Abstract
Chronic kidney disease (CKD) is a global health problem with rising incidence and prevalence. Among several pathogenetic mechanisms responsible for disease progression, lipid accumulation in the kidney parenchyma might drive inflammation and fibrosis, as has been described in fatty liver diseases. Lipids and their metabolites have several important structural and functional roles, as they are constituents of cell and organelle membranes, serve as signalling molecules and are used for energy production. However, although lipids can be stored in lipid droplets to maintain lipid homeostasis, lipid accumulation can become pathogenic. Understanding the mechanisms linking kidney parenchymal lipid accumulation to CKD of metabolic or non-metabolic origin is challenging, owing to the tremendous variety of lipid species and their functional diversity across different parenchymal cells. Nonetheless, multiple research reports have begun to emphasize the effect of dysregulated kidney lipid metabolism in CKD progression. For example, altered cholesterol and fatty acid metabolism contribute to glomerular and tubular cell injury. Newly developed lipid-targeting agents are being tested in clinical trials in CKD, raising expectations for further therapeutic development in this field.
Key points
-
Lipids and lipid-related enzymes have a major role in modulating the function of glomerular and tubular cells, and can drive chronic kidney disease (CKD) irrespective of circulating lipid levels.
-
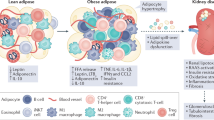
The mechanisms that initiate the accumulation of kidney lipids might differ between CKD of different aetiologies. Thus, the accumulation of kidney lipids in diabetic kidney disease is driven by increased glucose and fatty acids levels owing to insulin resistance, whereas in glomerulonephritis, inflammation can disrupt normal kidney lipid metabolism.
-
Several lipid species, such as cholesterol, triglycerides, fatty acids and phospholipids, are dysregulated in podocytes, endothelial and tubular cells, and contribute to CKD progression.
-
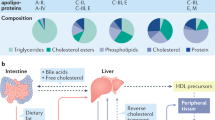
Accumulation of kidney parenchymal cholesterol occurs in association with impaired reverse cholesterol transport in diseases of both metabolic and non-metabolic origin, and contributes to CKD progression.
-
Accumulation of fatty acids triggers mitochondrial and kidney cell damage by promoting inflammation, including cellular sterile inflammation, via innate immune system activation and fibrosis; lipophagy has a protective effect.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
Shankland, S. J., Freedman, B. S. & Pippin, J. W. Can podocytes be regenerated in adults? Curr. Opin. Nephrol. Hypertens. 26, 154–164 (2017).
Lasagni, L. et al. Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Rep. 5, 248–263 (2015).
Kaverina, N. V., Eng, D. G., Schneider, R. R., Pippin, J. W. & Shankland, S. J. Partial podocyte replenishment in experimental FSGS derives from nonpodocyte sources. Am. J. Physiol. Renal Physiol. 310, F1397–F1413 (2016).
Hackl, M. J. et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat. Med. 19, 1661–1666 (2013).
Humphreys, B. D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 80, 309–326 (2018).
Emanuelsson, F., Nordestgaard, B. G. & Benn, M. Familial hypercholesterolemia and risk of peripheral arterial disease and chronic kidney disease. J. Clin. Endocrinol. Metab. 103, 4491–4500 (2018).
Weldegiorgis, M. & Woodward, M. Elevated triglycerides and reduced high-density lipoprotein cholesterol are independently associated with the onset of advanced chronic kidney disease: a cohort study of 911,360 individuals from the United Kingdom. BMC Nephrol. 23, 312 (2022).
Pauley, M. E. et al. Triglyceride content of lipoprotein subclasses and kidney hemodynamic function and injury in adolescents with type 1 diabetes. J. Diabetes Complicat. 37, 108384 (2023).
Rubinow, K. B. et al. Kidney function is associated with an altered protein composition of high-density lipoprotein. Kidney Int. 92, 1526–1535 (2017).
Herman-Edelstein, M., Scherzer, P., Tobar, A., Levi, M. & Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 55, 561–572 (2014).
Merscher-Gomez, S. et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes 62, 3817–3827 (2013).
Ducasa, G. M. et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J. Clin. Invest. 129, 3387–3400 (2019).
Proctor, G. et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in akita and OVE26 mice with type 1 diabetes. Diabetes 55, 2502–2509 (2006).
Meyrier, A. Nephrosclerosis: a term in quest of a disease. Nephron 129, 276–282 (2015).
Haruyama, N. et al. Subclinical nephrosclerosis is linked to left ventricular hypertrophy independent of classical atherogenic factors. Hypertens. Res. 37, 472–477 (2014).
Lovric, S. et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J. Clin. Invest. 127, 912–928 (2017).
Prasad, R. et al. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J. Clin. Invest. 127, 942–953 (2017).
Müller-Deile, J. et al. Novel diagnostic and therapeutic techniques reveal changed metabolic profiles in recurrent focal segmental glomerulosclerosis. Sci. Rep. 11, 4577 (2021).
Vivarelli, M., Massella, L., Ruggiero, B. & Emma, F. Minimal change disease. Clin. J. Am. Soc. Nephrol. 12, 332–345 (2017).
Mitrofanova, A. et al. Hydroxypropyl-β-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int. 94, 1151–1159 (2018).
Liu, X. et al. Sterol-O-acyltransferase-1 has a role in kidney disease associated with diabetes and Alport syndrome. Kidney Int. 98, 1275–1285 (2020).
Ding, W. et al. Osteopontin deficiency ameliorates Alport pathology by preventing tubular metabolic deficits. JCI Insight 3, e94818 (2018).
Su, X. et al. Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am. J. Kidney Dis. 67, 881–892 (2016).
Shamburek, R. D. et al. Familial lecithin:cholesterol acyltransferase deficiency: first-in-human treatment with enzyme replacement. J. Clin. Lipidol. 10, 356–367 (2016).
Rickards, E. Remarks on the fatty transformation of the kidney. Br. Med. J. 2, 2–3 (1883).
Minami, S. et al. Lipophagy maintains energy homeostasis in the kidney proximal tubule during prolonged starvation. Autophagy 13, 1629–1647 (2017).
Zhao, C. et al. PACS-2 deficiency in tubular cells aggravates lipid-related kidney injury in diabetic kidney disease. Mol. Med. 28, 117 (2022).
Csaki, L. S. et al. Lipin-1 and lipin-3 together determine adiposity in vivo. Mol. Metab. 3, 145–154 (2014).
Liu, X., Du, H., Sun, Y. & Shao, L. Role of abnormal energy metabolism in the progression of chronic kidney disease and drug intervention. Ren. Fail. 44, 790–805 (2022).
Czajka, A. & Malik, A. N. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Redox Biol. 10, 100–107 (2016).
Forbes, J. M. & Thorburn, D. R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 14, 291–312 (2018).
Khan, S. et al. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight 5, e136845 (2020).
Tsai, I. T. et al. FABP1 and FABP2 as markers of diabetic nephropathy. Int. J. Med. Sci. 17, 2338–2345 (2020).
Yokoi, H. & Yanagita, M. Targeting the fatty acid transport protein CD36, a class B scavenger receptor, in the treatment of renal disease. Kidney Int. 89, 740–742 (2016).
Alkhatatbeh, M. J., Enjeti, A. K., Acharya, S., Thorne, R. F. & Lincz, L. F. The origin of circulating CD36 in type 2 diabetes. Nutr. Diabetes 3, e59–e59 (2013).
Kim, H. J. et al. A novel index using soluble CD36 is associated with the prevalence of type 2 diabetes mellitus: comparison study with triglyceride-glucose index. Endocrinol. Metab. 32, 375–382 (2017).
Shiju, T. M., Mohan, V., Balasubramanyam, M. & Viswanathan, P. Soluble CD36 in plasma and urine: a plausible prognostic marker for diabetic nephropathy. J. Diabetes Complicat. 29, 400–406 (2015).
Ekici, M., Kisa, U., Arikan Durmaz, S., Ugur, E. & Nergiz-Unal, R. Fatty acid transport receptor soluble CD36 and dietary fatty acid pattern in type 2 diabetic patients: a comparative study. Br. J. Nutr. 119, 153–162 (2018).
Castelblanco, E. et al. Circulating soluble CD36 is similar in type 1 and type 2 diabetes mellitus versus non-diabetic subjects. J. Clin. Med. 8, 710 (2019).
Thi, T. N. D., Gia, B. N., Thi, H. L. L., Thi, T. N. C. & Thanh, H. P. Evaluation of urinary L-FABP as an early marker for diabetic nephropathy in type 2 diabetic patients. J. Med. Biochem. 39, 224–230 (2020).
Ito, H. et al. Current metabolic status affects urinary liver-type fatty-acid binding protein in normoalbuminuric patients with type 2 diabetes. J. Clin. Med. Res. 9, 366–373 (2017).
Tanaka, M. et al. Significance of urinary fatty acid-binding protein 4 level as a possible biomarker for the identification of minimal change disease in patents with nephrotic-range proteinuria. BMC Nephrol. 21, 459 (2020).
Su, H.-Y., Hsu, B.-G., Lin, Y.-L., Wang, C.-H. & Lai, Y.-H. Serum adipocyte fatty acid-binding protein level is positively associated with aortic stiffness in nondialysis chronic kidney disease patients: a cross-sectional study. Medicine 101, e29558 (2022).
Houten, S. M., Wanders, R. J. A. & Ranea-Robles, P. Metabolic interactions between peroxisomes and mitochondria with a special focus on acylcarnitine metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165720 (2020).
Violante, S. et al. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J. 33, 4355–4364 (2019).
Nakagawa, S. et al. Molecular markers of tubulointerstitial fibrosis and tubular cell damage in patients with chronic kidney disease. PLoS One 10, e0136994 (2015).
Woroniecka, K. I. et al. Transcriptome analysis of human diabetic kidney disease. Diabetes 60, 2354–2369 (2011).
Sun, H., Yuan, Y. & Sun, Z. L. Cholesterol contributes to diabetic nephropathy through SCAP-SREBP-2 pathway. Int. J. Endocrinol. 2013, 592576 (2013).
Yang, X. et al. CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 13, 769–781 (2017).
Yang, X. et al. CD36 promotes podocyte apoptosis by activating the pyrin domain-containing-3 (NLRP3) inflammasome in primary nephrotic syndrome. Med. Sci. Monit. 24, 6832–6839 (2018).
Kim, J. J. et al. Discoidin domain receptor 1 activation links extracellular matrix to podocyte lipotoxicity in Alport syndrome. EBioMedicine 63, 103162 (2021).
Wei Hua, L. P. et al. CD36-mediated podocyte lipotoxicity promotes foot process effacement. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-2454690/v1 (2023).
Gao, Q. et al. Overexpression of heart-type fatty acid binding protein enhances fatty acid-induced podocyte injury. Exp. Ther. Med. 15, 2054–2061 (2018).
Chen, H. M., Zheng, C. X., Gao, Q., Ge, Y. C. & Liu, Z. H. Heart-type fatty acid binding protein is associated with proteinuria in obesity. PLoS One 7, e45691 (2012).
Falkevall, A. et al. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab. 25, 713–726 (2017).
Bobulescu, I. A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 19, 393–402 (2010).
Rahman, M. et al. Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin. J. Am. Soc. Nephrol. 9, 1190–1198 (2014).
Feng, L., Gu, C., Li, Y. & Huang, J. High glucose promotes CD36 expression by upregulating peroxisome proliferator-activated receptor γ levels to exacerbate lipid deposition in renal tubular cells. Biomed. Res. Int. 2017, 1414070 (2017).
Kennedy, D. J. et al. CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension 61, 216–224 (2013).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Khan, S. et al. Kidney proximal tubule lipoapoptosis is regulated by fatty acid transporter-2 (FATP2). J. Am. Soc. Nephrol. 29, 81–91 (2018).
Chen, Y. et al. Involvement of FATP2-mediated tubular lipid metabolic reprogramming in renal fibrogenesis. Cell Death Dis. 11, 994 (2020).
Cheng, L. et al. Zoledronate dysregulates fatty acid metabolism in renal tubular epithelial cells to induce nephrotoxicity. Arch. Toxicol. 92, 469–485 (2018).
Bryant, C. et al. Alternatively spliced landscape of PPARγ mRNA in podocytes is distinct from adipose tissue. Cells 11, 3455 (2022).
Pistrosch, F. et al. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes 54, 2206–2211 (2005).
Agarwal, R. et al. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int. 68, 285–292 (2005).
Park, C. W. et al. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes 55, 885–893 (2006).
Matsushita, Y. et al. Activation of peroxisome proliferator-activated receptor delta inhibits streptozotocin-induced diabetic nephropathy through anti-inflammatory mechanisms in mice. Diabetes 60, 960–968 (2011).
Declèves, A. E. et al. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 85, 611–623 (2014).
Hong, Q. et al. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 93, 1330–1343 (2018).
Wang, Q. et al. Faster lipid β-oxidation rate by acetyl-CoA carboxylase 2 inhibition alleviates high-glucose-induced insulin resistance via SIRT1/PGC-1α in human podocytes. J. Biochem. Mol. Toxicol. 35, e22797 (2021).
Woo, C. Y. et al. Inhibition of ceramide accumulation in podocytes by myriocin prevents diabetic nephropathy. Diabetes Metab. J. 44, 581–591 (2020).
Fucho, R., Casals, N., Serra, D. & Herrero, L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J. 31, 1263–1272 (2017).
Chen, Y. et al. The inhibition of Nrf2 accelerates renal lipid deposition through suppressing the ACSL1 expression in obesity-related nephropathy. Ren. Fail. 41, 821–831 (2019).
Maeda, S. et al. A single nucleotide polymorphism within the acetyl-coenzyme A carboxylase beta gene is associated with proteinuria in patients with type 2 diabetes. PLoS Genet. 6, e1000842 (2010).
Shah, V. N. et al. ACACβ gene (rs2268388) and AGTR1 gene (rs5186) polymorphism and the risk of nephropathy in Asian Indian patients with type 2 diabetes. Mol. Cell Biochem. 372, 191–198 (2013).
Kayampilly, P., Roeser, N., Rajendiran, T. M., Pennathur, S. & Afshinnia, F. Acetyl Co-A carboxylase inhibition halts hyperglycemia induced upregulation of de novo lipogenesis in podocytes and proximal tubular cells. Metabolites 12, 940 (2022).
Lee, M. et al. Phosphorylation of acetyl-CoA carboxylase by AMPK reduces renal fibrosis and is essential for the anti-fibrotic effect of metformin. J. Am. Soc. Nephrol. 29, 2326–2336 (2018).
Dhillon, P. et al. The nuclear receptor ESRRA protects from kidney disease by coupling metabolism and differentiation. Cell Metab. 33, 379–394.e378 (2021).
Iwaki, T. et al. PPARα contributes to protection against metabolic and inflammatory derangements associated with acute kidney injury in experimental sepsis. Physiol. Rep. 7, e14078 (2019).
Jang, H. S. et al. Proximal tubule cyclophilin D regulates fatty acid oxidation in cisplatin-induced acute kidney injury. Kidney Int. 97, 327–339 (2020).
Chung, K. W. et al. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J. Am. Soc. Nephrol. 29, 1223–1237 (2018).
Li, J. et al. STAT6 contributes to renal fibrosis by modulating PPARα-mediated tubular fatty acid oxidation. Cell Death Dis. 13, 66 (2022).
Jao, T. M. et al. ATF6α downregulation of PPARα promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int. 95, 577–589 (2019).
Darshi, M. et al. Crabtree effect in kidney proximal tubule cells via late-stage glycolytic intermediates. iScience 26, 106462 (2023).
Song, J., Yang, X. & Yan, L. J. Role of pseudohypoxia in the pathogenesis of type 2 diabetes. Hypoxia 7, 33–40 (2019).
Menezes, L. F., Lin, C. C., Zhou, F. & Germino, G. G. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 5, 183–192 (2016).
Lakhia, R. et al. PPARα agonist fenofibrate enhances fatty acid β-oxidation and attenuates polycystic kidney and liver disease in mice. Am. J. Physiol. Renal Physiol. 314, F122–F131 (2018).
Jiang, T., Liebman, S. E., Lucia, M. S., Li, J. & Levi, M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 68, 2608–2620 (2005).
Chung, K. W. et al. PPARα/β activation alleviates age-associated renal fibrosis in Sprague Dawley rats. J. Gerontol. A Biol. Sci. Med. Sci. 75, 452–458 (2020).
Miguel, V. et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest, 131, e140695 (2021).
Piret, S. E. et al. Loss of proximal tubular transcription factor Krüppel-like factor 15 exacerbates kidney injury through loss of fatty acid oxidation. Kidney Int. 100, 1250–1267 (2021).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e785 (2019).
Hashimoto, T. Peroxisomal β-oxidation: enzymology and molecular biology. Ann. N. Y. Acad. Sci. 804, 86–98 (1996).
Chang, C. L. et al. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J. Cell Biol. 218, 2583–2599 (2019).
Ding, L. et al. Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nat. Metab. 3, 1648–1661 (2021).
Gulati, S. et al. Ischemia-reperfusion injury: biochemical alterations in peroxisomes of rat kidney. Arch. Biochem. Biophys. 295, 90–100 (1992).
Ibrahim, I. Y., Elbassuoni, E. A., Ragy, M. M. & Habeeb, W. N. Gender difference in the development of cardiac lesions following acute ischemic-reperfusion renal injury in albino rats. Gen. Physiol. Biophys. 32, 421–428 (2013).
Negishi, K. et al. A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int. 72, 348–358 (2007).
Wang, Y. et al. Peroxisome-generated succinate induces lipid accumulation and oxidative stress in the kidneys of diabetic mice. J. Biol. Chem. 298, 101660 (2022).
Dhaunsi, G. S. & Bitar, M. S. Antioxidants attenuate diabetes-induced activation of peroxisomal functions in the rat kidney. J. Biomed. Sci. 11, 566–570 (2004).
Hwang, I. et al. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 61, 728–738 (2012).
Sas, K. M. et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1, e86976 (2016).
Baek, J. et al. The deacylase sirtuin 5 reduces malonylation in nonmitochondrial metabolic pathways in diabetic kidney disease. J. Biol. Chem. 299, 102960 (2023).
Joven, J. et al. Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N. Engl. J. Med. 323, 579–584 (1990).
Valdete, T.-S. & Valdete, H. in Cellular Metabolism and Related Disorders Ch. 9 (eds Khan, J. & Hsieh, P.-S.) (IntechOpen, 2019).
Vaziri, N. D., Sato, T. & Liang, K. Molecular mechanisms of altered cholesterol metabolism in rats with spontaneous focal glomerulosclerosis. Kidney Int. 63, 1756–1763 (2003).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Jiang, T. et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 (2007).
Wang, X. X. et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am. J. Physiol. Renal Physiol. 297, F1587–F1596 (2009).
Wang, X. X. et al. FXR/TGR5 Dual agonist prevents progression of nephropathy in diabetes and obesity. J. Am. Soc. Nephrol. 29, 118–137 (2018).
Levy, E. et al. Intestinal cholesterol transport proteins: an update and beyond. Curr. Opin. Lipidol. 18, 310–318 (2007).
Caldas, Y. A. et al. Liver X receptor-activating ligands modulate renal and intestinal sodium-phosphate transporters. Kidney Int. 80, 535–544 (2011).
Liu, P. et al. Association between LXR-α and ABCA1 gene polymorphisms and the risk of diabetic kidney disease in patients with type 2 diabetes mellitus in a Chinese Han population. J. Diabetes Res. 2020, 8721536 (2020).
Pedigo, C. E. et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J. Clin. Invest. 126, 3336–3350 (2016).
Wright, M. B. et al. Compounds targeting OSBPL7 increase ABCA1-dependent cholesterol efflux preserving kidney function in two models of kidney disease. Nat. Commun. 12, 4662 (2021).
Byun, J. H. et al. Inhibitory antibodies against PCSK9 reduce surface CD36 and mitigate diet-induced renal lipotoxicity. Kidney360 3, 1394–1410 (2022).
Haas, M. E. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation 134, 61–72 (2016).
Buraczynska, M., Jacob, J., Gwiazda-Tyndel, K. & Ksiazek, A. LDLR gene polymorphism (rs688) affects susceptibility to cardiovascular disease in end-stage kidney disease patients. BMC Nephrol. 22, 316 (2021).
Guo, Q., Feng, X. & Zhou, Y. PCSK9 variants in familial hypercholesterolemia: a comprehensive synopsis. Front. Genet. 11, 1020 (2020).
Yang, Q. et al. Sirt6 deficiency aggravates angiotensin II-induced cholesterol accumulation and injury in podocytes. Theranostics 10, 7465–7479 (2020).
Fu, Y. et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metab. 32, 1052–1062.e1058 (2020).
Vaziri, N. D., Kim, C. H., Phan, D., Kim, S. & Liang, K. Up-regulation of hepatic Acyl CoA: diacylglycerol acyltransferase-1 (DGAT-1) expression in nephrotic syndrome. Kidney Int. 66, 262–267 (2004).
Zhong, F. et al. ANGPTL3 impacts proteinuria and hyperlipidemia in primary nephrotic syndrome. Lipids Health Dis. 21, 38 (2022).
Liu, J. et al. A novel role of angiopoietin-like-3 associated with podocyte injury. Pediatr. Res. 77, 732–739 (2015).
Chen, W. et al. Atgl deficiency induces podocyte apoptosis and leads to glomerular filtration barrier damage. FEBS J. 284, 1070–1081 (2017).
Freedman, B. I., Limou, S., Ma, L. & Kopp, J. B. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am. J. Kidney Dis. 72, S8–s16 (2018).
Ge, M. et al. APOL1 risk variants affect podocyte lipid homeostasis and energy production in focal segmental glomerulosclerosis. Hum. Mol. Genet. 30, 182–197 (2021).
Chun, J. et al. Recruitment of APOL1 kidney disease risk variants to lipid droplets attenuates cell toxicity. Proc. Natl Acad. Sci. USA 116, 3712–3721 (2019).
Zhao, K. et al. Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci. Rep. 6, 37234 (2016).
Kim, D. H. et al. Src-mediated crosstalk between FXR and YAP protects against renal fibrosis. FASEB J. 33, 11109–11122 (2019).
Zhou, W. & Anakk, S. Enterohepatic and non-canonical roles of farnesoid X receptor in controlling lipid and glucose metabolism. Mol. Cell. Endocrinol. 549, 111616 (2022).
Kim, D.-H. et al. The role of the farnesoid X receptor in kidney health and disease: a potential therapeutic target in kidney diseases. Exp. Mol. Med. 55, 304–312 (2023).
Zhou, B. et al. Activation of farnesoid X receptor downregulates visfatin and attenuates diabetic nephropathy. Mol. Cell Endocrinol. 419, 72–82 (2016).
Wu, D. et al. Vaccine against PCSK9 improved renal fibrosis by regulating fatty acid β‐oxidation. J. Am. Heart Assoc. 9, e014358 (2020).
Wu, M. M. et al. Lovastatin attenuates hypertension induced by renal tubule-specific knockout of ATP-binding cassette transporter A1, by inhibiting epithelial sodium channels. Br. J. Pharmacol. 176, 3695–3711 (2019).
Tsun, J. G. et al. Cellular cholesterol transport proteins in diabetic nephropathy. PLoS One 9, e105787 (2014).
Baumer, Y., McCurdy, S. G. & Boisvert, W. A. Formation and cellular impact of cholesterol crystals in health and disease. Adv. Biol. 5, 2100638 (2021).
Del Sordo, R. et al. Cholesterol crystals tubulointerstitial injury during nephrotic syndrome; can be classified as tubular crystallopathy? J. Nephropathol. 10, e20–e20 (2021).
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019).
Cui, L. & Liu, P. Two types of contact between lipid droplets and mitochondria. Front. Cell Dev. Biol. 8, 618322 (2020).
Yang, W. et al. Ectopic lipid accumulation: potential role in tubular injury and inflammation in diabetic kidney disease. Clin. Sci. 132, 2407–2422 (2018).
Kume, S. et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 18, 2715–2723 (2007).
Saito, K. et al. Lipid accumulation and transforming growth factor-β upregulation in the kidneys of rats administered angiotensin II. Hypertension 46, 1180–1185 (2005).
Kiss, E. et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by liver X receptors. Am. J. Pathol. 182, 727–741 (2013).
Pérez-Martí, A. et al. Reducing lipid bilayer stress by monounsaturated fatty acids protects renal proximal tubules in diabetes. eLife 11, e74391 (2022).
Xie, Y. H. et al. Role of the CTRP6/AMPK pathway in kidney fibrosis through the promotion of fatty acid oxidation. Eur. J. Pharmacol. 892, 173755 (2021).
Liu, L. et al. Twist1 downregulation of PGC-1α decreases fatty acid oxidation in tubular epithelial cells, leading to kidney fibrosis. Theranostics 12, 3758–3775 (2022).
Chen, Z. et al. Oxidative stress and lipid dysregulation in lipid droplets: a connection to chronic kidney disease revealed in human kidney cells. Antioxidants 11, 1387 (2022).
Lubojemska, A. et al. Adipose triglyceride lipase protects renal cell endocytosis in a Drosophila dietary model of chronic kidney disease. PLoS Biol. 19, e3001230 (2021).
Chen, R.-X. et al. The renal manifestations of type 4 familial partial lipodystrophy: a case report and review of literature. BMC Nephrol. 19, 111 (2018).
Wang, Y. et al. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes. Res. 11, 930–936 (2003).
Ju, L. et al. Obesity-associated inflammation triggers an autophagy–lysosomal response in adipocytes and causes degradation of perilipin 1. Cell Death Dis. 10, 121 (2019).
Jun, H. et al. In vivo and in vitro effects of SREBP-1 on diabetic renal tubular lipid accumulation and RNAi-mediated gene silencing study. Histochem. Cell Biol. 131, 327–345 (2009).
Kim, J. J. et al. British Society for Matrix Biology spring 2022 meeting. Int. J. Exp. Pathol. 103, A1–A8 (2022).
Mallela, S. K. et al. Sphingomyelin phosphodiesterase acid like 3B (SMPDL3b) regulates Perilipin5 (PLIN5) expression and mediates lipid droplet formation. Genes Dis. 9, 1397–1400 (2022).
Mitrofanova, A. et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat. Commun. 10, 2692 (2019).
Mallela, S. K., Mitrofanova, A., Merscher, S. & Fornoni, A. Regulation of the amount of ceramide-1-phosphate synthesized in differentiated human podocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 158517 (2019).
Feng, J. et al. Perilipin 5 ameliorates high-glucose-induced podocyte injury via Akt/GSK-3β/Nrf2-mediated suppression of apoptosis, oxidative stress, and inflammation. Biochem. Biophys. Res. Commun. 544, 22–30 (2021).
Yoshioka, K. et al. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 101, 510–526 (2022).
Li, H., Dixon, E. E., Wu, H. & Humphreys, B. D. Comprehensive single-cell transcriptional profiling defines shared and unique epithelial injury responses during kidney fibrosis. Cell Metab. 34, 1977–1998.e1979 (2022).
Sørensen, I. M. et al. Apolipoprotein M in patients with chronic kidney disease. Atherosclerosis 275, 304–311 (2018).
Drexler, Y. et al. Identification of glomerular and plasma apolipoprotein M as novel biomarkers in glomerular disease. Kidney Int. Rep. 8, 884–897 (2023).
Ferrans, V. J. & Fredrickson, D. S. The pathology of Tangier disease. A light and electron microscopic study. Am. J. Pathol. 78, 101–158 (1975).
Mende, C. & Einhorn, D. Fatty kidney disease: the importance of ectopic fat deposition and the potential value of imaging. J. Diabetes 14, 73–78 (2022).
Jiang, Z. et al. Obesity and chronic kidney disease. Am. J. Physiol. Endocrinol. Metab. 324, E24–E41 (2023).
Chughtai, H. L. et al. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension 56, 901–906 (2010).
Wagner, R. et al. The protective effect of human renal sinus fat on glomerular cells is reversed by the hepatokine fetuin-A. Sci. Rep. 7, 2261 (2017).
Wagner, R. et al. Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia 55, 2054–2058 (2012).
Shen, Y. et al. Renal fat fraction is significantly associated with the risk of chronic kidney disease in patients with type 2 diabetes. Front. Endocrinol. 13, 995028 (2022).
Spit, K. A. et al. Renal sinus fat and renal hemodynamics: a cross-sectional analysis. MAGMA 33, 73–80 (2020).
Krievina, G. et al. Ectopic adipose tissue storage in the left and the right renal sinus is asymmetric and associated with serum kidney injury molecule-1 and fibroblast growth factor-21 levels increase. EBioMedicine 13, 274–283 (2016).
Zelicha, H. et al. Changes of renal sinus fat and renal parenchymal fat during an 18-month randomized weight loss trial. Clin. Nutr. 37, 1145–1153 (2018).
Schaub, J. A. et al. SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth onset type 2 diabetes. J. Clin. Invest. 133, e164486 (2023).
Marzolla, V. et al. The novel non-steroidal MR antagonist finerenone improves metabolic parameters in high-fat diet-fed mice and activates brown adipose tissue via AMPK-ATGL pathway. FASEB J. 34, 12450–12465 (2020).
Ge, M. et al. Empagliflozin reduces podocyte lipotoxicity in experimental Alport syndrome. eLife 12, e83353 (2023).
Hayder, Z. S. & Kareem, Z. S. Resistin hormone in diabetic kidney disease and its relation to iron status and hepcidin. Int. Urol. Nephrol. 52, 749–756 (2020).
Ng, X.-N., Tang, C.-C., Wang, C.-H., Tsai, J.-P. & Hsu, B.-G. Positive correlation of serum resistin level with peripheral artery disease in patients with chronic kidney disease stage 3 to 5. Int. J. Environ. Res. Public. Health 18, 12746 (2021).
Li, H.-F., Liu, H.-T., Chen, P.-Y., Lin, H. & Tseng, T.-L. Role of PVAT in obesity-related cardiovascular disease through the buffering activity of ATF3. iScience 25, 105631 (2022).
Agabiti-Rosei, C. et al. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J. Hypertens. 32, 1264–1274 (2014).
Wang, M. et al. Deletion of seipin attenuates vascular function and the anticontractile effect of perivascular adipose tissue. Front. Cardiovasc. Med. 8, 706924 (2021).
Zou, L. et al. Spontaneous hypertension occurs with adipose tissue dysfunction in perilipin-1 null mice. Biochim. Biophys. Acta 1862, 182–191 (2016).
Qi, X. Y. et al. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc. Diabetol. 17, 134 (2018).
Ouyang, A., Olver, T. D., Emter, C. A. & Fleenor, B. S. Chronic exercise training prevents coronary artery stiffening in aortic-banded miniswine: Role of perivascular adipose-derived advanced glycation end products. J. Appl. Physiol. 127, 816–827 (2019).
Chen, X. et al. GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway. J. Int. Med. Res. 49, 300060521992981 (2021).
Hildebrand, S., Stümer, J. & Pfeifer, A. PVAT and its relation to brown, beige, and white adipose tissue in development and function. Front. Physiol. 9, 70 (2018).
Kim, S. J., Choi, Y., Choi, Y. H. & Park, T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J. Nutr. Biochem. 23, 113–122 (2012).
Song, M. J., Kim, K. H., Yoon, J. M. & Kim, J. B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 346, 739–745 (2006).
Kiernan, R. N., Maddie, N. & Carrillo-Sepulveda, M. A. Western diet-induced hypertension involves HMGB1/TLR4 signaling in perivascular adipose tissue of female rats. FASEB J. 34, 1–1 (2020).
Mao, Y. et al. STING-IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler. Thromb. Vasc. Biol. 37, 920–929 (2017).
Wu, J. et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J. Clin. Invest. 131, e136329 (2021).
Yu, B. C. et al. Minimal change disease is associated with mitochondrial injury and STING pathway activation. J. Clin. Med. 11, 577 (2022).
Mitrofanova, A. et al. Activation of stimulator of IFN genes (STING) causes proteinuria and contributes to glomerular diseases. J. Am. Soc. Nephrol. 33, 2153–2173 (2022).
Climent, E., Benaiges, D. & Pedro-Botet, J. Hydrophilic or lipophilic statins. Front. Cardiovasc. Med. 8, 687585 (2021).
Wanner, C., Tonelli, M. & Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int. 85, 1303–1309 (2014).
Jin Kang, H. et al. Effects of low-dose niacin on dyslipidemia and serum phosphorus in patients with chronic kidney disease. Kidney Res. Clin. Pract. 32, 21–26 (2013).
Pham, K. O. et al. Association between vitamin intake and chronic kidney disease according to a variant located upstream of the PTGS1 gene: a cross-sectional analysis of Shika study. Nutrients 14, 2082 (2022).
Robins, S. J. et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. J. Am. Med. Assoc. 285, 1585–1591 (2001).
Imai, E. & Imai, A. Effect of pemafibrate on serum creatinine in patients with chronic kidney disease. JMA J. 5, 328–333 (2022).
Hadjivasilis, A., Kouis, P., Kousios, A. & Panayiotou, A. The effect of fibrates on kidney function and chronic kidney disease progression: a systematic review and meta-analysis of randomised studies. J. Clin. Med. 11, 768 (2022).
Hakimizadeh, E. et al. Gemfibrozil, a lipid-lowering drug, improves hepatorenal damages in a mouse model of aging. Fundam. Clin. Pharmacol. 37, 599–605 (2023).
Aomura, D. et al. Pemafibrate protects against fatty acid-induced nephropathy by maintaining renal fatty acid metabolism. Metabolites 11, 372 (2021).
Zheng, F. et al. Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am. J. Physiol. Renal Physiol. 282, F639–F648 (2002).
Isshiki, K. et al. Thiazolidinedione compounds ameliorate glomerular dysfunction independent of their insulin-sensitizing action in diabetic rats. Diabetes 49, 1022–1032 (2000).
Okada, T. et al. Thiazolidinediones ameliorate diabetic nephropathy via cell cycle-dependent mechanisms. Diabetes 55, 1666–1677 (2006).
Ruan, X. Z. et al. PPAR agonists protect mesangial cells from interleukin 1β-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J. Am. Soc. Nephrol. 14, 593–600 (2003).
Zhu, X. et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283, 22930–22941 (2008).
Cid-Samamed, A., Rakmai, J., Mejuto, J. C., Simal-Gandara, J. & Astray, G. Cyclodextrins inclusion complex: preparation methods, analytical techniques and food industry applications. Food Chem. 384, 132467 (2022).
Lewandowski, C. T. et al. Metabolomic analysis of a selective ABCA1 inducer in obesogenic challenge provides a rationale for therapeutic development. EBioMedicine 66, 103287 (2021).
Yin, Q. H. et al. Exendin-4 ameliorates lipotoxicity-induced glomerular endothelial cell injury by improving ABC transporter A1-mediated cholesterol efflux in diabetic apoE knockout mice. J. Biol. Chem. 291, 26487–26501 (2016).
Schlackow, I. et al. Cost-effectiveness of lipid lowering with statins and ezetimibe in chronic kidney disease. Kidney Int. 96, 170–179 (2019).
Heinrich, N. S. et al. Evaluation of the effects of ezetimibe on albuminuria and kidney fat in individuals with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. https://doi.org/10.1111/dom.15146 (2023).
Hua, W. et al. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS One 10, e0127507 (2015).
Souza, A. C. P. et al. Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int. 89, 809–822 (2016).
Hou, Y. et al. The antioxidant peptide SS31 prevents oxidative stress, downregulates CD36 and improves renal function in diabetic nephropathy. Nephrol. Dial. Transplant. 33, 1908–1918 (2018).
Toth, P. P. et al. Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int. 93, 1397–1408 (2018).
Charytan, D. M. et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial. J. Am. Coll. Cardiol. 73, 2961–2970 (2019).
Warden, B. A. & Duell, P. B. Inclisiran: a novel agent for lowering apolipoprotein B-containing lipoproteins. J. Cardiovasc. Pharmacol. 78, e157–e174 (2021).
Sakurai, M., Muso, E., Matushima, H., Ono, T. & Sasayama, S. Rapid normalization of interleukin-8 production after low-density lipoprotein apheresis in steroid-resistant nephrotic syndrome. Kidney Int. Suppl. 71, S210–S212 (1999).
Wang, A. et al. Systematic review of low‐density lipoprotein cholesterol apheresis for the treatment of familial hypercholesterolemia. J. Am. Heart Assoc. 5, e003294 (2016).
Ai, J. Y., Zhao, P. C., Zhang, W. & Rao, G. W. Research progress in the clinical treatment of familial hypercholesterolemia. Curr. Med. Chem. https://doi.org/10.2174/0929867330666230202111849 (2023).
Al-Mousily, M., Nicoara, O., Selewski, D. T. & Twombley, K. Liposorber® LA-15 system for LDL apheresis in resistant nephrotic syndrome patients. Pediatr. Nephrol. 37, 585–592 (2022).
Muso, E. et al. Favorable therapeutic efficacy of low-density lipoprotein apheresis for nephrotic syndrome with impaired renal function. Ther. Apher. Dial. 26, 220–228 (2022).
Wang, X. X. et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J. Biol. Chem. 292, 5335–5348 (2017).
Sun, H., Chen, J., Hua, Y., Zhang, Y. & Liu, Z. New insights into the role of empagliflozin on diabetic renal tubular lipid accumulation. Diabetol. Metab. Syndr. 14, 121 (2022).
Wanner, C. et al. Consistent effects of empagliflozin on cardiovascular and kidney outcomes irrespective of diabetic kidney disease categories: Insights from the EMPA-REG OUTCOME trial. Diabetes Obes. Metab. 22, 2335–2347 (2020).
Wanner, C. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334 (2016).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
The EMPA-KIDNEY Collaborative Group et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388, 117–127 (2023).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Kwon, S. et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care 43, 948–955 (2020).
Lin, C.-X. et al. Metformin attenuates cyclosporine A-induced renal fibrosis in rats. Transplantation 103, e285–e296 (2019).
Satriano, J., Sharma, K., Blantz, R. C. & Deng, A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am. J. Physiol. Renal Physiol. 305, F727–F733 (2013).
Neven, E. et al. Metformin prevents the development of severe chronic kidney disease and its associated mineral and bone disorder. Kidney Int. 94, 102–113 (2018).
Abe, M., Okada, K. & Soma, M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr. Drug. Metab. 12, 57–69 (2011).
Roumie, C. L. et al. Association of treatment with metformin vs sulfonylurea with major adverse cardiovascular events among patients with diabetes and reduced kidney function. J. Am. Med. Assoc. 322, 1167–1177 (2019).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Hahr, A. J. & Molitch, M. E. Management of diabetes mellitus in patients with CKD: core curriculum 2022. Am. J. Kidney Dis. 79, 728–736 (2022).
Eom, M., Hudkins, K. L. & Alpers, C. E. Foam cells and the pathogenesis of kidney disease. Curr. Opin. Nephrol. Hypertens. 24, 245–251 (2015).
Kaseda, R. et al. Chronic kidney disease alters lipid trafficking and inflammatory responses in macrophages: effects of liver X receptor agonism. BMC Nephrol. 19, 17 (2018).
Yan, P. et al. Association of remnant cholesterol with chronic kidney disease in middle-aged and elderly Chinese: a population-based study. Acta Diabetol. 58, 1615–1625 (2021).
Nam, K. H. et al. Association between serum high‐density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW‐CKD. J. Am. Heart Assoc. 8, e011162 (2019).
Pavanello, C. et al. Progression of chronic kidney disease in familial LCAT deficiency: a follow-up of the Italian cohort. J. Lipid Res. 61, 1784–1788 (2020).
Tsuruya, K. et al. Impact of the triglycerides to high-density lipoprotein cholesterol ratio on the incidence and progression of CKD: a longitudinal study in a large Japanese population. Am. J. Kidney Dis. 66, 972–983 (2015).
Kim, J. et al. The ratio of triglycerides to high-density lipoprotein cholesterol is associated with the risk of chronic kidney disease in Korean men. Lipids 56, 475–483 (2021).
Kim, Y. et al. Predictive value of triglyceride/high-density lipoprotein cholesterol for major clinical outcomes in advanced chronic kidney disease: a nationwide population-based study. Clin. Kidney J. 14, 1961–1968 (2020).
Itabe, H., Yamaguchi, T., Nimura, S. & Sasabe, N. Perilipins: a diversity of intracellular lipid droplet proteins. Lipids Health Dis. 16, 83 (2017).
Schroeder, B. et al. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology 61, 1896–1907 (2015).
Kaushik, S. & Cuervo, A. M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 17, 759–770 (2015).
Kaushik, S. & Cuervo, A. M. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy 12, 432–438 (2016).
Shin, D. W. Lipophagy: molecular mechanisms and implications in metabolic disorders. Mol. Cell 43, 686–693 (2020).
Li, Y. et al. CD36 plays a negative role in the regulation of lipophagy in hepatocytes through an AMPK-dependent pathway. J. Lipid Res. 60, 844–855 (2019).
Zhang, H. et al. Dynamic MTORC1-TFEB feedback signaling regulates hepatic autophagy, steatosis and liver injury in long-term nutrient oversupply. Autophagy 14, 1779–1795 (2018).
Zhang, Z. et al. Lipophagy and liver disease: new perspectives to better understanding and therapy. Biomed. Pharmacother. 97, 339–348 (2018).
Han, Y. et al. Lipophagy deficiency exacerbates ectopic lipid accumulation and tubular cells injury in diabetic nephropathy. Cell Death Dis. 12, 1031 (2021).
Jung, H. S. et al. Loss of autophagy diminishes pancreatic β cell mass and function with resultant hyperglycemia. Cell Metab. 8, 318–324 (2008).
Zhao, Y. et al. High dose Vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front. Physiol. 9, 1939 (2018).
Liu, W. J. et al. Autophagy-lysosome pathway in renal tubular epithelial cells is disrupted by advanced glycation end products in diabetic nephropathy. J. Biol. Chem. 290, 20499–20510 (2015).
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501 (2019).
Shao, M. et al. Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885 (2019).
Cattaneo, P. et al. Parallel lineage-tracing studies establish fibroblasts as the prevailing in vivo adipocyte progenitor. Cell Rep. 30, 571–582.e572 (2020).
Grigoraș, A. et al. Perirenal adipose tissue-current knowledge and future opportunities. J. Clin. Med. 10, 1291 (2021).
Acknowledgements
We give special thanks to the Katz family for their continuous support.
Author information
Authors and Affiliations
Contributions
A.M. researched data for the article, made substantial contributions to discussions of the content and wrote the manuscript. S.M. and A.F. reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.F. and S.M. are inventors on pending or issued patents (US10.183.038, US10.052.345) aimed at diagnosing or treating proteinuric kidney diseases and therefore stand to gain royalties from their future commercialization. A.F. is Chief Scientific Officer of L&F Health LLC, holds equity interests in L&F Research and is the inventor of assets developed by ZyVersa Therapeutics. ZyVersa has licensed worldwide rights to develop and commercialize hydroxypropyl-β-cyclodextrin for the treatment of kidney disease from L&F Research. A.F. also holds equity in River 3 Renal Corporation. S.M. holds equity interest in L&F Research. A.M. declares no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks R. Inagi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mitrofanova, A., Merscher, S. & Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat Rev Nephrol 19, 629–645 (2023). https://doi.org/10.1038/s41581-023-00741-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00741-w
This article is cited by
-
Effect of sacubitril/valsartan on lipid metabolism in patients with chronic kidney disease combined with chronic heart failure: a retrospective study
Lipids in Health and Disease (2024)
-
Impact of resistance exercise on patients with chronic kidney disease
BMC Nephrology (2024)
-
Dysregulated lipid metabolism is associated with kidney allograft fibrosis
Lipids in Health and Disease (2024)