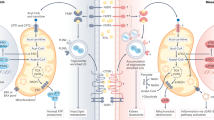
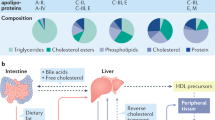
Abstract
Dyslipidaemia is a hallmark of chronic kidney disease (CKD). The severity of dyslipidaemia not only correlates with CKD stage but is also associated with CKD-associated cardiovascular disease and mortality. Understanding how lipids are dysregulated in CKD is, however, challenging owing to the incredible diversity of lipid structures. CKD-associated dyslipidaemia occurs as a consequence of complex interactions between genetic, environmental and kidney-specific factors, which to understand, requires an appreciation of perturbations in the underlying network of genes, proteins and lipids. Modern lipidomic technologies attempt to systematically identify and quantify lipid species from biological systems. The rapid development of a variety of analytical platforms based on mass spectrometry has enabled the identification of complex lipids at great precision and depth. Insights from lipidomics studies to date suggest that the overall architecture of free fatty acid partitioning between fatty acid oxidation and complex lipid fatty acid composition is an important driver of CKD progression. Available evidence suggests that CKD progression is associated with metabolic inflexibility, reflecting a diminished capacity to utilize free fatty acids through β-oxidation, and resulting in the diversion of accumulating fatty acids to complex lipids such as triglycerides. This effect is reversed with interventions that improve kidney health, suggesting that targeting of lipid abnormalities could be beneficial in preventing CKD progression.
Key points
-
Lipidomic analyses remain a challenge due to the numerous species that exist within each class; techniques that reduce analytical complexity while retaining important information about lipid structure are useful for elucidating biological significance from lipidomics studies.
-
The immense structural diversity of lipids means that lipidomic analyses are guided by a choice of untargeted and targeted analysis; sample preparation, extraction and separation methods may require optimization for specific classes of interest.
-
A key challenge with profiling technologies such as lipidomics is the ability to use the data to gain insights into biological processes, including mechanisms of disease onset and progression
-
Fatty acid profiles that are enriched in shorter and more saturated species are associated with later stages of chronic kidney disease (CKD) and are predictive of CKD progression.
-
Acylcarnitine profiles provide insights into mitochondrial function; a lower ratio of long-chain to medium-chain acylcarnitines is indicative of increased mitochondrial inefficiency and is associated with worsening CKD.
-
Patients with CKD or kidney failure demonstrate enrichment of complex lipids, such as triacylglycerols and phosphatidylethanolamines, with enrichment of longer and more unsaturated fatty acyl side-chains; an inverse association between mitochondrial efficiency and these complex lipids suggests a mechanistic link between fatty acid oxidation and fatty acid profiles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Johansen, K. L. et al. US Renal Data System 2020 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 77, A7–A8 (2021).
Sarnak, M. J. et al. Kidney disease as a risk factor for development of cardiovascular disease. Circulation 108, 2154–2169 (2003).
Noels, H., Lehrke, M., Vanholder, R. & Jankowski, J. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat. Rev. Nephrol. 17, 528–542 (2021).
Thompson, S. et al. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 26, 2504–2511 (2015).
Zhao, Y. Y., Wu, S. P., Liu, S., Zhang, Y. & Lin, R. C. Ultra-performance liquid chromatography–mass spectrometry as a sensitive and powerful technology in lipidomic applications. Chem. Biol. Interact. 220, 181–192 (2014).
Guijas, C. et al. METLIN: a technology platform for identifying knowns and unknowns. Anal. Chem. 90, 3156–3164 (2018).
Cotter, D., Maer, A., Guda, C., Saunders, B. & Subramaniam, S. LMPD: LIPID MAPS proteome database. Nucleic Acids Res. 34, D507–D510 (2006).
Richmond, G. S. & Smith, T. K. Phospholipases A1. Int. J. Mol. Sci. 12, 588–612 (2011).
Cajka, T. & Fiehn, O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 88, 524–545 (2016).
Züllig, T., Trötzmüller, M. & Köfeler, H. C. Lipidomics from sample preparation to data analysis: a primer. Anal. Bioanal. Chem. 412, 2191–2209 (2020).
Afshinnia, F. et al. Lipidomics and biomarker discovery in kidney disease. Semin. Nephrol. 38, 127–141 (2018).
Rhee, E. P. et al. Variability of two metabolomic platforms in CKD. Clin. J. Am. Soc. Nephrol. 14, 40–48 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Wishart, D. S. et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46, D608–D617 (2018).
Afshinnia, F. et al. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 4, e130317 (2019).
Afshinnia, F. et al. Impaired β-oxidation and altered complex lipid fatty acid partitioning with advancing CKD. J. Am. Soc. Nephrol. 29, 295–306 (2018).
Basu, S. et al. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 33, 1545–1553 (2017).
Krumsiek, J., Bartel, J. & Theis, F. J. Computational approaches for systems metabolomics. Curr. Opin. Biotechnol. 39, 198–206 (2016).
Ma, J. et al. Differential network enrichment analysis reveals novel lipid pathways in chronic kidney disease. Bioinformatics 35, 3441–3452 (2019).
Afshinnia, F. et al. Lipidomic signature of progression of chronic kidney disease in the chronic renal insufficiency cohort. Kidney Int. Rep. 1, 256–268 (2016).
Karnovsky, A. et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics 28, 373–380 (2012).
Subramanian, I., Verma, S., Kumar, S., Jere, A. & Anamika, K. Multi-omics data integration, interpretation, and its application. Bioinforma. Biol. Insights https://doi.org/10.1177/1177932219899051 (2020).
Brinkkoetter, P. T. et al. Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep. 27, 1551–1566.e5 (2019).
Forbes, J. M. & Thorburn, D. R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 14, 291–312 (2018).
Mandel, L. J. Metabolic substrates, cellular energy production, and the regulation of proximal tubular transport. Annu. Rev. Physiol. 47, 85–101 (1985).
Scerbo, D. et al. Kidney triglyceride accumulation in the fasted mouse is dependent upon serum free fatty acids. J. Lipid Res. 58, 1132–1142 (2017).
Khan, S. et al. Kidney proximal tubule lipoapoptosis is regulated by fatty acid transporter-2 (FATP2). J. Am. Soc. Nephrol. 29, 81–91 (2018).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells plays a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Kampe, K., Sieber, J., Orellana, J. M., Mundel, P. & Jehle, A. W. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am. J. Physiol. Ren. Physiol. 306, F401–F409 (2014).
Yuan, Y. et al. Activation of peroxisome proliferator-activated receptor-γ coactivator 1α ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 82, 771–789 (2012).
Tran, M. et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. 121, 4003–4014 (2011).
Zhang, H. et al. Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 295, F1071–F1081 (2008).
Miguel, V. et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest. 131, e140695 (2021).
Baines, R. J. et al. CD36 mediates proximal tubular binding and uptake of albumin and is upregulated in proteinuric nephropathies. Am. J. Physiol. Ren. Physiol. 303, F1006–F1014 (2012).
Herman-Edelstein, M., Scherzer, P., Tobar, A., Levi, M. & Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 55, 561–572 (2014).
Hua, W. et al. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS One 10, e0127507 (2015).
Okamura, D. M. et al. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J. Am. Soc. Nephrol. 20, 495–505 (2009).
Souza, A. C. P. et al. Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int. 89, 809–822 (2016).
Yang, P. et al. Inflammatory stress promotes the development of obesity-related chronic kidney disease via CD36 in mice. J. Lipid Res. 58, 1417–1427 (2017).
Yang, Y. L. et al. CD36 is a novel and potential anti-fibrogenic target in albumin-induced renal proximal tubule fibrosis. J. Cell. Biochem. 101, 735–744 (2007).
Li, L. C. et al. Palmitate aggravates proteinuria-induced cell death and inflammation via CD36-inflammasome axis in the proximal tubular cells of obese mice. Am. J. Physiol. Ren. Physiol. 315, F1720–F1731 (2018).
Cui, W. et al. Interaction of thrombospondin1 and CD36 contributes to obesity-associated podocytopathy. Biochim. Biophys. Acta 1852, 1323–1333 (2015).
Kim, J.-J. et al. Discoidin domain receptor 1 activation links extracellular matrix to podocyte lipotoxicity in Alport syndrome. EBioMedicine 63, 103162 (2021).
Kennedy, D. J. et al. CD36 and Na/K-ATPase-α1 form a pro-inflammatory signaling loop in kidney. Hypertension 61, 216–224 (2013).
Pennathur, S. et al. The macrophage phagocytic receptor CD36 promotes fibrogenic pathways on removal of apoptotic cells during chronic kidney injury. Am. J. Pathol. 185, 2232–2245 (2015).
Yang, X. et al. CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 13, 769–781 (2017).
Kuwahara, S. et al. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J. Am. Soc. Nephrol. 27, 1996–2008 (2016).
Khan, S. et al. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight 5, e136845 (2020).
Zager, R. A., Johnson, A. C. M. & Hanson, S. Y. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 67, 111–121 (2005).
Johnson, A. L. I. C. M., Stahl, A. & Zager, R. A. Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int. 67, 2196–2209 (2005).
Weinberg, J. M. Lipotoxicity. Kidney Int. 70, 1560–1566 (2006).
Jiang, T. et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J. Biol. Chem. 280, 32317–32325 (2005).
Lin, Y. C. et al. Nifedipine exacerbates lipogenesis in the kidney via KIM-1, CD36, and SREBP upregulation: implications from an animal model for human study. Int. J. Mol. Sci. 21, 4359 (2020).
Chen, H. et al. Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J. Proteome Res. 16, 1566–1578 (2017).
Szczuko, M. et al. Comparison of fatty acid profiles in a group of female patients with chronic kidney diseases (CKD) and metabolic syndrome (MetS)–similar trends of changes, different pathophysiology. Int. J. Mol. Sci. 20, 1719 (2019).
Szczuko, M. et al. The C18:3n6/C22:4n6 ratio is a good lipid marker of chronic kidney disease (CKD) progression. Lipids Health Dis. 19, 77 (2020).
Afshinnia, F. et al. Circulating free fatty acid and phospholipid signature predicts early rapid kidney function decline in patients with type 1 diabetes. Diabetes Care 44, 2098–2106 (2021).
Listenberger, L. L. et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl Acad. Sci. USA 100, 3077–3082 (2003).
Ackerman, D. et al. Triglycerides promote lipid homeostasis during hypoxic stress by balancing fatty acid saturation. Cell Rep. 24, 2596–2605.e5 (2018).
Czumaj, A. et al. Alterations of fatty acid profile may contribute to dyslipidemia in chronic kidney disease by influencing hepatocyte metabolism. Int. J. Mol. Sci. 20, 2470 (2019).
Wang, L. et al. Plasma lipidomics investigation of hemodialysis effects by using liquid chromatography-mass spectrometry. J. Proteome Res. 15, 1986 (2016).
Sieber, J. et al. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am. J. Pathol. 183, 735–744 (2013).
Iwai, T. et al. Stearoyl-CoA desaturase-1 protects cells against lipotoxicity-mediated apoptosis in proximal tubular cells. Int. J. Mol. Sci. 17, 1868 (2016).
de Luis, D. A., Izaola, O., Aller, R., de La Fuente, B. & Pacheco, D. Effects of C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) on weight loss, adipocytokines levels, and insulin resistance after a high polyunsaturated fat diet in obese patients. J. Endocrinol. Invest. 36, 965–969 (2013).
Stevenson, J. L., Paton, C. M. & Cooper, J. A. Hunger and satiety responses to high-fat meals after a high-polyunsaturated fat diet: a randomized trial. Nutrition 41, 14–23 (2017).
Cases, S. et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276, 38870–38876 (2001).
Hostetler, H. A., Petrescu, A. D., Kier, A. B. & Schroeder, F. Peroxisome proliferator-activated receptor α interacts with high affinity and is conformationally responsive to endogenous ligands. J. Biol. Chem. 280, 18667–18682 (2005).
Kliewer, S. A. et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl Acad. Sci. USA 94, 4318–4323 (1997).
Soumura, M. et al. Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem. Biophys. Res. Commun. 402, 265–271 (2010).
Kris-Etherton, P. M., Harris, W. S. & Appel, L. J. Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106, 2747–2757 (2002).
Kim, W. et al. Polyunsaturated fatty acid desaturation is a mechanism for glycolytic NAD+ recycling. Cell Metab. 29, 856–870.e7 (2019).
Crescenzo, R. et al. Fat quality influences the obesogenic effect of high fat diets. Nutrients 7, 9475–9491 (2015).
Ruiz-Ramírez, A., Barrios-Maya, M.-A., López-Acosta, O., Molina-Ortiz, D. & El-Hafidi, M. Cytochrome c release from rat liver mitochondria is compromised by increased saturated cardiolipin species induced by sucrose feeding. Am. J. Physiol. Endocrinol. Metab. 309, E777–E786 (2015).
Oemer, G. et al. Phospholipid acyl chain diversity controls the tissue-specific assembly of mitochondrial cardiolipins. Cell Rep. 30, 4281–4291.e4 (2020).
Szeto, H. H. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J. Am. Soc. Nephrol. 28, 2856–2865 (2017).
Cavaliere, G. et al. Polyunsaturated fatty acids attenuate diet induced obesity and insulin resistance, modulating mitochondrial respiratory uncoupling in rat skeletal muscle. PLoS One 11, e0149033 (2016).
Cortie, C. H. & Else, P. L. Dietary docosahexaenoic acid (22:6) incorporates into cardiolipin at the expense of linoleic acid (18:2): analysis and potential implications. Int. J. Mol. Sci. 13, 15447–15463 (2012).
Khairallah, R. J. et al. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One 7, e34402 (2012).
Herbst, Ea. F. et al. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J. Physiol. 592, 1341–1352 (2014).
Mulligan, C. M. et al. Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc. Res. 94, 460–468 (2012).
Abdelhamid, A. S. et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 7, CD012345 (2018).
Clifton, P. M. & Keogh, J. B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 27, 1060–1080 (2017).
St-Onge, M.-P., Zhang, S., Darnell, B. & Allison, D. B. Baseline serum C-reactive protein is associated with lipid responses to low-fat and high-polyunsaturated fat diets. J. Nutr. 139, 680–683 (2009).
Ibarra-González, I. et al. Optimization of kidney dysfunction prediction in diabetic kidney disease using targeted metabolomics. Acta Diabetol. 55, 1151–1161 (2018).
Liu, J.-J. et al. Profiling of plasma metabolites suggests altered mitochondrial fuel usage and remodeling of sphingolipid metabolism in individuals with type 2 diabetes and kidney disease. Kidney Int. Rep. 2, 470–480 (2016).
Kordalewska, M. et al. Multiplatform metabolomics provides insight into the molecular basis of chronic kidney disease. J. Chromatogr. B 1117, 49–57 (2019).
Reuter, S. E. et al. Impact of haemodialysis on individual endogenous plasma acylcarnitine concentrations in end-stage renal disease. Ann. Clin. Biochem. 42, 387–393 (2005).
Kalim, S. et al. A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2, e000542 (2013).
Looker, H. C. et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 88, 888–896 (2015).
Niewczas, M. A. et al. Uremic solutes and risk of end stage renal disease in type 2 diabetes. Kidney Int. 85, 1214–1224 (2014).
van der Kloet, F. M. et al. Discovery of early-stage biomarkers for diabetic kidney disease using MS-based metabolomics (FinnDiane study). Metabolomics 8, 109–119 (2012).
Goek, O.-N. et al. Serum metabolite concentrations and decreased GFR in the general population. Am. J. Kidney Dis. 60, 197–206 (2012).
Wang, F. et al. Associations of plasma amino acid and acylcarnitine profiles with incident reduced glomerular filtration rate. Clin. J. Am. Soc. Nephrol. 13, 560–568 (2018).
Koves, T. R. et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56 (2008).
Brass, E. P. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin. Ther. 17, 176–185 (1995).
Xu, G. et al. Liver and muscle contribute differently to the plasma acylcarnitine pool during fasting and exercise in humans. J. Clin. Endocrinol. Metab. 101, 5044–5052 (2016).
Fouque, D. et al. Relationship between serum carnitine, acylcarnitines, and renal function in patients with chronic renal disease. J. Ren. Nutr. 16, 125–131 (2006).
Murphy, W. J. A. et al. Altered carnitine metabolism in dialysis patients with reduced physical function may be due to dysfunctional fatty acid oxidation. Nephrol. Dial. Transplant. 27, 304–310 (2012).
Kamei, Y., Kamei, D., Tsuchiya, K., Mineshima, M. & Nitta, K. Association between 4-year all-cause mortality and carnitine profile in maintenance hemodialysis patients. PLoS One 13, e0201591 (2018).
Schooneman, M. G., Vaz, F. M., Houten, S. M. & Soeters, M. R. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62, 1–8 (2013).
Muoio, D. M. et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 15, 764–777 (2012).
Kruger, C. et al. Proximal tubular cell-specific ablation of carnitine acetyltransferase causes tubular disease and secondary glomerulosclerosis. Diabetes 68, 819 (2019).
Chen, Y. et al. L-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: a systematic review and meta-analysis. Am. J. Clin. Nutr. 99, 408–422 (2014).
Huang, H. et al. Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: a systematic review and meta-analysis. Kidney Blood Press. Res. 38, 31–41 (2013).
Naini, A. E. et al. Effects of oral L-carnitine supplementation on lipid profile, anemia, and quality of life in chronic renal disease patients under hemodialysis: a randomized, double-blinded, placebo-controlled trial. J. Nutr. Metab. 2012, 510483 (2012).
Mikolasevic, I., Žutelija, M., Mavrinac, V. & Orlic, L. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int. J. Nephrol. Renov. Dis. 10, 35–45 (2017).
Ho, C.-I. et al. Relationship between TG/HDL-C ratio and metabolic syndrome risk factors with chronic kidney disease in healthy adult population. Clin. Nutr. 34, 874–880 (2015).
Zoppini, G. et al. Triglyceride–high-density lipoprotein cholesterol is associated with microvascular complications in type 2 diabetes mellitus. Metabolism 61, 22–29 (2012).
Lamprea-Montealegre, J. A. et al. Apolipoprotein B, triglyceride-rich lipoproteins, and risk of cardiovascular events in persons with CKD. Clin. J. Am. Soc. Nephrol. 15, 47–60 (2020).
Rhee, E. P. et al. Metabolite profiling identifies markers of uremia. J. Am. Soc. Nephrol. 21, 1041–2051 (2010).
Chang, T. I. et al. Association of serum triglyceride to HDL cholesterol ratio with all-cause and cardiovascular mortality in incident hemodialysis patients. Clin. J. Am. Soc. Nephrol. 12, 591–602 (2017).
Zhan, X. et al. Triglyceride to high-density lipoprotein cholesterol ratio is associated with increased mortality in older patients on peritoneal dialysis. Lipids Health Dis. 18, 199 (2019).
Longo, N., Frigeni, M. & Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 1863, 2422–2435 (2016).
Davis, T. M. E. et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 54, 280–290 (2011).
Jun, M. et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 60, 2061–2071 (2012).
Sas, K. M. et al. Renin-angiotensin system inhibition reverses the altered triacylglycerol metabolic network in diabetic kidney disease. Metabolomics 17, 65 (2021).
Luo, S. et al. Serum metabolomic alterations associated with proteinuria in CKD. Clin. J. Am. Soc. Nephrol. 14, 342–353 (2019).
Afshinnia, F. et al. Plasma lipidomic profiling identifies a novel complex lipid signature associated with ischemic stroke in chronic kidney disease. J. Transl. Sci. 6, 419 (2020).
van der Veen, J. N. et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta 1859, 1558–1572 (2017).
Vance, J. E. & Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 1831, 543–554 (2013).
Chan, E. Y. L. & McQuibban, G. A. Phosphatidylserine decarboxylase 1 (Psd1) promotes mitochondrial fusion by regulating the biophysical properties of the mitochondrial membrane and alternative topogenesis of mitochondrial genome maintenance protein 1 (Mgm1). J. Biol. Chem. 287, 40131–40139 (2012).
Friedman, J. R. et al. Lipid homeostasis is maintained by dual targeting of the mitochondrial PE biosynthesis enzyme to the ER. Dev. Cell 44, 261–270.e6 (2018).
Tasseva, G. et al. Phosphatidylethanolamine deficiency in mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 288, 4158–4173 (2013).
Newsom, S. A. et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans. J. Appl. Physiol. 120, 1355–1363 (2016).
Lee, S. et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine respond to exercise and influence insulin sensitivity in men. Sci. Rep. 8, 6531 (2018).
van der Veen, J. N., Lingrell, S., da Silva, R. P., Jacobs, R. L. & Vance, D. E. The concentration of phosphatidylethanolamine in mitochondria can modulate ATP production and glucose metabolism in mice. Diabetes 63, 2620–2630 (2014).
Watanabe, M. et al. Pemt deficiency ameliorates endoplasmic reticulum stress in diabetic nephropathy. PLoS One 9, e92647 (2014).
Bhat, O. M., Yuan, X., Li, G., Lee, R. & Li, P.-L. Sphingolipids and redox signaling in renal regulation and chronic kidney diseases. Antioxid. Redox Signal. 28, 1008–1026 (2018).
Shayman, J. A. Targeting glucosylceramide synthesis in the treatment of rare and common renal disease. Semin. Nephrol. 38, 183–192 (2018).
Mesicek, J. et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell. Signal. 22, 1300–1307 (2010).
Rudd, A. K. & Devaraj, N. K. Traceless synthesis of ceramides in living cells reveals saturation-dependent apoptotic effects. Proc. Natl Acad. Sci. USA 115, 7485–7490 (2018).
Mantovani, A. et al. Association between increased plasma ceramides and chronic kidney disease in patients with and without ischemic heart disease. Diabetes Metab. 47, 101152 (2021).
Klein, R. L. et al. Decreased plasma levels of select very long chain ceramide species are associated with the development of nephropathy in type 1 diabetes. Metabolism 63, 1287–1295 (2014).
Sas, K. M. et al. Targeted lipidomic and transcriptomic analysis identifies dysregulated renal ceramide metabolism in a mouse model of diabetic kidney disease. J. Proteom. Bioinform. S14, 002 (2015).
Woo, C.-Y. et al. Inhibition of ceramide accumulation in podocytes by myriocin prevents diabetic nephropathy. Diabetes Metab. J. 44, 581–591 (2020).
Dupre, T. V. et al. Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury. J. Lipid Res. 58, 1439–1452 (2017).
Turpin-Nolan, S. M. et al. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 26, 1–10.e7 (2019).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Mitrofanova, A. et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat. Commun. 10, 2692 (2019).
Yoo, T.-H. et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J. Am. Soc. Nephrol. 26, 133–147 (2015).
Jimenez, F., Monte, M. J., El-Mir, M. Y., Pascual, M. J. & Marin, J. J. G. Chronic renal failure-induced changes in serum and urine bile acid profiles. Dig. Dis. Sci. 47, 2398–2406 (2002).
Li, R. et al. Targeted metabolomics study of serum bile acid profile in patients with end-stage renal disease undergoing hemodialysis. PeerJ 7, e7145 (2019).
Jovanovich, A. et al. Deoxycholic acid, a metabolite of circulating bile acids, and coronary artery vascular calcification in CKD. Am. J. Kidney Dis. 71, 27–34 (2018).
Chu, L., Zhang, K., Zhang, Y., Jin, X. & Jiang, H. Mechanism underlying an elevated serum bile acid level in chronic renal failure patients. Int. Urol. Nephrol. 47, 345–351 (2015).
Wang, X. et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 69, 2131–2142 (2020).
Wang, X. X. et al. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J. Am. Soc. Nephrol. 27, 1362–1378 (2016).
Wang, X. X. et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J. Am. Soc. Nephrol. 29, 118–137 (2018).
Wang, X. X. et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am. J. Physiol. Ren. Physiol. 297, F1587–F1596 (2009).
Jiang, T. et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 (2007).
Chianelli, D. et al. Nidufexor (LMB763), a novel FXR modulator for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 63, 3868–3880 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03804879 (2021).
Malhi, H. & Camilleri, M. Modulating bile acid pathways and TGR5 receptors for treating liver and GI diseases. Curr. Opin. Pharmacol. 37, 80–86 (2017).
Sas, K. M. et al. Shared and distinct lipid-lipid interactions in plasma and affected tissues in a diabetic mouse model. J. Lipid Res. 59, 173–183 (2018).
Eum, J. Y. et al. Aging-related lipidomic changes in mouse serum, kidney, and heart by nanoflow ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1618, 460849 (2020).
Tan, S. M. et al. Complement C5a induces renal injury in diabetic kidney disease by disrupting mitochondrial metabolic agility. Diabetes 69, 83–98 (2020).
Zhang, G. et al. DESI-MSI and METASPACE indicates lipid abnormalities and altered mitochondrial membrane components in diabetic renal proximal tubules. Metabolomics 16, 11 (2020).
Mizuguchi, Y. et al. A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am. J. Physiol. Ren. Physiol. 295, F1545–F1553 (2008).
Hou, Y. et al. Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 310, F547–F559 (2015).
Mitrofanova, A. et al. Hydroxypropyl-β-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int. 94, 1151–1159 (2018).
Ryu, J.-H. et al. APOL1 renal risk variants promote cholesterol accumulation in tissues and cultured macrophages from APOL1 transgenic mice. PLoS One 14, e0211559 (2019).
Isaac, G. in Metabolic Profiling: Methods and Protocols (ed. Metz, T. O.) 259–275 (Humana Press, 2011).
Qiao, X. et al. A tandem mass spectrometric study of bile acids: interpretation of fragmentation pathways and differentiation of steroid isomers. Steroids 77, 204–211 (2012).
van der Hooft, J. J. J., Ridder, L., Barrett, M. P. & Burgess, K. E. V. Enhanced acylcarnitine annotation in high-resolution mass spectrometry data: fragmentation analysis for the classification and annotation of acylcarnitines. Front. Bioeng. Biotechnol. 3, 204 (2015).
Ren, J. L., Zhang, A. H., Kong, L. & Wang, X. J. Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Adv. 8, 22335–22350 (2018).
Abbas, I. et al. Kidney lipidomics by mass spectrometry imaging: a focus on the glomerulus. Int. J. Mol. Sci. 20, 1623 (2019).
Wernisch, S. & Pennathur, S. Application of differential mobility-mass spectrometry for untargeted human plasma metabolomic analysis. Anal. Bioanal. Chem. 411, 6297–6308 (2019).
Acknowledgements
The authors’ work is supported by the NIH grants 5F30DK121463, T32GM007863, T32GM008322, 5T32DK101357, K08DK106523, R03DK121941, R56DK126647, R24 DK082841, P30DK089503, P30DK081943, P30DK020572, 1R01DK110541-01A1, 5U01CA235487-03 and 5R01GM114029-05, and the JDRF Center for Excellence (5-COE-2019-861-S-B). We apologize to our colleagues whose work could not be cited due to space constraints.
Author information
Authors and Affiliations
Contributions
J.B. wrote the first draft of the article. All authors contributed equally to all other aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks A. Fornoni, who co-reviewed with Y. Drexler; M. Levi; and Y.-Y. Zhao for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
LIPID MAPS database: https://www.lipidmaps.org/
Glossary
- Stereospecific number
-
(sn). Nomenclature that specifies the location of glycerol derivative side chains.
- Liquid chromatography–mass spectrometry
-
(LC–MS). A metabolomics and lipidomics technique that combines liquid chromatography separation with a mass spectrometry detection method.
- Features
-
Signals identified by the metabolomics study (e.g. in peaks in a LC–MS study defined by LC retention time and MS signal intensity).
- Isotopologues
-
Compounds that differ from each other only by a difference in the number of neutrons.
- Primary metabolic pathways
-
Metabolic pathways that generate products crucial to the biological function of the cell.
- Secondary metabolic pathways
-
Metabolic pathways that generate products that are not necessarily crucial to biological function but serve to supplement normal function (e.g. in the context of disease).
- Acylcarnitine
-
A conjugate of acyl-CoA and carnitine that can be exported between cellular membranes.
- Principal component analysis
-
A data dimensionality reduction method in which the number of variables is reduced by selecting the most significant variables.
- Cardiolipins
-
Phospholipids specific to the mitochondria that serve numerous functions including the regulation and structural organization of the electron transport chain.
- Pearson correlation coefficients
-
Measurements of the linear relationship between two continuous variables.
- Neutral lipids
-
Lipids that lack charged groups, such as triacylglycerols.
- Non-esterified fatty acids
-
Also known as free fatty acids. Fatty acids that are not conjugated to glycerol backbone.
- Kennedy pathway
-
An oestrogen receptor-based de novo phosphatidylcholine and phosphatidylethanolamine synthesis pathway.
Rights and permissions
About this article
Cite this article
Baek, J., He, C., Afshinnia, F. et al. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol 18, 38–55 (2022). https://doi.org/10.1038/s41581-021-00488-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00488-2
This article is cited by
-
Comparative lipidome study of maternal plasma, milk, and lamb plasma in sheep
Scientific Reports (2024)
-
A lipidome landscape of aging in mice
Nature Aging (2024)
-
Multi-molecular hyperspectral PRM-SRS microscopy
Nature Communications (2024)
-
The current use of proteomics and metabolomics in glomerulonephritis: a systematic literature review
Journal of Nephrology (2024)
-
Association between the triglyceride glucose index and in-hospital and 1-year mortality in patients with chronic kidney disease and coronary artery disease in the intensive care unit
Cardiovascular Diabetology (2023)