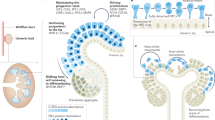
Abstract
Although the genetic basis of many kidney diseases is being rapidly elucidated, their experimental study remains problematic owing to the lack of suitable models. The fruitfly Drosophila melanogaster provides a rapid, ethical and cost-effective model system of the kidney. The unique advantages of D. melanogaster include ease and low cost of maintenance, comprehensive availability of genetic mutants and powerful transgenic technologies, and less onerous regulation, as compared with mammalian systems. Renal and excretory functions in D. melanogaster reside in three main tissues — the transporting renal (Malpighian) tubules, the reabsorptive hindgut and the endocytic nephrocytes. Tubules contain multiple cell types and regions and generate a primary urine by transcellular transport rather than filtration, which is then subjected to selective reabsorption in the hindgut. By contrast, the nephrocytes are specialized for uptake of macromolecules and equipped with a filtering slit diaphragm resembling that of podocytes. Many genes with key roles in the human kidney have D. melanogaster orthologues that are enriched and functionally relevant in fly renal tissues. This similarity has allowed investigations of epithelial transport, kidney stone formation and podocyte and proximal tubule function. Furthermore, a range of unique quantitative phenotypes are available to measure function in both wild type and disease-modelling flies.
Key points
-
The fruitfly Drosophila melanogaster has proved to be a valuable experimental model for the study of human biology and has major advantages, including low costs and a broad variety of established genetic tools and interventions.
-
The fly renal tubule — Malpighian tubule — secretes fluid faster (on a per-cell volume basis) than any other tissue, and is ideal for investigating secretory or transport phenotypes, such as nephrolithiasis.
-
Nephrocytes express many proteins that are also found in the slit diaphragm of podocytes and perform a specialized role by sequestering and breaking down filtered molecules such as proteins; the ability of nephrocytes to reabsorb filtered molecules is analogous to the reabsorptive capacity of the proximal tubule.
-
A wide range of models of kidney diseases have been developed in D. melanogaster.
-
D. melanogaster uniquely represents an organism with a recognizable renal system that is compatible with large-scale chemical or genetic screens.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
10 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41581-023-00788-9
References
Wigglesworth, V. B. The Principles of Insect Physiology. 7th edn (Chapman & Hall, 1972).
Simpson, S. J. & Douglas, A. E. (eds). The Insects: Structure and Function (Cambridge Univ, Press, 2013).
Berridge, M. J. A structural analysis of intestinal absorption. Symp. Rent. Soc. Lond. 5, 135–150 (1970).
Maddrell, S. H. P. The functional design of the insect excretory system. J. Exp. Biol. 90, 1–15 (1981).
Denholm, B. & Skaer, H. Bringing together components of the fly renal system. Curr. Opin. Genet. Dev. 19, 526–532 (2009).
Cohen, E., Sawyer, J. K., Peterson, N. G., Dow, J. A. T. & Fox, D. T. Physiology, development, and disease modeling in the Drosophila excretory system. Genetics 214, 235–264 (2020).
Adams, M. D. et al. The genome sequence of Drosophila melanogaster. Science 287, 2185–2195 (2000).
Pfeiffer, B. D. et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl Acad. Sci. USA 105, 9715–9720 (2008).
Pfeiffer, B. D. et al. Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755 (2010).
Gautam, N. K., Verma, P. & Tapadia, M. G. Drosophila Malpighian tubules: a model for understanding kidney development, function, and disease. Results Probl. Cell Differ. 60, 3–25 (2017).
Ugur, B., Chen, K. & Bellen, H. J. Drosophila tools and assays for the study of human diseases. Dis. Model. Mech. 9, 235–244 (2016).
Dow, J. A. T. & Romero, M. F. Drosophila provides rapid modeling of renal development, function, and disease. Am. J. Physiol. Renal Physiol. 299, F1237–F1244 (2010).
Bertoloni Meli, D. Mechanism, Experiment, Disease: Marcello Malpighi and Seventeenth-Century Anatomy. (Johns Hopkins University Press, 2011).
Sözen, M. A., Armstrong, J. D., Yang, M. Y., Kaiser, K. & Dow, J. A. T. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl Acad. Sci. USA 94, 5207–5212 (1997).
Beyenbach, K. W., Skaer, H. & Dow, J. A. T. The developmental, molecular, and transport biology of Malpighian tubules. Annu. Rev. Entomol. 55, 351–374 (2010).
O’Donnell, M. J. & Maddrell, S. H. P. Fluid reabsorption and ion transport by the lower Malpighian tubules of adult female Drosophila. J. Exp. Biol. 198, 1647–1653 (1995).
Maddrell, S. H. P. The fastest fluid-secreting cell known: the upper Malpighian tubule cell of Rhodnius. BioEssays 13, 357–362 (1991).
Hatton-Ellis, E. et al. Genetic regulation of patterned tubular branching in Drosophila. Proc. Natl Acad. Sci. USA 104, 169–174 (2007).
Skaer, H. Cell division in Malpighian tubule development in D. melanogaster is regulated by a single tip cell. Nature 342, 566–569 (1989).
Sudarsan, V., Pasalodos-Sanchez, S., Wan, S., Gampel, A. & Skaer, H. A genetic hierarchy establishes mitogenic signalling and mitotic competence in the renal tubules of Drosophila. Development 129, 935–944 (2002).
Campbell, K., Knust, E. & Skaer, H. Crumbs stabilises epithelial polarity during tissue remodelling. J. Cell Sci. 122, 2604–2612 (2009).
Grawe, F., Wodarz, A., Lee, B., Knust, E. & Skaer, H. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development 122, 951–959 (1996).
Denholm, B. et al. Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr. Biol. 13, 1052–1057 (2003).
Davies, J. A. Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat. 156, 187–201 (1996).
Slavotinek, A. M. The family of crumbs genes and human disease. Mol. Syndromol. 7, 274–281 (2016).
Attanasio, M. et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat. Genet. 39, 1018–1024 (2007).
Caubit, X. et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development 135, 3301–3310 (2008).
Dworak, H. A., Charles, M. A., Pellerano, L. B. & Sink, H. Characterization of Drosophila hibris, a gene related to human nephrin. Development 128, 4265–4276 (2001).
Day, J. P. et al. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J. Cell Sci. 121, 2612–2619 (2008).
Chintapalli, V. R. et al. Transport proteins NHA1 and NHA2 are essential for survival, but have distinct transport modalities. Proc. Natl Acad. Sci. USA 112, 11720–11725 (2015).
Cabrero, P. et al. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc. Natl Acad. Sci. USA 111, 14301–14306 (2014).
Cabrero, P. et al. Specialized stellate cells offer a privileged route for rapid water flux in Drosophila renal tubule. Proc. Natl Acad. Sci. USA 117, 1779–1787 (2020).
Brown, D., Hirsch, S. & Gluck, S. Localization of a proton-pumping ATPase in rat kidney. J. Clin. Invest. 82, 2114–2126 (1988).
Brown, D., Lui, B., Gluck, S. & Sabolic, I. A plasma membrane proton ATPase in specialized cells of rat epididymis. Am. J. Physiol. 263, C913–C916 (1992).
Chambrey, R. et al. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc. Natl Acad. Sci. USA 110, 7928–7933 (2013).
Dow, J. A. T. et al. The Malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J. Exp. Biol. 197, 421–428 (1994).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Traven, A., Jelicic, B. & Sopta, M. Yeast Gal4: a transcriptional paradigm revisited. EMBO Rep. 7, 496–499 (2006).
Duffy, J. B. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis 34, 1–15 (2002).
Rosay, P. et al. Cell-type specific calcium signalling in a Drosophila epithelium. J. Cell Sci. 110, 1683–1692 (1997).
Rossano, A. J., Kato, A., Minard, K. I., Romero, M. F. & Macleod, G. T. Na+/H+ exchange via the Drosophila vesicular glutamate transporter mediates activity-induced acid efflux from presynaptic terminals. J. Physiol. 595, 805–824 (2017).
Rossano, A. J. & Romero, M. F. Optical quantification of intracellular pH in Drosophila melanogaster Malpighian tubule epithelia with a fluorescent genetically-encoded pH indicator. J. Vis. Exp. https://doi.org/10.3791/55698 (2017).
Dionne, H., Hibbard, K. L., Cavallaro, A., Kao, J. C. & Rubin, G. M. Genetic reagents for making split-GAL4 lines in Drosophila. Genetics 209, 31–35 (2018).
Dow, J. A. T. Drosophila as an experimental organism for functional genomics. eLS https://doi.org/10.1002/9780470015902.a0000561 (2012).
Leung, B. & Waddell, S. Four-dimensional gene expression control: memories on the fly. Trends Neurosci. 27, 511–513 (2004).
Robles-Murguia, M., Hunt, L. C., Finkelstein, D., Fan, Y. & Demontis, F. Tissue-specific alteration of gene expression and function by RU486 and the GeneSwitch system. NPJ Aging Mech. Dis. 5, 6 (2019).
Bassett, A. R., Tibbit, C., Ponting, C. P. & Liu, J. L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4, 220–228 (2013).
Gratz, S. J. et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194, 1029–1035 (2013).
Gratz, S. J., Harrison, M. M., Wildonger, J. & O’Connor-Giles, K. M. Precise genome editing of Drosophila with CRISPR RNA-guided Cas9. Methods Mol. Biol. 1311, 335–348 (2015).
Xu, J. et al. A toolkit of CRISPR-based genome editing systems in Drosophila. J. Genet. Genomics 42, 141–149 (2015).
Chintapalli, V. R., Wang, J. & Dow, J. A. T. Using FlyAtlas to identify better Drosophila models of human disease. Nat. Genet. 39, 715–720 (2007).
Hildebrandt, F. Genetic kidney diseases. Lancet 375, 1287–1295 (2010).
Laing, C. M. & Unwin, R. J. Renal tubular acidosis. J. Nephrol. 19(Suppl 9), S46–S52 (2006).
Mazhab-Jafari, M. T. et al. Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 539, 118–122 (2016).
Gogarten, J. P. et al. Evolution of the vacuolar H+-ATPase — implications for the origin of eukaryotes. PNAS 86, 6661–6665 (1989).
Blair, H. C., Teitelbaum, S. L., Ghiselli, R. & Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245, 855–857 (1989).
Alper, S. L., Brown, D., Lodish, H. F., Natale, J. & Gluck, S. Subtypes of intercalated cells in rat-kidney collecting duct defined by antibodies against erythroid band-3 and renal vacuolar H+-ATPase. Proc. Natl Acad. Sci. USA 86, 5429–5433 (1989).
DeFranco, P. E., Haragsim, L., Schmitz, P. G., Bastani, B. & Li, J. P. Absence of vacuolar H+-ATPase pump in the collecting duct of a patient with hypokalemic distal renal tubular-acidosis and Sjögrens syndrome. J. Am. Soc. Nephrol. 6, 295–301 (1995).
Karet, F. E. et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat. Genet. 21, 84–90 (1999).
Smith, A. N. et al. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat. Genet. 26, 71–75 (2000).
Stover, E. H. et al. Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J. Med. Genet. 39, 796–803 (2002).
Allan, A. K., Du, J., Davies, S. A. & Dow, J. A. T. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol. Genomics 22, 128–138 (2005).
Wang, J. et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 5, R69 (2004).
Davies, S. A. et al. Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J. Biol. Chem. 271, 30677–30684 (1996).
Ares, G. R., Caceres, P. S. & Ortiz, P. A. Molecular regulation of NKCC2 in the thick ascending limb. Am. J. Physiol. Renal Physiol. 301, F1143–F1159 (2011).
Shekarabi, M. et al. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 25, 285–299 (2017).
Wilson, F. H. et al. Human hypertension caused by mutations in WNK kinases. Science 293, 1107–1112 (2001).
Choate, K. A., Kahle, K. T., Wilson, F. H., Nelson-Williams, C. & Lifton, R. P. WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl−-transporting epithelia. Proc. Natl Acad. Sci. USA 100, 663–668 (2003).
San-Cristobal, P. et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc. Natl Acad. Sci. USA 106, 4384–4389 (2009).
Welling, P. A., Chang, Y. P., Delpire, E. & Wade, J. B. Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int. 77, 1063–1069 (2010).
Grimm, P. R., Coleman, R., Delpire, E. & Welling, P. A. Constitutively Active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J. Am. Soc. Nephrol. 28, 2597–2606 (2017).
Grimm, P. R. et al. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J. Biol. Chem. 287, 37673–37690 (2012).
Lin, S. H. et al. Impaired phosphorylation of Na+-K+-2Cl− cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc. Natl Acad. Sci. USA 108, 17538–17543 (2011).
Rodan, A. R. WNK-SPAK/OSR1 signaling: lessons learned from an insect renal epithelium. Am. J. Physiol. Renal Physiol. 315, F903–F907 (2018).
Rodan, A. R. & Jenny, A. WNK kinases in development and disease. Curr. Top. Dev. Biol. 123, 1–47 (2017).
Wu, Y., Schellinger, J. N., Huang, C. L. & Rodan, A. R. Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the Ncc69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J. Biol. Chem. 289, 26131–26142 (2014).
Wu, Y., Baum, M., Huang, C. L. & Rodan, A. R. Two inwardly rectifying potassium channels, Irk1 and Irk2, play redundant roles in Drosophila renal tubule function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R747–R756 (2015).
Ramello, A., Vitale, C. & Marangella, M. Epidemiology of nephrolithiasis. J. Nephrol. 13 (Suppl 3), S45–S50 (2000).
Ziemba, J. B. & Matlaga, B. R. Epidemiology and economics of nephrolithiasis. Investig. Clin. Urol. 58, 299–306 (2017).
Shoag, J., Tasian, G. E., Goldfarb, D. S. & Eisner, B. H. The new epidemiology of nephrolithiasis. Adv. Chronic Kidney Dis. 22, 273–278 (2015).
Southall, T. D. et al. Novel subcellular locations and functions for secretory pathway Ca2+/Mn2+-ATPases. Physiol. Genomics 26, 35–45 (2006).
Knauf, F. & Preisig, P. A. Drosophila: a fruitful model for calcium oxalate nephrolithiasis? Kidney Int. 80, 327–329 (2011).
Amato, M., Lusini, M. L. & Nelli, F. Epidemiology of nephrolithiasis today. Urol. Int. 72 (Suppl 1), 1–5 (2004).
Hirata, T. et al. In vivo Drosophila genetic model for calcium oxalate nephrolithiasis. Am. J. Physiol. Renal Physiol. 303, F1555–F1562 (2012).
Chen, W. C. et al. Toward a new insight of calcium oxalate stones in Drosophila by micro-computerized tomography. Urolithiasis https://doi.org/10.1007/s00240-017-0967-0 (2017).
Khan, S. R., Glenton, P. A. & Byer, K. J. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-l-proline. Kidney Int. 70, 914–923 (2006).
Chen, Y. H. et al. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney Int. 80, 369–377 (2011).
Yang, H. et al. Efficacy of Hydroxy-l-proline (HYP) analogs in the treatment of primary hyperoxaluria in Drosophila melanogaster. BMC Nephrol. 19, 167 (2018).
Chi, T. et al. A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS One 10, e0124150 (2015).
Evan, A. P. et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J. Clin. Invest. 111, 607–616 (2003).
Alford, A., Furrow, E., Borofsky, M. & Lulich, J. Animal models of naturally occurring stone disease. Nat. Rev. Urol. 17, 691–705 (2020).
Richards, C. D. & Burke, R. A fly’s eye view of zinc homeostasis: novel insights into the genetic control of zinc metabolism from Drosophila. Arch. Biochem. Biophys. 611, 142–149 (2016).
Yin, S., Qin, Q. & Zhou, B. Functional studies of Drosophila zinc transporters reveal the mechanism for zinc excretion in Malpighian tubules. BMC Biol. 15, 12 (2017).
Landry, G. M. et al. Cloning, function, and localization of human, canine and Drosophila ZIP10 (SLC39A10), a Zn2+ transporter. Am. J. Physiol. Renal Physiol. 316, F263–F273 (2018).
Dow, J. A. T., Cabrero, P., Hirata, T., Chang, M. & Romero, M. F. A simple model for nephrolithiasis: Drosophila Prestin (Slc26a5) functions as a Cl−/oxalate exchanger in the gut with kinetics similar to mammalian Slc26a6. Proc. Physiol. Soc. 16, C15 (2009).
Hirata, T. et al. Ion and solute transport by Prestin in Drosophila and Anopheles. J. Insect Physiol. 58, 563–569 (2012).
Anderson, J. B., Hirata, T., Rossano, A. J., Landry, G. M. & Romero, M. F. Drosophila model indicates role of OSR1/SPAK signaling in calcium oxalate kidney stone formation via regulation of Slc26a6 mediated Cl/oxalate2 exchange. J. Am. Soc. Nephrol. 27, 465A (2016).
Landry, G. M. et al. Sulfate and thiosulfate inhibit oxalate transport via a dPrestin (Slc26a6)-dependent mechanism in an insect model of calcium oxalate nephrolithiasis. Am. J. Physiol. Renal Physiol. 310, F152–F159 (2016).
Chung, V. Y. et al. Proteomic changes in response to crystal formation in Drosophila Malpighian tubules. Fly 10, 91–100 (2016).
Chung, V. Y. & Turney, B. W. A Drosophila genetic model of nephrolithiasis: transcriptional changes in response to diet induced stone formation. BMC Urol. 17, 109 (2017).
Kanlaya, R., Singhto, N. & Thongboonkerd, V. EGCG decreases binding of calcium oxalate monohydrate crystals onto renal tubular cells via decreased surface expression of alpha-enolase. J. Biol. Inorg. Chem. 21, 339–346 (2016).
Abd El-Salam, M. et al. The synthesized plant metabolite 3,4,5-Tri-O-galloylquinic acid methyl ester inhibits calcium oxalate crystal growth in a Drosophila model, downregulates renal cell surface annexin A1 expression, and decreases crystal adhesion to cells. J. Med. Chem. 61, 1609–1621 (2018).
Ali, S. N. et al. Drosophila melanogaster as a function-based high-throughput screening model for anti-nephrolithiasis agents in kidney stone patients. Dis. Model. Mech. https://doi.org/10.1242/dmm.035873 (2018).
Tadmouri, G. O. et al. Consanguinity and reproductive health among Arabs. Reprod. Health 6, 17 (2009).
Kamleh, M. A., Hobani, Y., Dow, J. A. T. & Watson, D. G. Metabolomic profiling of Drosophila using liquid chromatography Fourier transform mass spectrometry. FEBS Lett. 582, 2916–2922 (2008).
Mitchell, H. K. & Glassman, E. Hypoxanthine in rosy and maroon-like mutants of Drosophila melanogaster. Science 129, 268 (1959).
Kamleh, M. A., Hobani, Y., Dow, J. A. T., Zheng, L. & Watson, D. G. Towards a platform for the metabonomic profiling of different strains of Drosophila melanogaster using liquid chromatography Fourier transform mass spectrometry. FEBS Lett. 276, 6798–6809 (2009).
Al Bratty, M., Hobani, Y., Dow, J. A. T. & Watson, D. G. Metabolomic profiling of the effects of allopurinol on Drosophila melanogaster. Metabolomics https://doi.org/10.1007/s11306-011-0275-6 (2011).
Harris, P. C. & Torres, V. E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Invest. 124, 2315–2324 (2014).
Hughes, J. et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151–160 (1995).
No Authors Listed. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium. Cell 81, 289–298 (1995).
Kim, D. Y. & Park, J. H. Genetic Mechanisms of ADPKD. Adv. Exp. Med. Biol. 933, 13–22 (2016).
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 13, 397–406 (2014).
Xiao, Z. S. & Quarles, L. D. Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann. N. Y. Acad. Sci. 1192, 410–421 (2010).
Douguet, D., Patel, A. & Honore, E. Structure and function of polycystins: insights into polycystic kidney disease. Nat. Rev. Nephrol. 15, 412–422 (2019).
Dalagiorgou, G., Basdra, E. K. & Papavassiliou, A. G. Polycystin-1: function as a mechanosensor. Int. J. Biochem. Cell Biol. 42, 1610–1613 (2010).
Luyten, A. et al. Aberrant regulation of planar cell polarity in polycystic kidney disease. J. Am. Soc. Nephrol. 21, 1521–1532 (2010).
McNeill, H. Planar cell polarity and the kidney. J. Am. Soc. Nephrol. 20, 2104–2111 (2009).
Chebib, F. T., Sussman, C. R., Wang, X., Harris, P. C. & Torres, V. E. Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat. Rev. Nephrol. 11, 451–464 (2015).
Torres, V. E. et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 377, 1930–1942 (2017).
Watnick, T. J., Jin, Y., Matunis, E., Kernan, M. J. & Montell, C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr. Biol. 13, 2179–2184 (2003).
Hofherr, A. et al. The mitochondrial transporter SLC25A25 links ciliary TRPP2 signaling and cellular metabolism. PLoS Biol. 16, e2005651 (2018).
Kraus, M. R. et al. Two mutations in human BICC1 resulting in Wnt pathway hyperactivity associated with cystic renal dysplasia. Hum. Mutat. 33, 86–90 (2012).
Bouvrette, D. J., Price, S. J. & Bryda, E. C. K homology domains of the mouse polycystic kidney disease-related protein, Bicaudal-C (Bicc1), mediate RNA binding in vitro. Nephron Exp. Nephrol. 108, e27–e34 (2008).
Bouvrette, D. J., Sittaramane, V., Heidel, J. R., Chandrasekhar, A. & Bryda, E. C. Knockdown of bicaudal C in zebrafish (Danio rerio) causes cystic kidneys: a nonmammalian model of polycystic kidney disease. Comp. Med. 60, 96–106 (2010).
Cogswell, C. et al. Positional cloning of jcpk/bpk locus of the mouse. Mamm. Genome 14, 242–249 (2003).
Tran, U. et al. The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 137, 1107–1116 (2010).
Gamberi, C., Hipfner, D. R., Trudel, M. & Lubell, W. D. Bicaudal C mutation causes myc and TOR pathway up-regulation and polycystic kidney disease-like phenotypes in Drosophila. PLoS Genet. 13, e1006694 (2017).
Knier, C. G., Landry, G. M. & Romero, M. F. Bicaudal-C in Drosophila as a model of polycystic kidney disease (PKD) and intersection of oxalate nephrolithiasis. FASEB J. 30(Suppl. 1), 1224.1227–1224.1227 (2016).
Tietz Bogert, P. S. et al. The zebrafish as a model to study polycystic liver disease. Zebrafish 10, 211–217 (2013).
Abu-Wasel, B., Walsh, C., Keough, V. & Molinari, M. Pathophysiology, epidemiology, classification and treatment options for polycystic liver diseases. World J. Gastroenterol. 19, 5775–5786 (2013).
Gallagher, A. R. & Somlo, S. Loss of cilia does not slow liver disease progression in mouse models of autosomal recessive polycystic kidney disease. Kidney360 1, 962–968 (2020).
Kramer-Zucker, A. G. et al. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 132, 1907–1921 (2005).
Obara, T. et al. Polycystin-2 immunolocalization and function in zebrafish. J. Am. Soc. Nephrol. 17, 2706–2718 (2006).
Mangos, S. et al. The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis. Model. Mech. 3, 354–365 (2010).
Zallen, J. A. & Wieschaus, E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343–355 (2004).
Saxena, A. et al. Epidermal growth factor signalling controls myosin II planar polarity to orchestrate convergent extension movements during Drosophila tubulogenesis. PLoS Biol. 12, e1002013 (2014).
Johnson, K., Knust, E. & Skaer, H. bloated tubules (blot) encodes a Drosophila member of the neurotransmitter transporter family required for organisation of the apical cytocortex. Dev. Biol. 212, 440–454 (1999).
Hadorn, E. & Schwinck, I. A mutant of Drosophila without isoxanthopterine which is non-autonomous for the red eye pigments. Nature 177, 940–941 (1956).
Beyenbach, K. W. et al. The septate junction protein Tetraspanin 2A is critical to the structure and function of Malpighian tubules in Drosophila melanogaster. Am. J. Physiol. Cell Physiol. 318, C1107–C1122 (2020).
Jonusaite, S. et al. The septate junction protein Mesh is required for epithelial morphogenesis, ion transport, and paracellular permeability in the Drosophila Malpighian tubule. Am. J. Physiol. Cell Physiol. 318, C675–C694 (2020).
Dornan, A. J., Halberg, K. A., Beuter, L.-K., Davies, S. A. & Dow, J. A. T. The septate junction protein Snakeskin is critical for epithelial barrier function and tissue homeostasis in the Malpighian tubules of adult Drosophila. Preprint at bioRxiv https://doi.org/10.1101/2020.12.14.422678 (2020).
Noble-Nesbitt, J. in Comparative Physiology (ed Schmidt-Nielsen, K. Maddrell, S. H. P. and Bolis, L.) 333–351 (Elsevier, 1973).
Hamaguchi, T. et al. Dorsoventral patterning of the Drosophila hindgut is determined by interaction of genes under the control of two independent gene regulatory systems, the dorsal and terminal systems. Mech. Dev. 129, 236–243 (2012).
Kumichel, A. & Knust, E. Apical localisation of crumbs in the boundary cells of the Drosophila hindgut is independent of its canonical interaction partner stardust. PLoS ONE 9, e94038 (2014).
Lengyel, J. A. & Iwaki, D. D. It takes guts: the Drosophila hindgut as a model system for organogenesis. Dev. Biol. 243, 1–19 (2002).
Murakami, R. & Shiotsuki, Y. Ultrastructure of the hindgut of Drosophila larvae, with special reference to the domains identified by specific gene expression patterns. J. Morphol. 248, 144–150 (2001).
Fox, D. T. & Spradling, A. C. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell 5, 290–297 (2009).
Takashima, S., Mkrtchyan, M., Younossi-Hartenstein, A., Merriam, J. R. & Hartenstein, V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454, 651–655 (2008).
Xie, T. Stem cell in the adult Drosophila hindgut: just a sleeping beauty. Cell Stem Cell 5, 227–228 (2009).
Luan, Z., Quigley, C. & Li, H. S. The putative Na+/Cl-dependent neurotransmitter/osmolyte transporter inebriated in the Drosophila hindgut is essential for the maintenance of systemic water homeostasis. Sci. Rep. 5, 7993 (2015).
Vanderveken, M. & O’Donnell, M. J. Effects of diuretic hormone 31, drosokinin, and allatostatin A on transepithelial K+ transport and contraction frequency in the midgut and hindgut of larval Drosophila melanogaster. Arch. Insect Biochem. Physiol. 85, 76–93 (2014).
Chang, H. C. et al. Hsc70 is required for endocytosis and clathrin function in Drosophila. J. Cell Biol. 159, 477–487 (2002).
Weavers, H. et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457, 322–326 (2009).
Zhuang, S. et al. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136, 2335–2344 (2009).
Crossley, A. C. The ultrastructure and function of pericardial cells and other nephrocytes in an insect: Calliphora erythrocephala. Tissue Cell 4, 529–560 (1972).
Helmstadter, M. & Simons, M. Using Drosophila nephrocytes in genetic kidney disease. Cell Tissue Res. 369, 119–126 (2017).
Sorensen, K. K. et al. The scavenger endothelial cell: a new player in homeostasis and immunity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R1217–R1230 (2012).
Helmstadter, M. et al. Functional study of mammalian Neph proteins in Drosophila melanogaster. PLoS One 7, e40300 (2012).
Hermle, T., Braun, D. A., Helmstadter, M., Huber, T. B. & Hildebrandt, F. Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J. Am. Soc. Nephrol. 28, 1521–1533 (2017).
Zhang, F., Zhao, Y., Chao, Y., Muir, K. & Han, Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J. Am. Soc. Nephrol. 24, 209–216 (2013).
Gleixner, E. M. et al. V-ATPase/mTOR signaling regulates Megalin-mediated apical endocytosis. Cell Rep. 8, 10–19 (2014).
Brankatschk, M., Dunst, S., Nemetschke, L. & Eaton, S. Delivery of circulating lipoproteins to specific neurons in the Drosophila brain regulates systemic insulin signaling. eLife https://doi.org/10.7554/eLife.02862 (2014).
Zheng, W., Ocorr, K. & Tatar, M. Extracellular matrix induced by steroids and aging through a G-protein-coupled receptor in a Drosophila model of renal fibrosis. Dis. Model Mech. https://doi.org/10.1242/dmm.041301 (2020).
Troha, K. et al. Nephrocytes remove microbiota-derived peptidoglycan from systemic circulation to maintain immune homeostasis. Immunity 51, 625–637 e623 (2019).
Soukup, S. F., Culi, J. & Gubb, D. Uptake of the necrotic serpin in Drosophila melanogaster via the lipophorin receptor-1. PLoS Genet. 5, e1000532 (2009).
Ivy, J. R. et al. Klf15 Is Critical for the development and differentiation of Drosophila nephrocytes. PLoS One 10, e0134620 (2015).
Mallipattu, S. K. et al. Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J. Biol. Chem. 287, 19122–19135 (2012).
Hurcombe, J. A. et al. Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function. Nat. Commun. 10, 403 (2019).
Carrasco-Rando, M., Prieto-Sanchez, S., Culi, J., Tutor, A. S. & Ruiz-Gomez, M. A specific isoform of Pyd/ZO-1 mediates junctional remodeling and formation of slit diaphragms. J. Cell Biol. 218, 2294–2308 (2019).
Schnabel, E., Anderson, J. M. & Farquhar, M. G. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J. Cell Biol. 111, 1255–1263 (1990).
Kriz, W., Shirato, I., Nagata, M., LeHir, M. & Lemley, K. V. The podocyte’s response to stress: the enigma of foot process effacement. Am. J. Physiol. Renal Physiol. 304, F333–F347 (2013).
Kurihara, H., Anderson, J. M. & Farquhar, M. G. Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am. J. Physiol. 268, F514–F524 (1995).
Kim, S., Kalappurakkal, J. M., Mayor, S. & Rosen, M. K. Phosphorylation of nephrin induces phase separated domains that move through actomyosin contraction. Mol. Biol. Cell 30, 2996–3012 (2019).
Beutel, O., Maraspini, R., Pombo-Garcia, K., Martin-Lemaitre, C. & Honigmann, A. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell 179, 923–936 e911 (2019).
Takahisa, M. et al. The Drosophila tamou gene, a component of the activating pathway of extramacrochaetae expression, encodes a protein homologous to mammalian cell-cell junction-associated protein ZO-1. Genes Dev. 10, 1783–1795 (1996).
Na, J., Sweetwyne, M. T., Park, A. S., Susztak, K. & Cagan, R. L. Diet-induced podocyte dysfunction in Drosophila and mammals. Cell Rep. 12, 636–647 (2015).
Hartley, P. S., Motamedchaboki, K., Bodmer, R. & Ocorr, K. SPARC-dependent cardiomyopathy in Drosophila. Circ. Cardiovasc. Genet. 9, 119–129 (2016).
Zhang, F., Zhao, Y. & Han, Z. An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J. Am. Soc. Nephrol. 24, 191–197 (2013).
Kopp, J. B. et al. Podocytopathies. Nat. Rev. Dis. Prim. 6, 68 (2020).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Fu, Y. et al. A Drosophila model system to assess the function of human monogenic podocyte mutations that cause nephrotic syndrome. Hum. Mol. Genet. 26, 768–780 (2017).
Gee, H. Y. et al. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J. Clin. Invest. 125, 2375–2384 (2015).
Gee, H. Y. et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J. Clin. Invest. 123, 3243–3253 (2013).
Ashraf, S. et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Invest. 123, 5179–5189 (2013).
Kampf, L. L. et al. TBC1D8B mutations implicate RAB11-dependent vesicular trafficking in the pathogenesis of nephrotic syndrome. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2019040414 (2019).
Hermle, T. et al. GAPVD1 and ANKFY1 mutations implicate RAB5 regulation in nephrotic syndrome. J. Am. Soc. Nephrol. 29, 2123–2138 (2018).
Lovric, S. et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J. Clin. Invest. 127, 912–928 (2017).
Goncalves, S. et al. A homozygous KAT2B variant modulates the clinical phenotype of ADD3 deficiency in humans and flies. PLoS Genet. 14, e1007386 (2018).
Teumer, A. et al. Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat. Commun. 10, 4130 (2019).
Marchesin, V. et al. Molecular basis for autosomal-dominant renal Fanconi syndrome caused by HNF4A. Cell Rep. 29, 4407–4421 e4405 (2019).
Fu, Y. et al. APOL1-G1 in nephrocytes induces hypertrophy and accelerates cell death. J. Am. Soc. Nephrol. 28, 1106–1116 (2017).
Kruzel-Davila, E. et al. APOL1-mediated cell injury involves disruption of conserved trafficking processes. J. Am. Soc. Nephrol. 28, 1117–1130 (2017).
Friedman, D. J. & Pollak, M. R. APOL1 and kidney disease: from genetics to biology. Annu. Rev. Physiol. 82, 323–342 (2020).
Rujano, M. A. et al. Mutations in the X-linked ATP6AP2 cause a glycosylation disorder with autophagic defects. J. Exp. Med. 214, 3707–3729 (2017).
Lee, P. T. et al. A gene-specific T2A-GAL4 library for Drosophila. eLife https://doi.org/10.7554/eLife.35574 (2018).
Yang, J. et al. A Drosophila systems approach to xenobiotic metabolism. Physiol. Genomics 30, 223–231 (2007).
McGettigan, J. et al. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem. Mol. Biol. 35, 741–754 (2005).
Denholm, B. et al. The tiptop/teashirt genes regulate cell differentiation and renal physiology in Drosophila. Development 140, 1100–1110 (2013).
Beyenbach, K. W. Kidneys sans glomeruli. Am. J. Physiol. Renal Physiol. 286, F811–F827 (2004).
Kolb, A. R., Buck, T. M. & Brodsky, J. L. Saccharomyces cerivisiae as a model system for kidney disease: what can yeast tell us about renal function? Am. J. Physiol. Renal Physiol. 301, F1–F11 (2011).
Ganner, A. & Neumann-Haefelin, E. Genetic kidney diseases: Caenorhabditis elegans as model system. Cell Tissue Res. 369, 105–118 (2017).
Rodan, A. R. The Drosophila Malpighian tubule as a model for mammalian tubule function. Curr. Opin. Nephrol. Hypertens. 28, 455–464 (2019).
Schenk, H., Muller-Deile, J., Kinast, M. & Schiffer, M. Disease modeling in genetic kidney diseases: zebrafish. Cell Tissue Res. 369, 127–141 (2017).
Elmonem, M. A. et al. Genetic renal diseases: the emerging role of zebrafish models. Cells https://doi.org/10.3390/cells7090130 (2018).
Hofmeister, A. F., Komhoff, M., Weber, S. & Grgic, I. Disease modeling in genetic kidney diseases: mice. Cell Tissue Res. 369, 159–170 (2017).
Ruiz-Gómez, M., Coutts, N., Price, A., Taylor, M. V. & Bate, M. Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell 102, 189–198 (2000).
Kestila, M. et al. Positionally cloned gene for a novel glomerular protein — nephrin — is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Bour, B. A., Chakravarti, M., West, J. M., & Abmayr, S. M. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 14, 1498–1511 (2000).
Donoviel, D. B. et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol. Cell Biol. 21, 4829–4836 (2001).
Shih, N. Y. et al. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am. J. Pathol. 159, 2303–2308 (2001).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Xu, J. et al. A cell atlas of the fly kidney. Preprint at bioRxiv https://doi.org/10.1101/2021.09.03.458871 (2021).
Inoue, T. et al. FAT is a component of glomerular slit diaphragms. Kidney Int. 59, 1003–1012 (2001).
Conti, S., Perico, L., Grahammer, F. & Huber, T. B. The long journey through renal filtration: new pieces in the puzzle of slit diaphragm architecture. Curr. Opin. Nephrol. Hypertens. 26, 148–153 (2017).
Leader, D. P., Krause, S. A., Pandit, A., Davies, S. A. & Dow, J. A. T. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 46, D809–D815 (2017).
Chen, Z. X. et al. Comparative validation of the D. melanogaster modENCODE transcriptome annotation. Genome Res. 24, 1209–1223 (2014).
Chintapalli, V. R. et al. Functional correlates of positional and gender-specific renal asymmetry in Drosophila. PLoS One 7, e32577 (2012).
O’Donnell, M.J. et al. J. Exp. Biol. 199, 1163-75 (1996).
Terhzaz, S. et al. Mechanism and function of Drosophila capa GPCR: a desiccation stress-responsive receptor with functional homology to human neuromedinU receptor. PLoS One 7, e29897 (2012).
Wessing, A. & Eichelberg, D. in The Genetics and Biology of Drosophila Vol. 2c (eds Ashburner, A. & Wright, T. R. F.) 1–42 (Academic Press, 1978).
Acknowledgements
J.A.T.D. and M.F.R. were supported by grants from the US National Institutes of Health (NIH) R01-DK092408, U54-DK100227. M.F.R. was also supported by the Mayo Foundation, the Oxalosis & Hyperoxaluria Foundation (OHF) and NIH grants P30-DK090728 and R25-DK101405. J.A.T.D. was further supported by grants from the UK BBSRC, the European Commission Horizon 2020, and Marie Slodowska-Curie Actions. The authors thank Shireen Davies, Alan Jardine, Peter Harris, John Lieske, Steve Ekker, Nicholas LaRusso and Carli Sussman for critical reading of the manuscript before submission.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content, wrote the manuscript, and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks P. Hartley, A. Rodan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FlyAtlas database: http://www.flyatlas.org/
FlyBase database: https://flybase.org/
NCBI Homologene database: https://www.ncbi.nlm.nih.gov/homologene
Supplementary information
Glossary
- Specification
-
A stage in differentiation where a cell has sufficient information to differentiate in a particular way in a neutral environment; differs from the determination stage, at which a cell is committed to a specific fate.
- Eversion
-
The out-pushing of a group of cells during the early stages of tubular epithelium formation.
- Metanephric kidney
-
The fully differentiated mammalian kidney.
- S-shaped bodies
-
An intermediate stage in the development of the mammalian nephron, in which proximal, medial and distal segments can already be identified.
- Inward-rectifying K+ channels
-
K+-specific ion channels that display rectification (that is, K+ can enter the cell through the channels but cannot exit even under a favourable electrochemical gradient).
- Euteleostomi
-
Phylogenetic group that includes nearly all living vertebrates.
- Bilateria
-
A phylogenetic clade comprising animals with bilateral symmetry.
- Morpholinos
-
Synthetic antisense nucleotides that interfere with complementary mRNAs (act in a similar manner to antisense RNAs or siRNAs); widely used in zebrafish studies.
- Translocon
-
A complex of proteins that allow nascent peptides to cross a lipid bilayer, typically in the endoplasmic reticulum.
- Peripheral membrane protein
-
A membrane-associated protein that is tethered to, but that does not cross, a plasma membrane.
- Septate junction
-
Insect analogue of vertebrate tight junction with similarity in protein composition; these junctions appear as ladder-like septa in transmission electron microscopy.
- Phase separation
-
The creation of two distinct phases from a homogeneous mixture.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dow, J.A.T., Simons, M. & Romero, M.F. Drosophila melanogaster: a simple genetic model of kidney structure, function and disease. Nat Rev Nephrol 18, 417–434 (2022). https://doi.org/10.1038/s41581-022-00561-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00561-4