Abstract
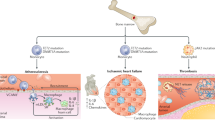
Clonal haematopoiesis of indeterminate potential (CHIP) is a preclinical condition wherein a sizeable proportion of an individual’s circulating blood cells are derived from a single mutated haematopoietic stem cell. CHIP occurs frequently with ageing — more than 10% of individuals over 65 years of age are affected — and is associated with an increased risk of disease across several organ systems and premature death. Emerging evidence suggests that CHIP has a role in kidney health, including associations with predisposition to acute kidney injury, impaired recovery from acute kidney injury and kidney function decline, both in the general population and among those with chronic kidney disease. Beyond its direct effect on the kidney, CHIP elevates the susceptibility of individuals to various conditions that can detrimentally affect the kidneys, including cardiovascular disease, obesity and insulin resistance, liver disease, gout, osteoporosis and certain autoimmune diseases. Aberrant pro-inflammatory signalling, telomere attrition and epigenetic ageing are potential causal pathophysiological pathways and mediators that underlie CHIP-related disease risk. Experimental animal models have shown that inhibition of inflammatory cytokine signalling can ameliorate many of the pathological effects of CHIP, and assessment of the efficacy and safety of this class of medications for human CHIP-associated pathology is ongoing.
Key points
-
Clonal haematopoiesis of indeterminate potential (CHIP) is a common, acquired condition wherein mutated white blood cells form an expanded clonal population in the blood and cause chronic organ damage through dysregulated inflammation.
-
CHIP has been associated with a greater risk of acute kidney injury (AKI) and impaired recovery from AKI in human population cohorts and in mouse models, as well as loss of kidney function in the general population and in those with chronic kidney disease.
-
In addition to its direct effects on the kidney, CHIP predisposes individuals to several conditions that impact kidney health, including cardiovascular disease, gout, osteoporosis and insulin resistance.
-
CHIP affects 10–20% of individuals aged 65 and older; other than age, risk factors include smoking, male sex, chronic inflammation, cytotoxic therapies and certain inherited genetic variants.
-
In preclinical models, cytokine blockade strategies mitigate many of the pathological effects of CHIP; these strategies are being evaluated in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cagan, A. et al. Somatic mutation rates scale with lifespan across mammals. Nature 604, 517–524 (2022).
Blokzijl, F. et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 (2016).
Martincorena, I. et al. Universal patterns of selection in cancer and somatic tissues. Cell 171, 1029–1041.e21 (2017).
Martincorena, I. et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015).
Martincorena, I. et al. Somatic mutant clones colonize the human esophagus with age. Science 362, 911–917 (2018).
Lee-Six, H. et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature 574, 532–537 (2019).
Lawson, A. R. J. et al. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science 370, 75–82 (2020).
Yoshida, K. et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272 (2020).
Moore, L. et al. The mutational landscape of human somatic and germline cells. Nature 597, 381–386 (2021).
Mustjoki, S. & Young, N. S. Somatic mutations in “benign” disease. N. Engl. J. Med. 384, 2039–2052 (2021).
Beck, D. B. et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 383, 2628–2638 (2020).
Anglesio, M. S. et al. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 376, 1835–1848 (2017).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Mass, E., Nimmerjahn, F., Kierdorf, K. & Schlitzer, A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat. Rev. Immunol. 23, 563–579 (2023).
Mitchell, E. et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 606, 343–350 (2022).
Niroula, A. et al. Distinction of lymphoid and myeloid clonal hematopoiesis. Nat. Med. 27, 1921–1927 (2021).
Liu, A. et al. Population analyses of mosaic X chromosome loss identify genetic drivers and widespread signatures of cellular selection. Preprint at medRxiv https://www.medrxiv.org/content/10.1101/2023.01.28.23285140v1 (2023).
Thompson, D. J. et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature 575, 652–657 (2019).
Haitjema, S. et al. Loss of Y chromosome in blood is associated with major cardiovascular events during follow-up in men after carotid endarterectomy. Circ. Cardiovasc. Genet. 10, e001544 (2017).
Sano, S. et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science 377, 292–297 (2022).
Stewart, B. J. et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019).
Vlasschaert, C., Moran, S. M. & Rauh, M. J. The myeloid–kidney interface in health and disease. CJASN 17, 323–331 (2022).
Nikolich-Žugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10–19 (2018).
Zhang, H., Weyand, C. M. & Goronzy, J. J. Hallmarks of the aging T-cell system. FEBS J. 288, 7123–7142 (2021).
de Mol, J., Kuiper, J., Tsiantoulas, D. & Foks, A. C. The dynamics of B cell aging in health and disease. Front. Immunol. 12, 733566 (2021).
Mogilenko, D. A., Shchukina, I. & Artyomov, M. N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 22, 484–498 (2022).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Buscarlet, M. et al. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood 132, 277–280 (2018).
Arends, C. M. et al. Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia 32, 1908–1919 (2018).
Fuster, J. J. et al. TET2-loss-of-function-driven clonal hematopoiesis exacerbates experimental insulin resistance in aging and obesity. Cell Rep. 33, 108326 (2020).
Nam, A. S. et al. Single-cell multi-omics of human clonal hematopoiesis reveals that DNMT3A R882 mutations perturb early progenitor states through selective hypomethylation. Nat. Genet. 54, 1514–1526 (2022).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005).
Beerman, I. et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107, 5465–5470 (2010).
Pang, W. W. et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108, 20012–20017 (2011).
Cull, A. H., Snetsinger, B., Buckstein, R., Wells, R. A. & Rauh, M. J. Tet2 restrains inflammatory gene expression in macrophages. Exp. Hematol. 55, 56–70.e13 (2017).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Sano, S. et al. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ. Res. 123, 335–341 (2018).
Cai, Z. et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell 23, 833–849.e5 (2018).
Sano, S. et al. JAK2 V617F-mediated clonal hematopoiesis accelerates pathological remodeling in murine heart failure. JACC Basic. Transl. Sci. 4, 684–697 (2019).
Abplanalp, W. T. et al. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol. 5, 1170–1175 (2020).
Abplanalp, W. T. et al. Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ. Res. 128, 216–228 (2021).
Agrawal, M. et al. TET2-mutant clonal hematopoiesis and risk of gout. Blood 140, 1094–1103 (2022).
Zhang, Q. et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 (2015).
Cobo, I. et al. DNA methyltransferase 3 alpha and TET methylcytosine dioxygenase 2 restrain mitochondrial DNA-mediated interferon signaling in macrophages. Immunity 55, 1386–1401.e10 (2022).
Cook, E. K. et al. Impact of Tet2 deficiency, and of TET2 mutations in clonal hematopoiesis, on neutrophil/granulocyte immune function. Blood 138, 2159 (2021).
von Beck, K., von Beck, T., Ferrell, P. B., Bick, A. G. & Kishtagari, A. Lymphoid clonal hematopoiesis: implications for malignancy, immunity, and treatment. Blood Cancer J. 13, 5 (2023).
Moran-Crusio, K. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20, 11–24 (2011).
Hormaechea-Agulla, D. et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell 28, 1428–1442 (2021).
Zioni, N. et al. Inflammatory signals from fatty bone marrow support DNMT3A driven clonal hematopoiesis. Nat. Commun. 14, 2070 (2023).
Challen, G. A. & Goodell, M. A. Clonal hematopoiesis: mechanisms driving dominance of stem cell clones. Blood 136, 1590–1598 (2020).
Avagyan, S. et al. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 374, 768–772 (2021).
Caiado, F. et al. Aging drives Tet2+/− clonal hematopoiesis via IL-1 signaling. Blood 141, 886–903 (2023).
Arber, D. A. et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood 140, 1200–1228 (2022).
Khoury, J. D. et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 36, 1703–1719 (2022).
Steensma, D. P. et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16 (2015).
Vlasschaert, C. et al. A practical approach to curate clonal hematopoiesis of indeterminate potential in human genetic datasets. Blood 141, 2214–2223 (2023).
Turesson, I. et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood 123, 338–345 (2014).
Kyle, R. A. et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 378, 241–249 (2018).
D’Souza, A. & Costa, L. J. MGIP, MGUS, and the PROMISE of meaning in small things. Lancet Haematol. 9, e315–e317 (2022).
Weeks, L. D. et al. Prediction of risk for myeloid malignancy in clonal hematopoiesis. NEJM Evid. 2, EVIDoa2200310 (2023).
Bick, A. G. et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768 (2020).
Fairchild, L. et al. Clonal hematopoiesis detection in patients with cancer using cell-free DNA sequencing. Sci. Transl. Med. 15, eabm8729 (2023).
Wong, W. J. et al. Clonal haematopoiesis and risk of chronic liver disease. Nature 616, 747–754 (2023).
Gao, L. et al. Comprehensive structure-function characterization of DNMT3B and DNMT3A reveals distinctive de novo DNA methylation mechanisms. Nat. Commun. 11, 3355 (2020).
Anteneh, H., Fang, J. & Song, J. Structural basis for impairment of DNA methylation by the DNMT3A R882H mutation. Nat. Commun. 11, 2294 (2020).
Abdel-Wahab, O. et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell 22, 180–193 (2012).
Coombs, C. C. et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and impacts clinical outcome. Cell Stem Cell 21, 374–382.e4 (2017).
Fabre, M. A. et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature 606, 335–342 (2022).
Levine, R. L., Pardanani, A., Tefferi, A. & Gilliland, D. G. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat. Rev. Cancer 7, 673–683 (2007).
Dawoud, A. A. Z., Gilbert, R. D., Tapper, W. J. & Cross, N. C. P. Clonal myelopoiesis promotes adverse outcomes in chronic kidney disease. Leukemia 36, 507–515 (2022).
Kestenbaum, B. et al. Clonal hematopoiesis of indeterminate potential and kidney function decline in the general population. Am. J. Kidney Dis. 81, 329–335 (2023).
Larsen, M. K. et al. Clonal haematopoiesis of indeterminate potential and impaired kidney function—a Danish general population study with 11 years follow-up. Eur. J. Haematol. 109, 576–585 (2022).
Vlasschaert, C. et al. Association of clonal hematopoiesis of indeterminate potential with worse kidney function and anemia in two cohorts of patients with advanced chronic kidney disease. J. Am. Soc. Nephrol. 33, 985–995 (2022).
Denicolò, S. et al. Clonal hematopoiesis of indeterminate potential and diabetic kidney disease: a nested case–control study. Kidney Int. Rep. 7, 876–888 (2022).
Vlasschaert, C., Rauh, M. J. & Lanktree, M. B. Response to: “Clonal hematopoiesis of indeterminate potential and diabetic kidney disease: a nested case-control study”. Kidney Int. Rep. 7, 2543 (2022).
Vlasschaert, C. et al. Clonal hematopoiesis of indeterminate potential is associated with acute kidney injury. Preprint at medRxiv https://www.medrxiv.org/content/10.1101/2023.05.16.23290051v1 (2023).
Hsu, C.-Y. et al. Post-acute kidney injury proteinuria and subsequent kidney disease progression: the Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) study. JAMA Intern. Med. 180, 402–410 (2020).
Wang, Y. et al. Murine models of clonal haematopoiesis to assess mechanisms of cardiovascular disease. Cardiovasc. Res. 118, 1413–1432 (2021).
Wang, Y. et al. Tet2-mediated clonal hematopoiesis in nonconditioned mice accelerates age-associated cardiac dysfunction. JCI Insight 5, e135204 (2020).
Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137–144 (2020).
Heimlich, J. B. et al. Clonal hematopoiesis of indeterminate potential status is associated with left main artery stenosis. Preprint at medRxiv https://www.medrxiv.org/content/10.1101/2023.02.10.23285708v1 (2023).
Zekavat, S. M. et al. TP53-mediated clonal hematopoiesis confers increased risk for incident atherosclerotic disease. Nat. Cardiovasc. Res. 2, 144–158 (2023).
Bhattacharya, R. et al. Clonal hematopoiesis is associated with higher risk of stroke. Stroke 53, 788–797 (2022).
Arends, C. M. et al. Associations of clonal hematopoiesis with recurrent vascular events and death in patients with incident ischemic stroke. Blood 141, 787–799 (2023).
Gumuser, E. D. et al. Clonal hematopoiesis of indeterminate potential predicts adverse outcomes in patients with atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 81, 1996–2009 (2023).
Yu, B. et al. Supplemental association of clonal hematopoiesis with incident heart failure. J. Am. Coll. Cardiol. 78, 42–52 (2021).
Dorsheimer, L. et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 4, 25–33 (2019).
Cremer, S. et al. Multiple somatic mutations for clonal hematopoiesis are associated with increased mortality in patients with chronic heart failure. Circ. Genom. Precis. Med. 13, e003003 (2020).
Pascual-Figal, D. A. et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J. Am. Coll. Cardiol. 77, 1747–1759 (2021).
Mas-Peiro, S. et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur. Heart J. 41, 933–939 (2020).
Mas-Peiro, S. et al. Long-term risk associated with clonal hematopoiesis in patients with severe aortic valve stenosis undergoing TAVR. Clin. Res. Cardiol. 112, 585–593 (2023).
Nakao, T. et al. Increased risk of thoracic aortic aneurysms with JAK2 V617F. J. Am. Coll. Cardiol. 81, 2128–2130 (2023).
Sano, S. et al. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI Insight 6, 146076 (2021).
Nakao, T. et al. Mendelian randomization supports bidirectional causality between telomere length and clonal hematopoiesis of indeterminate potential. Sci. Adv. 8, eabl6579 (2022).
Nachun, D. et al. Clonal hematopoiesis associated with epigenetic aging and clinical outcomes. Aging Cell 20, e13366 (2021).
Sano, S. et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J. Am. Coll. Cardiol. 71, 875–886 (2018).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Svensson, E. C. et al. TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 7, 521–528 (2022).
Fidler, T. P. et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592, 296–301 (2021).
Wang, W. et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 V617F mice. Circ. Res. 123, e35–e47 (2018).
Yura, Y. et al. The cancer therapy-related clonal hematopoiesis driver gene Ppm1d promotes inflammation and non-ischemic heart failure in mice. Circ. Res. 129, 684–698 (2021).
Rauch, P. J. et al. Loss-of-function mutations in Dnmt3a and Tet2 lead to accelerated atherosclerosis and concordant macrophage phenotypes. Nat. Cardiovasc. Res. 2, 805–818 (2023).
Bick, A. G. et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 141, 124–131 (2020).
Vlasschaert, C., Heimlich, J. B., Rauh, M. J., Natarajan, P. & Bick, A. G. Interleukin-6 receptor polymorphism attenuates clonal hematopoiesis-mediated coronary artery disease risk among 451 180 individuals in the UK Biobank. Circulation 147, 358–360 (2023).
Heimlich, J. B. et al. Mutated cells mediate distinct inflammatory responses in clonal hematopoiesis. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2022.12.01.518580v2 (2022).
O’Sullivan, R. J. & Karlseder, J. Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 11, 171–181 (2010).
Benetos, A. & Aviv, A. Ancestry, telomere length, and atherosclerosis risk. Circ. Cardiovasc. Genet. 10, e001718 (2017).
Ameh, O. I., Okpechi, I. G., Dandara, C. & Kengne, A.-P. Association between telomere length, chronic kidney disease, and renal traits: a systematic review. OMICS 21, 143–155 (2017).
Cheng, F. et al. Shortened leukocyte telomere length is associated with glycemic progression in type 2 diabetes: a prospective and Mendelian randomization analysis. Diabetes Care 45, 701–709 (2022).
DeBoy, E. A. et al. Familial clonal hematopoiesis in a long telomere syndrome. N. Engl. J. Med. 388, 2422–2433 (2023).
Kar, S. P. et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat. Genet. 54, 1155–1166 (2022).
Roetker, N. S., Pankow, J. S., Bressler, J., Morrison, A. C. & Boerwinkle, E. A prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the atherosclerosis risk in communities (ARIC) study. Circ. Genom. Precis. Med. 11, e001937 (2018).
Joyce, B. T. et al. Epigenetic age acceleration reflects long-term cardiovascular health. Circ. Res. 129, 770–781 (2021).
Yusipov, I. et al. Accelerated epigenetic aging and inflammatory/immunological profile (ipAGE) in patients with chronic kidney disease. GeroScience 44, 817–834 (2022).
Pan, Y. et al. Effects of epigenetic age acceleration on kidney function: a Mendelian randomization study. Clin. Epigenetics 15, 61 (2023).
Robertson, N. A. et al. Age-related clonal haemopoiesis is associated with increased epigenetic age. Curr. Biol. 29, R786–R787 (2019).
Uddin, M. D. M. et al. Clonal hematopoiesis of indeterminate potential, DNA methylation, and risk for coronary artery disease. Nat. Commun. 13, 5350 (2022).
Haring, B. et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the women’s health initiative. J. Am. Heart Assoc. 10, e018789 (2021).
Pasupuleti, S. K. et al. Obesity induced inflammation exacerbates clonal hematopoiesis. J. Clin. Invest. 133, e163968 (2023).
Tovy, A. et al. Constitutive loss of DNMT3A causes morbid obesity through misregulation of adipogenesis. eLife 11, e72359 (2022).
Wu, D. et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641 (2018).
van Deuren, R. C. et al. Expansion of mutation-driven haematopoietic clones is associated with insulin resistance and low HDL-cholesterol in individuals with obesity. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2021.05.12.443095v2 (2021).
Andersson-Assarsson, J. C. et al. Evolution of age-related mutation-driven clonal haematopoiesis over 20 years is associated with metabolic dysfunction in obesity. eBioMedicine 92, 104621 (2023).
Bhattacharya, R. et al. Association of diet quality with prevalence of clonal hematopoiesis and adverse cardiovascular events. JAMA Cardiol. 6, 1069–1077 (2021).
Cimmino, L. et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 170, 1079–1095.e20 (2017).
Taira, A. et al. Vitamin C boosts DNA demethylation in TET2 germline mutation carriers. Clin. Epigenetics 15, 7 (2023).
Vargas-Santos, A. B. & Neogi, T. Management of gout and hyperuricemia in CKD. Am. J. Kidney Dis. 70, 422–439 (2017).
Krishnan, E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS ONE 7, e50046 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 7, 1–59 (2017).
Nair, S. S. et al. Temporal trends in the incidence, treatment, and outcomes of hip fracture in older patients initiating dialysis in the United States. Clin. J. Am. Soc. Nephrol. 8, 1336–1342 (2013).
Kim, P. G. et al. Dnmt3a-mutated clonal hematopoiesis promotes osteoporosis. J. Exp. Med. 218, e20211872 (2021).
Hecker, J. S. et al. CHIP & HIPs: clonal hematopoiesis is common in hip arthroplasty patients and associates with autoimmune disease. Blood 138, 1727–1732 (2021).
Arends, C. M. et al. Clonal hematopoiesis in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Haematologica 105, e264–e267 (2020).
Wells, K. et al. Clonal hematopoiesis across the age spectrum in patients with systemic vasculitis. ACR Meeting Abstracts https://acrabstracts.org/abstract/clonal-hematopoiesis-across-the-age-spectrum-in-patients-with-systemic-vasculitis/ (2021).
Gutierrez-Rodrigues, F. et al. Spectrum of clonal hematopoiesis in VEXAS syndrome. Blood 142, 244–259 (2023).
De Langhe, E. et al. TET2-driver and NLRC4-passenger variants in adult-onset autoinflammation. N. Engl. J. Med. 388, 1626–1629 (2023).
Vlasschaert, C. et al. Infection risk associated with clonal hematopoiesis of indeterminate potential is partly mediated by hematologic cancer transformation in the UK Biobank. Leukemia, https://doi.org/10.1038/s41375-023-02023-7 (2023).
Dharan, N. J. et al. HIV is associated with an increased risk of age-related clonal hematopoiesis among older adults. Nat. Med. 27, 1006–1011 (2021).
Bick, A. G. et al. Increased prevalence of clonal hematopoiesis of indeterminate potential amongst people living with HIV. Sci. Rep. 12, 577 (2022).
Bolton, K. L. et al. Clonal hematopoiesis is associated with risk of severe Covid-19. Nat. Commun. 12, 5975 (2021).
Kessler, M. D. et al. Common and rare variant associations with clonal haematopoiesis phenotypes. Nature 612, 301–309 (2022).
Miller, P. G. et al. Clonal hematopoiesis of indeterminate potential and risk of death from COVID-19. Blood 140, 1993–1997 (2022).
Zhou, Y. et al. Clonal hematopoiesis is not significantly associated with COVID-19 disease severity. Blood 140, 1650–1655 (2022).
Choudhri, Y., Maslove, D. M. & Rauh, M. J. COVID-19 and the genetics of inflammation. Crit. Care Med. 51, 817–825 (2023).
Bouzid, H. et al. Clonal hematopoiesis is associated with protection from Alzheimer’s disease. Nat. Med. 29, 1662–1670 (2023).
Hansen, D. V., Hanson, J. E. & Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 217, 459–472 (2018).
Zhang, C.-Y., He, F.-F., Su, H., Zhang, C. & Meng, X.-F. Association between chronic kidney disease and Alzheimer’s disease: an update. Metab. Brain Dis. 35, 883–894 (2020).
Krishnan, A. V. & Kiernan, M. C. Neurological complications of chronic kidney disease. Nat. Rev. Neurol. 5, 542–551 (2009).
Fang, C. et al. Chronic kidney disease promotes cerebral microhemorrhage formation. J. Neuroinflammation 20, 51 (2023).
Young, A. L., Challen, G. A., Birmann, B. M. & Druley, T. E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 7, 12484 (2016).
Huang, Z. et al. Emerging evidence on the role of clonal hematopoiesis of indeterminate potential in chronic kidney disease. Transl. Res. 256, 87–94 (2022).
Dawoud, A. A. Z., Tapper, W. J. & Cross, N. C. P. Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia 34, 2660–2672 (2020).
Pich, O. et al. The evolution of hematopoietic cells under cancer therapy. Nat. Commun. 12, 4803 (2021).
Weinstock, J. S. et al. Aberrant activation of TCL1A promotes stem cell expansion in clonal haematopoiesis. Nature 616, 755–763 (2023).
Bolton, K. L. et al. The clinical management of clonal hematopoiesis: creation of a clonal hematopoiesis clinic. Hematol. Oncol. Clin. North. Am. 34, 357–367 (2020).
Rossi, M. et al. Clinical relevance of clonal hematopoiesis in the oldest-old population. Blood 138, 2093–2105 (2021).
Min, K., Polizio, A. H., Kour, A., Thel, M. C. & Walsh, K. Experimental ASXL1‐mediated clonal hematopoiesis promotes inflammation and accelerates heart failure. J. Am. Heart Assoc. 11, e026154 (2022).
Yokokawa, T. et al. Crucial role of hematopoietic JAK2 V617F in the development of aortic aneurysms. Haematologica 106, 1910–1922 (2021).
Zekavat, S. M. et al. Hematopoietic mosaic chromosomal alterations increase the risk for diverse types of infection. Nat. Med. 27, 1012–1024 (2021).
Tanaka, T., Narazaki, M. & Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 (2014).
Kaneko, N., Kurata, M., Yamamoto, T., Morikawa, S. & Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 39, 12 (2019).
Kalliolias, G. D. & Ivashkiv, L. B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62 (2016).
Hughes, C. E. & Nibbs, R. J. B. A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 (2018).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 2060–2069 (2021).
Pergola, P. E. et al. Ziltivekimab for treatment of anemia of inflammation in patients on hemodialysis: results from a phase 1/2 multicenter, randomized, double-blind, placebo-controlled trial. JASN 32, 211–222 (2021).
Yu, Z., Zekavat, S. M., Honigberg, M. C. & Natarajan, P. Genetic IL-6 signaling modifies incident coronary artery disease risk in chronic kidney disease. J. Am. Coll. Cardiol. 79, 415–416 (2022).
Author information
Authors and Affiliations
Contributions
C.V. researched data for the article and wrote the manuscript. P.N., C.V., M.B.L. and T.N.K. made substantial contributions to discussions of the content. All authors reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.N. reports research grants from Allelica, Apple, Amgen, Boston Scientific, Genentech/Roche and Novartis, personal fees from Allelica, Apple, AstraZeneca, Blackstone Life Sciences, Foresite Labs, Genentech/Roche, GV, HeartFlow, Magnet Biomedicine and Novartis, scientific advisory board membership of Esperion Therapeutics, Preciseli and TenSixteen Bio, scientific co-founder of TenSixteen Bio, equity in Preciseli and TenSixteen Bio, and spousal employment at Vertex Pharmaceuticals, all unrelated to the present work. M.B.L. reports received speaking and advisory board fees from Bayer, Otsuka, Reata and Sanofi. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks F. Damm, T. Speer and A. M. Zeiher for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Passenger variants
-
Acquired genetic changes that accumulate in cells over time but are not expected to affect cell fitness or drive clonal expansion, in contrast to driver mutations.
- Variant allele fraction
-
(VAF). The proportion of sequencing reads that contain the variant, which serves as an estimate of the fraction of cells that containing the variant (for autosomal chromosomes and X chromosomes in female individuals, VAF × 2 = the cell fraction).
- VEXAS syndrome
-
First described in 2020, VEXAS syndrome (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) is a severe, adult-onset autoinflammatory disease caused by acquired mutations in the ubiquitin ligase enzyme gene (UBA1) in circulating blood cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vlasschaert, C., Lanktree, M.B., Rauh, M.J. et al. Clonal haematopoiesis, ageing and kidney disease. Nat Rev Nephrol 20, 161–174 (2024). https://doi.org/10.1038/s41581-023-00778-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00778-x
This article is cited by
-
Acquired blood mutations cause acute kidney injury via dysregulated inflammation
Nature Medicine (2024)