Abstract
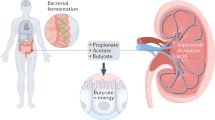
Uraemic syndrome (also known as uremic syndrome) in patients with advanced chronic kidney disease involves the accumulation in plasma of small-molecule uraemic solutes and uraemic toxins (also known as uremic toxins), dysfunction of multiple organs and dysbiosis of the gut microbiota. As such, uraemic syndrome can be viewed as a disease of perturbed inter-organ and inter-organism (host–microbiota) communication. Multiple biological pathways are affected, including those controlled by solute carrier (SLC) and ATP-binding cassette (ABC) transporters and drug-metabolizing enzymes, many of which are also involved in drug absorption, distribution, metabolism and elimination (ADME). The remote sensing and signalling hypothesis identifies SLC and ABC transporter-mediated communication between organs and/or between the host and gut microbiota as key to the homeostasis of metabolites, antioxidants, signalling molecules, microbiota-derived products and dietary components in body tissues and fluid compartments. Thus, this hypothesis provides a useful perspective on the pathobiology of uraemic syndrome. Pathways considered central to drug ADME might be particularly important for the body’s attempts to restore homeostasis, including the correction of disturbances due to kidney injury and the accumulation of uraemic solutes and toxins. This Review discusses how the remote sensing and signalling hypothesis helps to provide a systems-level understanding of aspects of uraemia that could lead to novel approaches to its treatment.
Key points
-
The uraemic syndrome (also known as uremic syndrome) associated with chronic kidney disease (CKD) is characterized by complex local and systemic derangements in metabolism and signalling.
-
CKD involves aberrant inter-organ (gut–liver–kidney–brain) and inter-organism (host–gut microbiota) remote communication via small molecules, including uraemic solutes, metabolites and signalling molecules.
-
Aspects of uraemic syndrome can be considered disordered remote sensing and signalling mediated by a multi-organ network of solute carrier (SLC) and ATP-binding cassette (ABC) transporters and drug-metabolizing enzymes (DMEs).
-
The remote sensing and signalling hypothesis provides a systems biology framework for understanding the role of these transporters and DMEs in small-molecule-mediated inter-organ and inter-organism communication.
-
Transported uraemic solutes (including gut-microbiota-derived indoxyl sulfate) can affect multiple signalling pathways.
-
Viewing CKD and uraemic syndrome through the lens of the remote sensing and signalling hypothesis provides fresh perspectives on the metabolic derangements of CKD that might lead to novel therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wu, W., Dnyanmote, A. V. & Nigam, S. K. Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol. Pharmacol. 79, 795–805 (2011).
Liu, X. & Dai, C. Advances in understanding and management of residual renal function in patients with chronic kidney disease. Kidney Dis. 2, 187–196 (2017).
Lowenstein, J. & Grantham, J. J. Residual renal function: a paradigm shift. Kidney Int. 91, 561–565 (2017).
Shafi, T., Mullangi, S., Toth-Manikowski, S. M., Hwang, S. & Michels, W. M. Residual kidney function: implications in the era of personalized medicine. Semin. Dial. 30, 241–245 (2017).
Nigam, S. K. What do drug transporters really do? Nat. Rev. Drug Discov. 14, 29–44 (2015).
Nigam, S. K. et al. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin. J. Am. Soc. Nephrol. 10, 2039–2049 (2015).
Wikoff, W. R., Nagle, M. A., Kouznetsova, V. L., Tsigelny, I. F. & Nigam, S. K. Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J. Proteome Res. 10, 2842–2851 (2011).
Wu, W., Bush, K. T. & Nigam, S. K. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci. Rep. 7, 4939 (2017).
Kim, S. H., Yu, M. A., Ryu, E. S., Jang, Y. H. & Kang, D. H. Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab. Invest. 92, 488–498 (2012).
Sun, C. Y., Chang, S. C. & Wu, M. S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLOS ONE 7, e34026 (2012).
Bolati, D., Shimizu, H., Higashiyama, Y., Nishijima, F. & Niwa, T. Indoxyl sulfate induces epithelial-to-mesenchymal transition in rat kidneys and human proximal tubular cells. Am. J. Nephrol. 34, 318–323 (2011).
van den Brand, J. A. et al. Uremic solutes in chronic kidney disease and their role in progression. PLOS ONE 11, e0168117 (2016).
Nigam, S. K. The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol. 58, 663–687 (2018).
Ahn, S. Y. & Nigam, S. K. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol. Pharmacol. 76, 481–490 (2009).
Nigam, S. K. et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol. Rev. 95, 83–123 (2015).
Kaler, G. et al. Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J. Biol. Chem. 282, 23841–23853 (2007).
Pavlova, A. et al. Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and ROCT. Am. J. Physiol. Renal Physiol. 278, F635–F643 (2000).
Kaler, G. et al. Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem. Biophys. Res. Commun. 351, 872–876 (2006).
Monte, J. C., Nagle, M. A., Eraly, S. A. & Nigam, S. K. Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem. Biophys. Res. Commun. 323, 429–436 (2004).
Bush, K. T., Wu, W., Lun, C. & Nigam, S. K. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J. Biol. Chem. 292, 15789–15803 (2017).
Nigam, S. K. & Bhatnagar, V. The systems biology of uric acid transporters: the role of remote sensing and signaling. Curr. Opin. Nephrol. Hypertens. 27, 305–313 (2018).
Li, T. & Apte, U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv. Pharmacol. 74, 263–302 (2015).
Chiang, J. Y. Recent advances in understanding bile acid homeostasis. F1000Res. 6, 2029 (2017).
Molinaro, A., Wahlstrom, A. & Marschall, H. U. Role of bile acids in metabolic control. Trends Endocrinol. Metab. 29, 31–41 (2018).
Evenepoel, P., Poesen, R. & Meijers, B. The gut–kidney axis. Pediatr. Nephrol. 32, 2005–2014 (2017).
Wing, M. R., Patel, S. S., Ramezani, A. & Raj, D. S. Gut microbiome in chronic kidney disease. Exp. Physiol. 101, 471–477 (2016).
Spector, R., Robert Snodgrass, S. & Johanson, C. E. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp. Neurol. 273, 57–68 (2015).
Stieger, B. & Gao, B. Drug transporters in the central nervous system. Clin. Pharmacokinet. 54, 225–242 (2015).
Jabbari, B. & Vaziri, N. D. The nature, consequences, and management of neurological disorders in chronic kidney disease. Hemodial. Int. 22, 150–160 (2018).
Seifter, J. L. & Samuels, M. A. Uremic encephalopathy and other brain disorders associated with renal failure. Semin. Neurol. 31, 139–143 (2011).
Ebah, L. M. et al. Subcutaneous interstitial pressure and volume characteristics in renal impairment associated with edema. Kidney Int. 84, 980–988 (2013).
Mayerl, S. et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J. Clin. Invest. 124, 1987–1999 (2014).
Prentice, K. J. et al. The furan fatty acid metabolite CMPF is elevated in diabetes and induces beta cell dysfunction. Cell Metab. 19, 653–666 (2014).
Ahn, S. Y. et al. Linkage of organic anion transporter-1 to metabolic pathways through integrated “omics”-driven network and functional analysis. J. Biol. Chem. 286, 31522–31531 (2011).
Liu, H. C. et al. An organic anion transporter 1 (OAT1)-centered metabolic network. J. Biol. Chem. 291, 19474–19486 (2016).
Wu, W. et al. Multispecific drug transporter Slc22a8 (Oat3) regulates multiple metabolic and signaling pathways. Drug Metab. Dispos. 41, 1825–1834 (2013).
Liu, B. et al. Metabolic enzyme system and transport pathways in chronic kidney diseases. Curr. Drug Metab. 19, 568–576 (2018).
Takada, T. et al. Identification of ABCG2 as an exporter of uremic toxin indoxyl sulfate in mice and as a crucial factor influencing CKD progression. Sci. Rep. 8, 11147 (2018).
Matsuo, H. et al. Hyperuricemia in acute gastroenteritis is caused by decreased urate excretion via ABCG2. Sci. Rep. 6, 31003 (2016).
Bhatnagar, V. et al. Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling. Clin. Kidney J. 9, 444–453 (2016).
Jing, J. et al. Genetics of serum urate concentrations and gout in a high-risk population, patients with chronic kidney disease. Sci. Rep. 8, 13184 (2018).
Takada, T. et al. ABCG2 dysfunction increases serum uric acid by decreased intestinal urate excretion. Nucleosides Nucleotides Nucleic Acids 33, 275–281 (2014).
Yano, H., Tamura, Y., Kobayashi, K., Tanemoto, M. & Uchida, S. Uric acid transporter ABCG2 is increased in the intestine of the 5/6 nephrectomy rat model of chronic kidney disease. Clin. Exp. Nephrol. 18, 50–55 (2014).
Pahl, M. V. & Vaziri, N. D. The chronic kidney disease–colonic axis. Semin. Dial. 28, 459–463 (2015).
Velenosi, T. J., Fu, A. Y., Luo, S., Wang, H. & Urquhart, B. L. Down-regulation of hepatic CYP3A and CYP2C mediated metabolism in rats with moderate chronic kidney disease. Drug Metab. Dispos. 40, 1508–1514 (2012).
Naud, J., Nolin, T. D., Leblond, F. A. & Pichette, V. Current understanding of drug disposition in kidney disease. J. Clin. Pharmacol. 52, 10S–22S (2012).
Philips, B. J., Lane, K., Dixon, J. & Macphee, I. The effects of acute renal failure on drug metabolism. Expert Opin. Drug Metab. Toxicol. 10, 11–23 (2014).
Ladda, M. A. & Goralski, K. B. The effects of CKD on cytochrome P450-mediated drug metabolism. Adv. Chronic Kidney Dis. 23, 67–75 (2016).
Michaud, J. et al. Effect of hemodialysis on hepatic cytochrome P450 functional expression. J. Pharmacol. Sci. 108, 157–163 (2008).
Ma, X., Idle, J. R. & Gonzalez, F. J. The pregnane X receptor: from bench to bedside. Expert Opin. Drug Metab. Toxicol. 4, 895–908 (2008).
Martovetsky, G., Bush, K. T. & Nigam, S. K. Kidney versus liver specification of SLC and ABC drug transporters, tight junction molecules, and biomarkers. Drug Metab. Dispos. 44, 1050–1060 (2016).
Martovetsky, G., Tee, J. B. & Nigam, S. K. Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol. Pharmacol. 84, 808–823 (2013).
Staudinger, J. L. et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA 98, 3369–3374 (2001).
Xie, W. et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406, 435–439 (2000).
Santana Machado, T. et al. Indoxyl sulfate upregulates liver P-glycoprotein expression and activity through aryl hydrocarbon receptor signaling. J. Am. Soc. Nephrol. 29, 906–918 (2018).
Xu, C., Li, C. Y. & Kong, A. N. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 28, 249–268 (2005).
Leong, S. C. & Sirich, T. L. Indoxyl sulfate — review of toxicity and therapeutic strategies. Toxins 8, E358 (2016).
Camacho, O. et al. Effect of a sustained difference in hemodialytic clearance on the plasma levels of p-cresol sulfate and indoxyl sulfate. Nephrol. Dial. Transplant. 31, 1335–1341 (2016).
Poesen, R. et al. The influence of prebiotic arabinoxylan oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: a randomized controlled trial. PLOS ONE 11, e0153893 (2016).
Chiavaroli, L., Mirrahimi, A., Sievenpiper, J. L., Jenkins, D. J. & Darling, P. B. Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 69, 761–768 (2015).
Cha, R. H. et al. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin. J. Am. Soc. Nephrol. 11, 559–567 (2016).
Rossi, M. et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin. J. Am. Soc. Nephrol. 11, 223–231 (2016).
Schulman, G. et al. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J. Am. Soc. Nephrol. 26, 1732–1746 (2015).
Sirich, T. L., Plummer, N. S., Gardner, C. D., Hostetter, T. H. & Meyer, T. W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 9, 1603–1610 (2014).
Prokopienko, A. J. & Nolin, T. D. Microbiota-derived uremic retention solutes: perpetrators of altered nonrenal drug clearance in kidney disease. Expert Rev. Clin. Pharmacol. 11, 71–82 (2018).
Vanholder, R., Schepers, E., Pletinck, A., Nagler, E. V. & Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J. Am. Soc. Nephrol. 25, 1897–1907 (2014).
Kaminski, T., Michalowska, M. & Pawlak, D. Aryl hydrocarbon receptor (AhR) and its endogenous agonist - indoxyl sulfate in chronic kidney disease. Postepy Hig. Med. Dosw. 71, 624–632 (2017).
Gao, H. & Liu, S. Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci. 185, 23–29 (2017).
Liu, H., Narayanan, R., Hoffmann, M. & Surapaneni, S. The uremic toxin indoxyl-3-sulfate induces CYP1A2 in primary human hepatocytes. Drug Metab. Lett. 10, 195–199 (2016).
Wu, Y., Han, X., Wang, L., Diao, Z. & Liu, W. Indoxyl sulfate promotes vascular smooth muscle cell calcification via the JNK/Pit-1 pathway. Ren. Fail. 38, 1702–1710 (2016).
Muteliefu, G. et al. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation ofp53, p21, and prelamin A through oxidative stress. Am. J. Physiol. Cell Physiol. 303, C126–C134 (2012).
Mozar, A. et al. Uremic toxin indoxyl sulfate inhibits human vascular smooth muscle cell proliferation. Ther. Apher. Dial. 15, 135–139 (2011).
Shimizu, H., Hirose, Y., Nishijima, F., Tsubakihara, Y. & Miyazaki, H. ROS and PDGF-β [corrected] receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am. J. Physiol. Cell Physiol. 297, C389–C396 (2009).
Yamamoto, H. et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 69, 1780–1785 (2006).
Mair, R. D., Sirich, T. L. & Meyer, T. W. Uremic toxin clearance and cardiovascular toxicities. Toxins 10, E226 (2018).
Schroeder, J. C. et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49, 393–400 (2010).
Esser, C. & Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 67, 259–279 (2015).
Roman, A. C., Carvajal-Gonzalez, J. M., Merino, J. M., Mulero-Navarro, S. & Fernandez-Salguero, P. M. The aryl hydrocarbon receptor in the crossroad of signalling networks with therapeutic value. Pharmacol. Ther. 185, 50–63 (2017).
Wakamatsu, T. et al. Indoxyl sulfate promotes macrophage IL-1β production by activating aryl hydrocarbon receptor/NF-κ/MAPK cascades, but the NLRP3 inflammasome was not activated. Toxins 10, E124 (2018).
Wanchai, K. et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin. Sci. 132, 1545–1563 (2018).
Czuba, L. C., Hillgren, K. M. & Swaan, P. W. Post-translational modifications of transporters. Pharmacol. Ther. 192, 88–99 (2018).
Murray, M. & Zhou, F. Trafficking and other regulatory mechanisms for organic anion transporting polypeptides and organic anion transporters that modulate cellular drug and xenobiotic influx and that are dysregulated in disease. Br. J. Pharmacol. 174, 1908–1924 (2017).
Xu, D. & You, G. Loops and layers of post-translational modifications of drug transporters. Adv. Drug Deliv. Rev. 116, 37–44 (2017).
Hong, M. et al. Human organic anion transporter hOAT1 forms homooligomers. J. Biol. Chem. 280, 32285–32290 (2005).
Miyazaki, H. et al. Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J. Am. Soc. Nephrol. 16, 3498–3506 (2005).
Endou, H. & Anzai, N. Urate transport across the apical membrane of renal proximal tubules. Nucleosides Nucleotides Nucleic Acids 27, 578–584 (2008).
Zhang, Q., Pan, Z. & You, G. Regulation of human organic anion transporter 4 by protein kinase C and NHERF-1: altering the endocytosis of the transporter. Pharm. Res. 27, 589–596 (2010).
Xue, P., Crum, C. M. & Thibodeau, P. H. Regulation of ABCC6 trafficking and stability by a conserved C-terminal PDZ-like sequence. PLOS ONE 9, e97360 (2014).
Schaletzki, Y. et al. Several adaptor proteins promote intracellular localisation of the transporter MRP4/ABCC4 in platelets and haematopoietic cells. Thromb. Haemost. 117, 105–115 (2017).
Ferreira, C. et al. The scaffold protein PDZK1 modulates expression and function of the organic anion transporting polypeptide 2B1. Eur. J. Pharm. Sci. 120, 181–190 (2018).
Venot, Q. et al. A PDZ-like motif in the biliary transporter ABCB4 interacts with the scaffold protein EBP50 and regulates ABCB4 cell surface expression. PLOS ONE 11, e0146962 (2016).
Vanholder, R. et al. The role of EUTox in uremic toxin research. Semin. Dial. 22, 323–328 (2009).
Zhao, Y. Y. Metabolomics in chronic kidney disease. Clin. Chim. Acta 422, 59–69 (2013).
Lau, W. L., Savoj, J., Nakata, M. B. & Vaziri, N. D. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin. Sci. 132, 509–522 (2018).
Mahmoodpoor, F., Rahbar Saadat, Y., Barzegari, A., Ardalan, M. & Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 93, 412–419 (2017).
Vogt, S. L., Pena-Diaz, J. & Finlay, B. B. Chemical communication in the gut: effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens. Anaerobe 34, 106–115 (2015).
Kennedy, P. J., Cryan, J. F., Dinan, T. G. & Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112, 399–412 (2017).
Xu, L. et al. Furan fatty acids — beneficial or harmful to health? Prog. Lipid Res. 68, 119–137 (2017).
El Ridi, R. & Tallima, H. Physiological functions and pathogenic potential of uric acid: a review. J. Adv. Res. 8, 487–493 (2017).
Bae, D. H., Lane, D. J. R., Jansson, P. J. & Richardson, D. R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subj. 1862, 2053–2068 (2018).
Lopez-Nieto, C. E. et al. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J. Biol. Chem. 272, 6471–6478 (1997).
Eraly, S. A. et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J. Biol. Chem. 281, 5072–5083 (2006).
Sweeney, D. E. et al. Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney. Mol. Pharmacol. 80, 147–154 (2011).
Nagle, M. A. et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J. Biol. Chem. 286, 243–251 (2011).
Nagle, M. A., Wu, W., Eraly, S. A. & Nigam, S. K. Organic anion transport pathways in antiviral handling in choroid plexus in Oat1 (Slc22a6) and Oat3 (Slc22a8) deficient tissue. Neurosci. Lett. 534, 133–138 (2013).
Truong, D. M., Kaler, G., Khandelwal, A., Swaan, P. W. & Nigam, S. K. Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J. Biol. Chem. 283, 8654–8663 (2008).
Vallon, V. et al. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am. J. Physiol. Renal Physiol. 294, F867–F873 (2008).
VanWert, A. L., Gionfriddo, M. R. & Sweet, D. H. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm. Drug Dispos. 31, 1–71 (2010).
Rubino, F. M. Toxicity of glutathione-binding metals: a review of targets and mechanisms. Toxics 3, 20–62 (2015).
Torres, A. M., Dnyanmote, A. V., Bush, K. T., Wu, W. & Nigam, S. K. Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J. Biol. Chem. 286, 26391–26395 (2011).
Zalups, R. K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 52, 113–143 (2000).
Wikoff, W. R. et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl Acad. Sci. USA 106, 3698–3703 (2009).
Liu, H. C. et al. Molecular properties of drugs interacting with SLC22 transporters OAT1, OAT3, OCT1, and OCT2: a machine-learning approach. J. Pharmacol. Exp. Ther. 359, 215–229 (2016).
Rhee, E. P. et al. Metabolite profiling identifies markers of uremia. J. Am. Soc. Nephrol. 21, 1041–1051 (2010).
Sun, C. Y. et al. A novel SNP in the 5’ regulatory region of organic anion transporter 1 is associated with chronic kidney disease. Sci. Rep. 8, 8085 (2018).
Schophuizen, C. M. et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch. 465, 1701–1714 (2013).
Ahn, S. Y., Eraly, S. A., Tsigelny, I. & Nigam, S. K. Interaction of organic cations with organic anion transporters. J. Biol. Chem. 284, 31422–31430 (2009).
Sager, G., Smaglyukova, N. & Fuskevaag, O. M. The role of OAT2 (SLC22A7) in the cyclic nucleotide biokinetics of human erythrocytes. J. Cell. Physiol. 233, 5972–5980 (2018).
Dias, G. F. et al. Indoxyl sulfate, a uremic toxin, stimulates reactive oxygen species production and erythrocyte cell death supposedly by an organic anion transporter 2 (OAT2) and NADPH oxidase activity-dependent pathways. Toxins 10, E280 (2018).
Toyohara, T. et al. SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J. Am. Soc. Nephrol. 20, 2546–2555 (2009).
Subramaniam, S. & Fletcher, C. Trimethylamine N-oxide: breathe new life. Br. J. Pharmacol. 175, 1344–1353 (2018).
Tang, W. H. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 116, 448–455 (2015).
Teft, W. A. et al. Identification and characterization of trimethylamine-N-oxide uptake and efflux transporters. Mol. Pharm. 14, 310–318 (2017).
Masuda, S. et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 17, 2127–2135 (2006).
Motohashi, H. & Inui, K. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 15, 581–588 (2013).
Nies, A. T., Koepsell, H., Damme, K. & Schwab, M. Organic cation transporters (OCTs, MATEs), in vitroand in vivo evidence for the importance in drug therapy. Handb. Exp. Pharmacol. 201, 105–167 (2011).
Sato, T., Yamaguchi, H., Kogawa, T., Abe, T. & Mano, N. Organic anion transporting polypeptides 1B1 and 1B3 play an important role in uremic toxin handling and drug-uremic toxin interactions in the liver. J. Pharm. Pharm. Sci. 17, 475–484 (2014).
Katsube, Y. et al. Cooperative inhibitory effects of uremic toxins and other serum components on OATP1B1-mediated transport of SN-38. Cancer Chemother. Pharmacol. 79, 783–789 (2017).
Fu, Z. D., Selwyn, F. P., Cui, J. Y. & Klaassen, C. D. RNA-seq profiling of intestinal expression of xenobiotic processing genes in germ-free mice. Drug Metab. Dispos. 45, 1225–1238 (2017).
Selwyn, F. P., Cheng, S. L., Klaassen, C. D. & Cui, J. Y. Regulation of hepatic drug-metabolizing enzymes in germ-free mice by conventionalization and probiotics. Drug Metab. Dispos. 44, 262–274 (2016).
Selwyn, F. P. et al. Developmental regulation of drug-processing genes in livers of germ-free mice. Toxicol. Sci. 147, 84–103 (2015).
Claus, S. P. et al. Colonization-induced host–gut microbial metabolic interaction. mBio 2, e00271–10 (2011).
Meinl, W., Sczesny, S., Brigelius-Flohe, R., Blaut, M. & Glatt, H. Impact of gut microbiota on intestinal and hepatic levels of phase 2 xenobiotic-metabolizing enzymes in the rat. Drug Metab. Dispos. 37, 1179–1186 (2009).
Jansen, J., Jankowski, J., Gajjala, P. R., Wetzels, J. F. M. & Masereeuw, R. Disposition and clinical implications of protein-bound uremic toxins. Clin. Sci. 131, 1631–1647 (2017).
Manley, H. J. et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol. Dial. Transplant. 19, 1842–1848 (2004).
Clayton, T. A., Baker, D., Lindon, J. C., Everett, J. R. & Nicholson, J. K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl Acad. Sci. USA 106, 14728–14733 (2009).
Kong, F. et al. Increased plasma exposures of conjugated metabolites of morinidazole in renal failure patients: a critical role of uremic toxins. Drug Metab. Dispos. 45, 593–603 (2017).
Cihlar, T. & Ho, E. S. Fluorescence-based assay for the interaction of small molecules with the human renal organic anion transporter 1. Anal. Biochem. 283, 49–55 (2000).
Khamdang, S. et al. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 303, 534–539 (2002).
Yu, C. P. et al. Effects of nonsteroidal anti-inflammatory drugs on the renal excretion of indoxyl sulfate, a nephro-cardiovascular toxin, in rats. Eur. J. Pharm. Sci. 101, 66–70 (2017).
Morrissey, K. M., Stocker, S. L., Wittwer, M. B., Xu, L. & Giacomini, K. M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 53, 503–529 (2013).
DiNatale, B. C. et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand thatsynergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 115, 89–97 (2010).
Wang, J. et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 281, 22021–22028 (2006).
Wirthgen, E., Hoeflich, A., Rebl, A. & Gunther, J. Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 8, 1957 (2017).
Moroni, F., Cozzi, A., Sili, M. & Mannaioni, G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 119, 133–139 (2012).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H. Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012).
Fujigaki, H., Yamamoto, Y. & Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: focus on cell type differences. Neuropharmacology 112, 264–274 (2017).
Schwarcz, R. & Stone, T. W. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology 112, 237–247 (2017).
Mandi, Y. & Vecsei, L. The kynurenine system and immunoregulation. J. Neural Transm. 119, 197–209 (2012).
Stone, T. W., Stoy, N. & Darlington, L. G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 34, 136–143 (2013).
Agudelo, L. Z. et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 27, 378–392 (2018).
Prentice, K. J. et al. CMPF, a metabolite formed upon prescription omega-3-acid ethyl ester supplementation, prevents and reverses steatosis. EBioMedicine 27, 200–213 (2018).
Tanaka, H. et al. p-Cresyl sulfate induces osteoblast dysfunction through activating JNK and p38 MAPK pathways. Bone 56, 347–354 (2013).
Yang, K. et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol. Lett. 234, 110–119 (2015).
Park, J. S., Choi, H. I., Bae, E. H., Ma, S. K. & Kim, S. W. Paricalcitol attenuates indoxyl sulfate-induced apoptosis through the inhibition of MAPK, Akt, and NF-kB activation in HK-2 cells. Korean J. Intern. Med. 34, 146–155 (2019).
Pegg, A. E. Functions of polyamines in mammals. J. Biol. Chem. 291, 14904–14912 (2016).
Ramani, D., De Bandt, J. P. & Cynober, L. Aliphatic polyamines in physiology and diseases. Clin. Nutr. 33, 14–22 (2014).
Park, M. H., Nishimura, K., Zanelli, C. F. & Valentini, S. R. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38, 491–500 (2010).
Weiger, T. M. & Hermann, A. Cell proliferation, potassium channels, polyamines and their interactions: a mini review. Amino Acids 46, 681–688 (2014).
Tassone, E. J. et al. Uric acid impairs insulin signaling by promoting ENPP1 binding to insulin receptor in human umbilical vein endothelial cells. Front. Endocrinol. 9, 98 (2018).
Spiga, R. et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 37, 1241–1249 (2017).
Liang, W. Y. et al. Uric acid promotes chemokine and adhesion molecule production in vascular endothelium via nuclear factor-κB signaling. Nutr. Metab. Cardiovasc. Dis. 25, 187–194 (2015).
Filiopoulos, V., Hadjiyannakos, D. & Vlassopoulos, D. New insights into uric acid effects on the progression and prognosis of chronic kidney disease. Ren. Fail. 34, 510–520 (2012).
Kielstein, J. T., Fliser, D. & Veldink, H. Asymmetric dimethylarginine and symmetric dimethylarginine: axis of evil or useful alliance? Semin. Dial. 22, 346–350 (2009).
De Deyn, P. P., Vanholder, R. & D’Hooge, R. Nitric oxide in uremia: effects of several potentially toxic guanidino compounds. Kidney Int. Suppl. 84, S25–S28 (2003).
De Deyn, P. P., D’Hooge, R., Van Bogaert, P. P. & Marescau, B. Endogenous guanidino compounds as uremic neurotoxins. Kidney Int. Suppl. 78, S77–S83 (2001).
Chen, J. Y. et al. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 97, 423–428 (2018).
Velenosi, T. J. et al. Untargeted plasma and tissue metabolomics in rats with chronic kidney disease given AST-120. Sci. Rep. 6, 22526 (2016).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Lafaye, A. et al. Profiling of sulfoconjugates in urine by using precursor ion and neutral loss scans in tandem mass spectrometry. Application to the investigation of heavy metal toxicity in rats. J. Mass Spectrom. 39, 655–664 (2004).
John, G. K. et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes 9, E167 (2018).
Yamaguchi, J., Tanaka, T. & Inagi, R. Effect of AST-120 in chronic kidney disease treatment: still a controversy? Nephron 135, 201–206 (2017).
Masereeuw, R. et al. The kidney and uremic toxin removal: glomerulus or tubule? Semin. Nephrol. 34, 191–208 (2014).
Lowenstein, J. & Grantham, J. J. The rebirth of interest in renal tubular function. Am. J. Physiol. Renal Physiol. 310, F1351–F1355 (2016).
Wang, K. & Kestenbaum, B. Proximal tubular secretory clearance: a neglected partner of kidney function. Clin. J. Am. Soc. Nephrol. 13, 1291–1296 (2018).
Leong, S. C. et al. Residual function effectively controls plasma concentrations of secreted solutes in patients on twice weekly hemodialysis. J. Am. Soc. Nephrol. 29, 1992–1999 (2018).
Bhatnagar, V. et al. Analyses of 5′ regulatory region polymorphisms in human SLC22A6 (OAT1) and SLC22A8 (OAT3). J. Hum. Genet. 51, 575–580 (2006).
Xu, G. et al. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)]. Kidney Int. 68, 1491–1499 (2005).
Stanley, L. A. in Pharmacognosy (ed. Delgoda, R.) 527–545 (Academic, 2017).
Almazroo, O. A., Miah, M. K. & Venkataramanan, R. Drug metabolism in the liver. Clin. Liver Dis. 21, 1–20 (2017).
Ishikawa, T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem. Sci. 17, 463–468 (1992).
Döring, B. & Petzinger, E. Phase 0 and phase III transport in various organs: combined concept of phases in xenobiotic transport and metabolism. Drug Metab. Rev. 46, 261–282 (2014).
Petzinger, E. & Geyer, J. Drug transporters in pharmacokinetics. Naunyn Schmiedebergs Arch. Pharmacol. 372, 465–475 (2006).
Eraly, S. A. et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genomics 33, 180–192 (2008).
Vallon, V. et al. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am. J. Physiol. Renal Physiol. 302, F1293–F1299 (2012).
Acknowledgements
The authors’ work referred to in this Review was partly supported by US National Institutes of Health grants DK109392 and HD090259 (U54) to S.K.N.
Reviewer information
Nature Reviews Nephrology thanks T. D. Nolin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, contributed substantially to discussions of the article content, wrote the manuscript and participated in the review or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nigam, S.K., Bush, K.T. Uraemic syndrome of chronic kidney disease: altered remote sensing and signalling. Nat Rev Nephrol 15, 301–316 (2019). https://doi.org/10.1038/s41581-019-0111-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0111-1
This article is cited by
-
AHR is a master regulator of diverse pathways in endogenous metabolism
Scientific Reports (2022)
-
Drug transporters OAT1 and OAT3 have specific effects on multiple organs and gut microbiome as revealed by contextualized metabolic network reconstructions
Scientific Reports (2022)
-
Effects of the l-tyrosine-derived bacterial metabolite p-cresol on colonic and peripheral cells
Amino Acids (2022)
-
Metabolic profiling in children and young adults with autosomal dominant polycystic kidney disease
Scientific Reports (2021)
-
Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study
International Urology and Nephrology (2021)