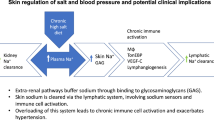
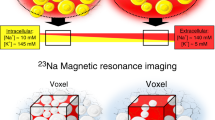
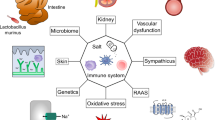
Abstract
Sodium intake is undoubtedly indispensable for normal body functions but can be detrimental when taken in excess of dietary requirements. The consequences of excessive salt intake are becoming increasingly clear as high salt consumption persists across the globe. Salt has long been suspected to promote the development of hypertension and cardiovascular diseases and is now also recognized as a potential modulator of inflammatory and autoimmune diseases through its direct and indirect effects on immune cells. The finding that, in addition to the kidneys, other organs such as the skin regulate sodium levels in the body prompted new hypotheses, including the concept that skin-resident macrophages might participate in tissue sodium regulation through their interactions with lymphatic vessels. Moreover, immune cells such as macrophages and different T cell subsets are found in sodium-rich interstitial microenvironments, where sodium levels modulate their function. Alterations to the intestinal bacterial community induced by excess dietary salt represent another relevant axis whereby salt indirectly modulates immune cell function. Depending on the inflammatory context, sodium might either contribute to protective immunity (for example, by enhancing host responses against cutaneous pathogens) or it might contribute to immune dysregulation and promote the development of cardiovascular and autoimmune diseases.
Key points
-
Salt (sodium chloride) is an important environmental factor that influences various immune cells at biological barriers, including the skin, intestine and kidney.
-
Innate and adaptive immune cells can sense hypertonic sodium in the interstitium, which subsequently affects their differentiation and/or function.
-
Salt-enhanced pro-inflammatory responses may be beneficial for the clearance of invasive pathogens but they might also promote hypertension and chronic immune-mediated diseases.
-
Salt also affects immune cells through its effects on the composition of the gut microbiome.
-
Clinical studies on the role of salt in immune-mediated diseases partly confirm experimental findings, but further investigation is needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brodin, P. et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015).
Brodin, P. & Davis, M. M. Human immune system variation. Nat. Rev. Immunol. 17, 21–29 (2017).
Bogdanos, D. P. et al. Twin studies in autoimmune disease: genetics, gender and environment. J. Autoimmun. 38, J156–J169 (2012).
Glaser, R. & Kiecolt-Glaser, J. K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 (2005).
Dong, T. S. & Gupta, A. Influence of early life, diet, and the environment on the microbiome. Clin. Gastroenterol. Hepatol. 17, 231–242 (2019).
Scheiermann, C., Kunisaki, Y. & Frenette, P. S. Circadian control of the immune system. Nat. Rev. Immunol. 13, 190–198 (2013).
Leone, V. et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17, 681–689 (2015).
Lambrecht, B. N. & Hammad, H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat. Immunol. 18, 1076–1083 (2017).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175 (2018).
Kloss, L., Meyer, J. D., Graeve, L. & Vetter, W. Sodium intake and its reduction by food reformulation in the European Union — a review. NFS. Journal 1, 9–19 (2015).
Anderson, C. A. M. et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J. Am. Diet. Assoc. 110, 736–745 (2010).
Ni Mhurchu, C. et al. Sodium content of processed foods in the United Kingdom: analysis of 44,000 foods purchased by 21,000 households1–3. Am. J. Clin. Nutr. 93, 594–600 (2011).
Yu, L. et al. Secular trends in salt and soy sauce intake among Chinese adults, 1997–2011. Int. J. Food Sci. Nutr. 69, 215–222 (2018).
Wiig, H., Luft, F. C. & Titze, J. M. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol. 222, e13006 (2018).
Kitada, K. et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J. Clin. Invest. 127, 1944–1959 (2017).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Kopp, C. et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61, 635–640 (2013).
Titze, J. et al. Osmotically inactive skin Na+ storage in rats. Am. J. Physiol. Renal Physiol. 285, F1108–F1117 (2003).
Titze, J. et al. Internal sodium balance in DOCA-salt rats: a body composition study. Am. J. Physiol. Renal Physiol. 289, F793–F802 (2005).
Titze, J. et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am. J. Physiol. Heart. Circ. Physiol. 287, H203–H208 (2004).
Wiig, H. & Swartz, M. A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 (2012).
Fischereder, M. et al. Sodium storage in human tissues is mediated by glycosaminoglycan expression. Am. J. Physiol. Renal Physiol. 313, F319–F325 (2017).
Schafflhuber, M. et al. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am. J. Physiol. Renal Physiol. 292, F1490–F1500 (2007).
Titze, J. et al. Reduced osmotically inactive Na storage capacity and hypertension in the Dahl mode. Am. J. Physiol. Renal Physiol. 283, F134–F141 (2002).
Titze, J. et al. Extrarenal Na+ balance, volume, and blood pressure homeostasis in intact and ovariectomized deoxycorticosterone-acetate salt rats. Hypertension 47, 1101–1107 (2006).
Muller, S. et al. Salt-dependent chemotaxis of macrophages. PLOS ONE 8, e73439 (2013).
Jantsch, J. et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 21, 493–501 (2015).
Wiig, H. et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J. Clin. Invest. 123, 2803–2815 (2013).
Berry, M. R. et al. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 170, 860–874 (2017).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Wei, Y. et al. High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice. Oncotarget 8, 70–82 (2017).
Hernandez, A. L. et al. Sodium chloride inhibits the suppressive function of FOXP3+regulatory T cells. J. Clin. Invest. 125, 4212–4222 (2015).
Safa, K. et al. Salt accelerates allograft rejection through serum- and glucocorticoid-regulated kinase-1-dependent inhibition of regulatory T cells. J. Am. Soc. Nephrol. 26, 2341–2347 (2015).
Miranda, P. M. et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 6, 57 (2018).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Brown, I. J., Tzoulaki, I., Candeias, V. & Elliott, P. Salt intakes around the world: implications for public health. Int. J. Epidemiol. 38, 791–813 (2009).
Mente, A. et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 371, 601–611 (2014).
He, F. J. & MacGregor, G. A. Role of salt intake in prevention of cardiovascular disease: controversies and challenges. Nat. Rev. Cardiol. 15, 371–377 (2018).
Mozaffarian, D. et al. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 371, 624–634 (2014).
Norlander, A. E., Madhur, M. S. & Harrison, D. G. The immunology of hypertension. J. Exp. Med. 215, 21–33 (2018).
Farez, M. F., Fiol, M. P., Gaitan, M. I., Quintana, F. J. & Correale, J. Sodium intake is associated with increased disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 86, 26–31 (2015).
Fitzgerald, K. C. et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann. Neurol. 82, 20–29 (2017).
Muller, D. N., Wilck, N., Haase, S., Kleinewietfeld, M. & Linker, R. A. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat. Rev. Immunol. 19, 243–254 (2019).
Junger, W. G., Liu, F. C., Loomis, W. H. & Hoyt, D. B. Hypertonic saline enhances cellular immune function. Circ. Shock 42, 190–196 (1994).
Shapiro, L. & Dinarello, C. A. Osmotic regulation of cytokine synthesis in vitro. Proc. Natl Acad. Sci. USA 92, 12230–12234 (1995).
Ip, W. K. & Medzhitov, R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat. Commun. 6, 6931 (2015).
Binger, K. J. et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J. Clin. Invest. 125, 4223–4238 (2015).
Byles, V. et al. The TSC-mTOR pathway regulates macrophage polarization. Nat. Commun. 4, 2834 (2013).
Huber, S., Hoffmann, R., Muskens, F. & Voehringer, D. Alternatively activated macrophages inhibit T cell proliferation by Stat6-dependent expression of PD-L2. Blood 116, 3311–3320 (2010).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Zhang, W. C. et al. High salt primes a specific activation state of macrophages, M(Na). Cell Res. 25, 893–910 (2015).
Hucke, S. et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J. Autoimmun. 67, 90–101 (2016).
Pennock, N. D. et al. T cell responses: naive to memory and everything in between. Adv. Physiol. Educ. 37, 273–283 (2013).
Steinman, L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13, 139–145 (2007).
Kaiko, G. E., Horvat, J. C., Beagley, K. W. & Hansbro, P. M. Immunological decision-making: how does the immune system decide to mount a helper T cell response? Immunology 123, 326–338 (2008).
Ivanov, I. I. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+T helper cells. Cell 126, 1121–1133 (2006).
Coccia, M. et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J. Exp. Med. 209, 1595–1609 (2012).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Sano, T. et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 163, 381–393 (2015).
Ladinsky, M. S. et al. Endocytosis of commensal antigens by intestinal epithelial cells regulates mucosal T cell homeostasis. Science 363, eaat4042 (2019).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Vallon, V. et al. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R395–R401 (2005).
Chen, S. et al. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J. Clin. Invest. 119, 1647–1658 (2009).
Krementsov, D. N., Case, L. K., Hickey, W. F. & Teuscher, C. Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. FASEB J. 29, 3446–3457 (2015).
Markle, J. G. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Onishi, Y., Fehervari, Z., Yamaguchi, T. & Sakaguchi, S. Foxp3+natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl Acad. Sci. USA 105, 10113–10118 (2008).
Schmidt, A., Oberle, N. & Krammer, P. H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 3, 51 (2012).
Shevach, E. M. Mechanisms of foxp3+T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009).
Crome, S. Q. et al. Inflammatory effects of ex vivo human Th17 cells are suppressed by regulatory T cells. J. Immunol. 185, 3199–3208 (2010).
Luo, Y. et al. Negligible effect of sodium chloride on the development and function of TGF-beta-induced CD4+ Foxp3+ regulatory T cells. Cell Rep. 26, 1869–1879 (2019).
Aguiar, S. L. F. et al. High-salt diet induces IL-17-dependent gut inflammation and exacerbates colitis in mice. Front. Immunol. 8, 1969 (2017).
Monteleone, I. et al. Sodium chloride-enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J. Crohns Colitis 11, 237–245 (2017).
Faraco, G. et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 21, 240–249 (2018).
Matthias, J. et al. Sodium chloride is an ionic checkpoint for human TH2 cells and shapes the atopic skin microenvironment. Sci. Transl Med. 11, eaau0683 (2019).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46, 562–576 (2017).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl Med. 1, 6ra14 (2009).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L. & Gordon, J. I. Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 (2011).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Laurans, L. et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat. Med. 24, 1113–1120 (2018).
Wang, C. et al. High-salt diet has a certain impact on protein digestion and gut microbiota: a sequencing and proteome combined study. Front. Microbiol. 8, 1838 (2017).
Kurdi, A. T. et al. Tiam1/Rac1 complex controls Il17a transcription and autoimmunity. Nat. Commun. 7, 13048 (2016).
Shen, F. & Gaffen, S. L. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine 41, 92–104 (2008).
Kotla, S., Singh, N. K., Heckle, M. R., Tigyi, G. J. & Rao, G. N. The transcription factor CREB enhances interleukin-17A production and inflammation in a mouse model of atherosclerosis. Sci. Signal 6, ra83 (2013).
Chang, P. V., Hao, L., Offermanns, S. & Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 111, 2247–2252 (2014).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 504, 451–455 (2013).
Stamler, J. The INTERSALT study: background, methods, findings, and implications. Am. J. Clin. Nutr. 65, 626S–642S (1997).
O’Donnell, M. et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med. 371, 612–623 (2014).
Itani, H. A. et al. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ. Res. 118, 1233–1243 (2016).
De Miguel, C., Das, S., Lund, H. & Mattson, D. L. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1136–R1142 (2010).
Mattson, D. L. et al. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R407–R414 (2013).
Rodriguez-Iturbe, B. et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 59, 2222–2232 (2001).
Luft, F. C., Dechend, R. & Muller, D. N. Immune mechanisms in angiotensin II-induced target-organ damage. Ann. Med. 44 (Suppl. 1), S49–S54 (2012).
Rucker, A. J., Rudemiller, N. P. & Crowley, S. D. Salt, hypertension, and immunity. Annu. Rev. Physiol. 80, 283–307 (2018).
Wenzel, P. et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124, 1370–1381 (2011).
De Ciuceis, C. et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler. Thromb. Vasc. Biol. 25, 2106–2113 (2005).
Ko, E. A. et al. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 292, H1789–H1795 (2007).
Lankhorst, S. et al. Salt sensitivity of angiogenesis inhibition-induced blood pressure rise: role of interstitial sodium accumulation? Hypertension 69, 919–926 (2017).
Machnik, A. et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension 55, 755–761 (2010).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Pons, H. et al. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am. J. Physiol. Renal Physiol. 304, F289–F299 (2013).
Kirabo, A. et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest. 124, 4642–4656 (2014).
Trott, D. W. et al. Oligoclonal CD8+T cells play a critical role in the development of hypertension. Hypertension 64, 1108–1115 (2014).
McDonnell, W. J. et al. High CD8 T-cell receptor clonality and altered CDR3 properties are associated with elevated isolevuglandins in adipose tissue during diet-induced obesity. Diabetes 67, 2361–2376 (2018).
Youn, J. C. et al. Immunosenescent CD8+T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133 (2013).
Liu, Y. et al. CD8+ T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nat. Commun. 8, 14037 (2017).
Madhur, M. S. et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010).
Kamat, N. V. et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A−/− mice. Hypertension 65, 569–576 (2015).
Norlander, A. E. et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 68, 167–174 (2016).
Norlander, A. E. et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI insight 2, 92801 (2017).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Shah, K. H. et al. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ. Res. 117, 858–869 (2015).
Vinh, A. et al. Inhibition and genetic ablation of the B7/CD28 T cell costimulation axis prevents experimental hypertension. Circulation 122, 2529–2537 (2010).
Barbaro, N. R. et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 21, 1009–1020 (2017).
Yi, B. et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res. 166, 103–110 (2015).
Zhou, X. et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation. PLOS ONE 8, e60332 (2013).
Luo, T. et al. Th17/Treg imbalance induced by dietary salt variation indicates inflammation of target organs in humans. Sci. Rep. 6, 26767 (2016).
Rakova, N. et al. Long-term space flight simulation reveals infradian rhythmicity in human Na+ balance. Cell Metab. 17, 125–131 (2013).
Lerchl, K. et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension 66, 850–857 (2015).
McDonald, J. et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult. Scler. Relat. Disord. 6, 87–92 (2016).
Nourbakhsh, B. et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 87, 1350–1353 (2016).
McLean, R. M. et al. Assessment of dietary sodium intake using a food frequency questionnaire and 24-hour urinary sodium excretion: a systematic literature review. J. Clin. Hypertens. 19, 1214–1230 (2017).
Salgado, E., Bes-Rastrollo, M., de Irala, J., Carmona, L. & Gomez-Reino, J. J. High sodium intake is associated with self-reported rheumatoid arthritis: a cross sectional and case control analysis within the SUN cohort. Medicine 94, e924 (2015).
Jiang, X. et al. High sodium chloride consumption enhances the effects of smoking but does not interact with SGK1 polymorphisms in the development of ACPA-positive status in patients with RA. Ann. Rheum. Dis. 75, 943–946 (2016).
Sundstrom, B., Johansson, I. & Rantapaa-Dahlqvist, S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: results from a nested case-control study. Rheumatology 54, 487–493 (2015).
Marouen, S. et al. Sodium excretion is higher in patients with rheumatoid arthritis than in matched controls. PLOS ONE 12, e0186157 (2017).
Khalili, H. et al. Identification and characterization of a novel association between dietary potassium and risk of Crohn’s disease and ulcerative colitis. Front. Immunol. 7, 554 (2016).
Schmieder, R. E. Dietary salt intake and left ventricular hypertrophy. Nephrol. Dial. Transplant. 12, 245–248 (1997).
Jin, Y. et al. Independent relations of left ventricular structure with the 24-hour urinary excretion of sodium and aldosterone. Hypertension 54, 489–495 (2009).
Rodriguez, C. J. et al. Association of sodium and potassium intake with left ventricular mass: coronary artery risk development in young adults. Hypertension 58, 410–416 (2011).
Farquhar, W. B., Edwards, D. G., Jurkovitz, C. T. & Weintraub, W. S. Dietary sodium and health: more than just blood pressure. J. Am. Coll. Cardiol. 65, 1042–1050 (2015).
Elijovich, F. et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 68, e7–e46 (2016).
Kurtz, T. W., DiCarlo, S. E., Pravenec, M. & Morris, R. C. Jr. An appraisal of methods recently recommended for testing salt sensitivity of blood pressure. J. Am. Heart Assoc. 6, e005653 (2017).
Kotchen, T. A., Cowley, A. W. Jr & Frohlich, E. D. Salt in health and disease—a delicate balance. N. Engl. J. Med. 368, 1229–1237 (2013).
Luft, F. C. & Weinberger, M. H. Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am. J. Clin. Nutr. 65, 612S–617S (1997).
Weinberger, M. H. Salt sensitivity of blood pressure in humans. Hypertension 27, 481–490 (1996).
Johnson, R. J., Herrera-Acosta, J., Schreiner, G. F. & Rodriguez-Iturbe, B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N. Engl. J. Med. 346, 913–923 (2002).
Luzardo, L., Noboa, O. & Boggia, J. Mechanisms of salt-sensitive hypertension. Curr. Hypertens. Rev. 11, 14–21 (2015).
Itani, H. A. et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68, 123–132 (2016).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380, 752–762 (2019).
Dalekos, G. N., Elisaf, M., Bairaktari, E., Tsolas, O. & Siamopoulos, K. C. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J. Lab. Clin. Med. 129, 300–308 (1997).
Dorffel, Y. et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 34, 113–117 (1999).
Schneider, M. P. et al. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J. Am. Soc. Nephrol. 28, 1867–1876 (2017).
Hammon, M. et al. 3 Tesla 23Na magnetic resonance imaging during acute kidney injury. Acad. Radiol. 24, 1086–1093 (2017).
Dahlmann, A. et al. Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int. 87, 434–441 (2015).
Hammon, M. et al. 23Na magnetic resonance imaging of the lower leg of acute heart failure patients during diuretic treatment. PLOS ONE 10, e0141336 (2015).
Kopp, C. et al. Na+ deposition in the fibrotic skin of systemic sclerosis patients detected by 23Na-magnetic resonance imaging. Rheumatology 56, 556–560 (2017).
Colonna, M. Innate lymphoid cells: diversity, plasticity, and unique functions in immunity. Immunity 48, 1104–1117 (2018).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Acknowledgements
The authors thank E. G. Avery (Max Delbruck Center for Molecular Medicine, Berlin, Germany) for critically reviewing the manuscript before submission. N.W. is funded by the Berlin Institute of Health (Clinician Scientist Program), Berlin, Germany.
Peer review information
Nature Reviews Nephrology thanks A. Kirabo, P. Mehrotra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
N.W. and D.N.M. researched the data and wrote the article with major input from H.B., L.M. and A.B. All authors discussed the content, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Western diet
-
Diet characterized, for example, by the consumption of high amounts of red meat, dairy products, sugars, unsaturated fats and salt (typically consumed in pre-processed foods).
- Glycosaminoglycans
-
(GAGs). Linear, negatively charged carbohydrates, often bound to glycoproteins in the extracellular matrix, where they determine the biomechanical properties of the interstitial space.
- Intestinal microbiome
-
Collective genomes of the community of intestinal microorganisms, which include bacteria, viruses and fungi.
- Acute cellulitis
-
Acute bacterial infection of the skin affecting the dermis and adjacent subcutaneous fat.
- Osmolytes
-
Osmotically active particles (for example, ions or proteins) that are dissolved in the cytosol and the extracellular fluid and affect cell volume and other cellular processes.
- Hypertonicity
-
A state in which the extracellular space contains more solutes than the cytosol.
- Naive T cells
-
Mature T cells (helper T cells or cytotoxic T cells) that have not encountered their cognate antigens in peripheral tissues.
- 16S ribosomal RNA (rRNA) sequencing
-
Sequencing of the 16S rRNA amplicon, a common, culture-independent method used to identify and compare bacterial phylogeny and taxonomy in a microbial sample.
- Essential hypertension
-
Chronic elevation of blood pressure in the systemic circulation that cannot be assigned to an identifiable cause; also known as primary or idiopathic hypertension, it affects approximately 90% of all patients with high blood pressure.
- Isoketal-modified proteins
-
Proteins oxidatively modified by highly reactive γ-ketoaldehydes (also referred to as isoketals, formed by peroxidation of arachidonic acid induced by free radicals). Isoketals adduct to lysine residues on proteins and extensively crosslink proteins.
- Subpressor dose
-
Low dose of a given substance that leads to an increase in blood pressure.
- Clinically isolated syndrome
-
A single episode of neurological symptoms and inflammatory demyelination in humans that is isolated in time and compatible with a possible future development of multiple sclerosis.
- 23Na MRI
-
Non-invasive method for imaging of tissue sodium using the nuclear magnetic resonance signal of sodium nuclei; quantification is performed by placing phantoms of known sodium concentration within the field of view.
Rights and permissions
About this article
Cite this article
Wilck, N., Balogh, A., Markó, L. et al. The role of sodium in modulating immune cell function. Nat Rev Nephrol 15, 546–558 (2019). https://doi.org/10.1038/s41581-019-0167-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0167-y
This article is cited by
-
The immunomodulatory effect of oral NaHCO3 is mediated by the splenic nerve: multivariate impact revealed by artificial neural networks
Journal of Neuroinflammation (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
T helper cell polarity determines salt sensitivity and hypertension development
Hypertension Research (2023)
-
High salt diet does not impact the development of acute myeloid leukemia in mice
Cancer Immunology, Immunotherapy (2023)
-
Salt Behind the Scenes of Systemic Lupus Erythematosus and Rheumatoid Arthritis
Current Nutrition Reports (2023)