endothelium the origin of endothelial progenitor cells? Arterioscler. Thromb. Vasc. Biol. 30, 1094–1103 (2010).
Lin, R. Z., Hatch, A., Antontsev, V. G., Murthy, S. K. & Melero-Martin, J. M. Microfluidic capture of endothelial colony-forming cells from human adult peripheral blood: phenotypic and functional validation in vivo. Tissue Eng. C. Methods 21, 274–283 (2015).
Karlsson, G., Sommarin, M. N. E. & Böiers, C. Defining the emerging blood system during development at single-cell resolution. Front. Cell Dev. Biol. 9, 660350 (2021).
Bian, Z. et al. Deciphering human macrophage development at single-cell resolution. Nature 582, 571–576 (2020).
Tselios, K., Sheane, B. J., Gladman, D. D. & Urowitz, M. B. Optimal monitoring for coronary heart disease risk in patients with systemic lupus erythematosus: a systematic review. J. Rheumatol. 43, 54–65 (2016).
Sella, E. M., Sato, E. I., Leite, W. A., Oliveira Filho, J. A. & Barbieri, A. Myocardial perfusion scintigraphy and coronary disease risk factors in systemic lupus erythematosus. Ann. Rheum. Dis. 62, 1066–1070 (2003).
Schanberg, L. E. et al. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 60, 1496–1507 (2009).
Lu, X. et al. Patients with systemic lupus erythematosus face a high risk of cardiovascular disease: a systematic review and meta-analysis. Int. Immunopharmacol. 94, 107466 (2021).
Munguia-Realpozo, P. et al. Systemic lupus erythematosus and hypertension. Autoimmun. Rev. 18, 102371 (2019).
Molina, M. J. et al. Prevalence of systemic lupus erythematosus and associated comorbidities in Puerto Rico. J. Clin. Rheumatol. 13, 202–204 (2007).
Font, J. et al. Cardiovascular risk factors and the long-term outcome of lupus nephritis. QJM 94, 19–26 (2001).
Quinones, A., Lobach, I., Maduro, G. A. Jr., Smilowitz, N. R. & Reynolds, H. R. Diabetes and ischemic heart disease death in people age 25–54: a multiple-cause-of-death analysis based on over 400 000 deaths from 1990 to 2008 in New York City. Clin. Cardiol. 38, 114–120 (2015).
Yang, L. et al. Prevalence and correlation of conventional and lupus-specific risk factors for cardiovascular disease in Chinese systemic lupus erythematosus patients. J. Eur. Acad. Dermatol. Venereol. 26, 95–101 (2012).
Paolisso, G. et al. Evidence for peripheral impaired glucose handling in patients with connective tissue diseases. Metabolism 40, 902–907 (1991).
Zeng, Y. J. et al. Characteristics and risk factors for hyperglycemia in Chinese female patients with systemic lupus erythematosus. Lupus 19, 1344–1350 (2010).
Moroni, G. et al. Oxidative stress and homocysteine metabolism in patients with lupus nephritis. Lupus 19, 65–72 (2010).
Lazzerini, P. E. et al. Hyperhomocysteinemia: a cardiovascular risk factor in autoimmune diseases? Lupus 16, 852–862 (2007).
Bruce, I. N., Urowitz, M. B., Gladman, D. D., Ibañez, D. & Steiner, G. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 48, 3159–3167 (2003).
Teh, P., Zakhary, B. & Sandhu, V. K. The impact of obesity on SLE disease activity: findings from the Southern California Lupus Registry (SCOLR). Clin. Rheumatol. 38, 597–600 (2019).
Tedeschi, S. K. et al. Obesity and the risk of systemic lupus erythematosus among women in the Nurses’ Health Studies. Semin. Arthritis Rheum. 47, 376–383 (2017).
Ghaussy, N. O., Sibbitt, W. L. Jr & Qualls, C. R. Cigarette smoking, alcohol consumption, and the risk of systemic lupus erythematosus: a case-control study. J. Rheumatol. 28, 2449–2453 (2001).
Legge, A., Blanchard, C. & Hanly, J. G. Physical activity, sedentary behaviour and their associations with cardiovascular risk in systemic lupus erythematosus. Rheumatology 59, 1128–1136 (2020).
Mak, A. Physical exercise and systemic lupus erythematosus. Rheumatology 59, 921–922 (2020).
Manzi, S. et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 42, 51–60 (1999).
López-Pedrera, C. et al. Oxidative stress in the pathogenesis of atherothrombosis associated with anti-phospholipid syndrome and systemic lupus erythematosus: new therapeutic approaches. Rheumatology 55, 2096–2108 (2016).
Chorin, E. et al. Soluble ST2 and CXCL-10 may serve as biomarkers of subclinical diastolic dysfunction in SLE and correlate with disease activity and damage. Lupus 29, 1430–1437 (2020).
Sagar, D. et al. LOX-1: A potential driver of cardiovascular risk in SLE patients. PLoS One 15, e0229184 (2020).
Tripi, L. M. et al. Relationship of serum paraoxonase 1 activity and paraoxonase 1 genotype to risk of systemic lupus erythematosus. Arthritis Rheum. 54, 1928–1939 (2006).
Giannelou, M. & Mavragani, C. P. Cardiovascular disease in systemic lupus erythematosus: a comprehensive update. J. Autoimmun. 82, 1–12 (2017).
Apel, F., Zychlinsky, A. & Kenny, E. F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 14, 467–475 (2018).
Al Gadban, M. M., Alwan, M. M., Smith, K. J. & Hammad, S. M. Accelerated vascular disease in systemic lupus erythematosus: role of macrophage. Clin. Immunol. 157, 133–144 (2015).
Parra, S. et al. Circulating FABP4 is a marker of metabolic and cardiovascular risk in SLE patients. Lupus 23, 245–254 (2014).
Nived, O. et al. Disease duration, age at diagnosis and organ damage are important factors for cardiovascular disease in SLE. Lupus Sci. Med. 7, e000398 (2020).
Kow, N. Y. & Mak, A. Costimulatory pathways: physiology and potential therapeutic manipulation in systemic lupus erythematosus. Clin. Dev. Immunol. 2013, 245928 (2013).
Ganjali, S., Shirmohammadi, L., Read, M. I. & Sahebkar, A. High-density lipoprotein functionality in systemic lupus erythematosus. Semin. Arthritis Rheum. 50, 769–775 (2020).
Frostegård, J. Autoimmunity, oxidized LDL and cardiovascular disease. Autoimmun. Rev. 1, 233–237 (2002).
Acknowledgements
The authors would like to thank A-M. Fairhurst, for her expert contributions of flow cytometry evaluation of CD34+–CD133+–CD309+ circulating angiogenic cells, and L-H. Ling, and his team for their contributions of flow-mediated dilation measurement of our patients with SLE, and for his expert input into the information presented in Table 1.
Author information
Authors and Affiliations
Contributions
A.M. conceptualized the framework and content of the article; A.M. and J.K.Y.C. researched data for the article, made substantial contributions to discussions of the content, co-wrote the article, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.M. received consulting fees from Janssen and GlaxoSmithKline, and a research fund from GlaxoSmithKline for investigator-sponsored research through the GSK Supported Studies Programme (Proposal ID 10743). J.K.Y.C. received salary support from Singapore’s Ministry of Health’s National Medical Research Council (NMRC-CSA-SI-008/2016).
Peer review
Peer review information
Nature Reviews Rheumatology thanks C. Mendoza-Pinto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mak, A., Chan, J.K.Y. Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nat Rev Rheumatol 18, 286–300 (2022). https://doi.org/10.1038/s41584-022-00770-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00770-y
This article is cited by
-
Endothelial Progenitor Cells in Autoimmune Disorders
Stem Cell Reviews and Reports (2023)