Abstract
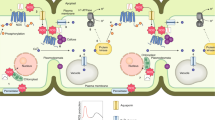
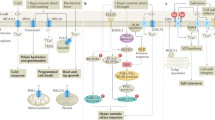
Reactive oxygen species (ROS) are key signalling molecules that enable cells to rapidly respond to different stimuli. In plants, ROS play a crucial role in abiotic and biotic stress sensing, integration of different environmental signals and activation of stress-response networks, thus contributing to the establishment of defence mechanisms and plant resilience. Recent advances in the study of ROS signalling in plants include the identification of ROS receptors and key regulatory hubs that connect ROS signalling with other important stress-response signal transduction pathways and hormones, as well as new roles for ROS in organelle-to-organelle and cell-to-cell signalling. Our understanding of how ROS are regulated in cells by balancing production, scavenging and transport has also increased. In this Review, we discuss these promising developments and how they might be used to increase plant resilience to environmental stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bailey-Serres, J., Parker, J. E., Ainsworth, E. A., Oldroyd, G. E. D. & Schroeder, J. I. Genetic strategies for improving crop yields. Nature 575, 109–118 (2019).
Lesk, C., Rowhani, P. & Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 529, 84–87 (2016).
Intergovernmental Panel on Climate Change. Climate Change 2021: the physical science basis (IPCC, 2021).
Zandalinas, S. I., Fritschi, F. B. & Mittler, R. Global warming, climate change, and environmental pollution: recipe for a multifactorial stress combination disaster. Trends Plant Sci. 26, 588–599 (2021).
Halliwell, B. & Gutteridge, J. M. C. Free Radicals in Biology and Medicine (Oxford Univ. Press, 2015).
Mittler, R. ROS are good. Trends Plant Sci. 22, 11–19 (2017).
Waszczak, C., Carmody, M. & Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 69, 209–236 (2018).
Smirnoff, N. & Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 221, 1197–1214 (2019).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Rodrigues, O. et al. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl Acad. Sci. USA 114, 9200–9205 (2017). This study reveals the physiological importance of AQPs for H2O2 mobilization in plants.
Zou, J. J. et al. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 27, 1445–1460 (2015).
Nie, W. F. et al. Silencing of tomato RBOH1 and MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant Cell Environ. 36, 789–803 (2013).
Suzuki, N., Miller, G., Sejima, H., Harper, J. & Mittler, R. Enhanced seed production under prolonged heat stress conditions in Arabidopsis thaliana plants deficient in cytosolic ascorbate peroxidase 2. J. Exp. Bot. 64, 253–263 (2013).
Kim, M. J., Ciani, S. & Schachtman, D. P. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol. Plant 3, 420–427 (2010).
Liu, D. et al. Tobacco transcription factor bHLH123 improves salt tolerance by activating NADPH oxidase NtRbohE expression. Plant Physiol. 186, 1706–1720 (2021).
Miura, K. et al. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J. 73, 91–104 (2013).
Hu, Y. et al. Silencing of OsGRXS17 in rice improves drought stress tolerance by modulating ROS accumulation and stomatal closure. Sci. Rep. 7, 15950 (2017).
Yin, Y. et al. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 59, 2239–2254 (2018).
Shi, Y. et al. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 39, 1767–1784 (2020).
Martins, L. et al. Redox modification of the iron-sulfur glutaredoxin GRXS17 activates holdase activity and protects plants from heat stress. Plant Physiol. 184, 676–692 (2020).
Chae, H. B. et al. Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Mol. Plant 6, 323–336 (2013).
Wang, L. et al. The Arabidopsis SAFEGUARD1 suppresses singlet oxygen-induced stress responses by protecting grana margins. Proc. Natl Acad. Sci. USA 117, 6918–6927 (2020).
Galvez-Valdivieso, G. et al. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21, 2143–2162 (2009).
Kerchev, P. et al. Lack of GLYCOLATE OXIDASE1, but not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2-deficient Arabidopsis. Plant Physiol. 171, 1704–1719 (2016).
Hipsch, M. et al. Sensing stress responses in potato with whole-plant redox imaging. Plant Physiol. 187, 618–631 (2021).
Koffler, B. E., Luschin-Ebengreuth, N., Stabentheiner, E., Müller, M. & Zechmann, B. Compartment specific response of antioxidants to drought stress in Arabidopsis. Plant Sci. 227, 133–144 (2014).
Zia, A. et al. Protection of the photosynthetic apparatus against dehydration stress in the resurrection plant Craterostigma pumilum. Plant J. 87, 664–680 (2016).
Schwarzländer, M., Fricker, M. D. & Sweetlove, L. J. Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta Bioenerg. 1787, 468–475 (2009).
Babbar, R., Karpinska, B., Grover, A. & Foyer, C. H. Heat-induced oxidation of the nuclei and cytosol. Front. Plant Sci. 11, 617779 (2021).
Torres, M. A., Dangl, J. L. & Jones, J. D. G. G. Arabidopsis gp91 phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl Acad. Sci. USA 99, 517–522 (2002). This study unravels the role of RBOHs in generating ROS during early responses to pathogens.
Kámán-Tóth, E. et al. Contribution of cell wall peroxidase- and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Mol. Plant Pathol. 20, 485–499 (2019).
Xu, Q. et al. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nat. Commun. 10, 5571 (2019).
Liu, R. et al. A Plasmopara viticola RXLR effector targets a chloroplast protein PsbP to inhibit ROS production in grapevine. Plant J. 106, 1557–1570 (2021).
Ding, X., Jimenez-Gongora, T., Krenz, B. & Lozano-Duran, R. Chloroplast clustering around the nucleus is a general response to pathogen perception in Nicotiana benthamiana. Mol. Plant Pathol. 20, 1298–1306 (2019).
Yang, M. et al. Barley stripe mosaic virus γb interacts with glycolate oxidase and inhibits peroxisomal ROS production to facilitate virus infection. Mol. Plant 11, 338–341 (2018).
Bratt, A., Rosenwasser, S., Meyer, A. & Fluhr, R. Organelle redox autonomy during environmental stress. Plant Cell Environ. 39, 1909–1919 (2016).
Mor, A. et al. Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant Physiol. 165, 249–261 (2014).
Niemeyer, J., Scheuring, D., Oestreicher, J., Morgan, B. & Schroda, M. Real-time monitoring of subcellular H2O2 distribution in Chlamydomonas reinhardtii. Plant Cell 33, 2935–2949 (2021).
Exposito-Rodriguez, M., Laissue, P. P., Yvon-Durocher, G., Smirnoff, N. & Mullineaux, P. M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 8, 49 (2017). This article reports chloroplast-to-nucleus signalling during light stress.
Ugalde, J. M. et al. Chloroplast-derived photo-oxidative stress causes changes in H2O2 and EGSH in other subcellular compartments. Plant Physiol. 186, 125–141 (2021).
Mizrachi, A., Creveld, S. G., Shapiro, O. H., Rosenwasser, S. & Vardi, A. Light-dependent single-cell heterogeneity in the chloroplast redox state regulates cell fate in a marine diatom. eLife 8, e47732 (2019).
Nietzel, T. et al. The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol. 221, 1649–1664 (2019).
Marty, L. et al. Arabidopsis glutathione reductase 2 is indispensable in plastids, while mitochondrial glutathione is safeguarded by additional reduction and transport systems. New Phytol. 224, 1569–1584 (2019).
Müller-Schüssele, S. J. et al. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. Plant J. 103, 1140–1154 (2020).
Haber, Z. et al. Resolving diurnal dynamics of the chloroplastic glutathione redox state in Arabidopsis reveals its photosynthetically derived oxidation. Plant Cell 33, 1828–1844 (2021).
Shapiguzov, A. et al. Arabidopsis RCD1 coordinates chloroplast and mitochondrial functions through interaction with ANAC transcription factors. eLife 8, e43284 (2019).
Nomura, H. et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 3, 926 (2012).
Bienert, G. P. & Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596–1604 (2014).
Estavillo, G. M. et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23, 3992–4012 (2011). Together with Shapiguzov et al. (2019), this study reveals a role for ROS in retrograde signalling.
Inupakutika, M. A., Sengupta, S., Devireddy, A. R., Azad, R. K. & Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 67, 5933–5943 (2016).
Jabłońska, J. & Tawfik, D. S. The evolution of oxygen-utilizing enzymes suggests early biosphere oxygenation. Nat. Ecol. Evol. 5, 442–448 (2021).
Huang, J. et al. Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. Proc. Natl Acad. Sci. USA 116, 20256–20261 (2019).
Nietzel, T. et al. Redox-mediated kick-start of mitochondrial energy metabolism drives resource-efficient seed germination. Proc. Natl Acad. Sci. USA 117, 741–751 (2020).
De Smet, B. et al. In vivo detection of protein cysteine sulfenylation in plastids. Plant J. 97, 765–778 (2019).
Leferink, N. G. H., Duijn, E., van, Barendregt, A., Heck, A. J. R. & van Berkel, W. J. H. Galactonolactone dehydrogenase requires a redox-sensitive thiol for optimal production of vitamin C. Plant Physiol. 150, 596–605 (2009).
Chan, K. X. et al. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc. Natl Acad. Sci. USA 113, E4567–E4576 (2016).
Zaffagnini, M. et al. Tuning cysteine reactivity and sulfenic acid stability by protein microenvironment in glyceraldehyde-3-phosphate dehydrogenases of Arabidopsis thaliana. Antioxid. Redox Signal. 24, 502–517 (2016).
Akter, S. et al. Chemical proteomics reveals new targets of cysteine sulfinic acid reductase. Nat. Chem. Biol. 14, 995–1004 (2018).
Puerto-Galán, L., Pérez-Ruiz, J. M., Guinea, M. & Cejudo, F. J. The contribution of NADPH thioredoxin reductase C (NTRC) and sulfiredoxin to 2-Cys peroxiredoxin overoxidation in Arabidopsis thaliana chloroplasts. J. Exp. Bot. 66, 2957–2966 (2015).
Iglesias-Baena, I., Barranco-Medina, S., Sevilla, F. & Lázaro, J.-J. The dual-targeted plant sulfiredoxin retroreduces the sulfinic form of atypical mitochondrial peroxiredoxin. Plant Physiol. 155, 944–955 (2011).
Bender, K. W. et al. Glutaredoxin AtGRXC2 catalyses inhibitory glutathionylation of Arabidopsis BRI1-associated receptor-like kinase 1 (BAK1) in vitro. Biochem. J. 467, 399–413 (2015).
Niazi, A. K. et al. Cytosolic isocitrate dehydrogenase from Arabidopsis thaliana is regulated by glutathionylation. Antioxidants 8, 16 (2019).
Jacques, S. et al. Protein methionine sulfoxide dynamics in Arabidopsis thaliana under oxidative stress. Mol. Cell. Proteom. 14, 1217–1229 (2015).
Trnka, D. et al. Molecular basis for the distinct functions of redox-active and FeS-transfering glutaredoxins. Nat. Commun. 11, 3445 (2020).
Tarrago, L. et al. Regeneration mechanisms of Arabidopsis thaliana methionine sulfoxide reductases B by glutaredoxins and thioredoxins. J. Biol. Chem. 284, 18963–18971 (2009).
Tossounian, M. A. et al. Disulfide bond formation protects Arabidopsis thaliana glutathione transferase tau 23 from oxidative damage. Biochim. Biophys. Acta Gen. Subj. 1862, 775–789 (2018).
Daloso, D. M. et al. Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proc. Natl Acad. Sci. USA 112, E1392–E1400 (2015).
Attacha, S. et al. Glutathione peroxidase-like enzymes cover five distinct cell compartments and membrane surfaces in Arabidopsis thaliana. Plant Cell Environ. 40, 1281–1295 (2017).
Liebthal, M., Schuetze, J., Dreyer, A., Mock, H.-P. & Dietz, K.-J. Redox conformation-specific protein–protein interactions of the 2-cysteine peroxiredoxin in Arabidopsis. Antioxidants 9, 515 (2020).
Delaunay, A., Pflieger, D., Barrault, M. B., Vinh, J. & Toledano, M. B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471–481 (2002). This study discovers that thiol peroxidases function as sensors of H2O2.
Miao, Y. et al. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18, 2749–2766 (2006).
Foyer, C. H. & Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 18, 2087–2090 (2013).
Rattanawong, K. et al. Regulatory functions of ROS dynamics via glutathione metabolism and glutathione peroxidase activity in developing rice zygote. Plant J. 108, 1097–1115 (2021).
Han, Y. et al. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 18, 2106–2121 (2013).
Terai, Y. et al. Dehydroascorbate reductases and glutathione set a threshold for high-light–induced ascorbate accumulation. Plant Physiol. 183, 112–122 (2020).
Zhang, T. et al. Glutathione-dependent denitrosation of GSNOR1 promotes oxidative signalling downstream of H2O2. Plant Cell Environ. 43, 1175–1191 (2020).
Gupta, R. & Luan, S. Redox control of protein tyrosine phosphatases and mitogen-activated protein kinases in plants. Plant Physiol. 132, 1149–1152 (2003).
Murata, Y., Pei, Z. M., Mori, I. C. & Schroeder, J. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13, 2513–2523 (2001).
Silver, D. M. et al. Insight into the redox regulation of the phosphoglucan phosphatase SEX4 involved in starch degradation. FEBS J. 280, 538–548 (2013).
Kovtun, Y., Chiu, W. L., Tena, G. & Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl Acad. Sci. USA 97, 2940–2945 (2000).
Matsushita, M., Nakamura, T., Moriizumi, H., Miki, H. & Takekawa, M. Stress-responsive MTK1 SAPKKK serves as a redox sensor that mediates delayed and sustained activation of SAPKs by oxidative stress. Sci. Adv. 6, eaay9778 (2020).
Byrne, D. P. et al. Aurora A regulation by reversible cysteine oxidation reveals evolutionarily conserved redox control of Ser/Thr protein kinase activity. Sci. Signal. 13, eaax2713 (2020).
Després, C. et al. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15, 2181–2191 (2003).
Lindermayr, C., Sell, S., Müller, B., Leister, D. & Durner, J. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 22, 2894–2907 (2010).
Tavares, C. P. et al. S-nitrosylation influences the structure and DNA binding activity of AtMYB30 transcription factor from Arabidopsis thaliana. Biochim. Biophys. Acta Prot. Proteom. 1844, 810–817 (2014).
Liu, H. & Charng, Y. Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol. 163, 276–290 (2013).
Albertos, P. et al. Redox feedback regulation of ANAC089 signaling alters seed germination and stress response. Cell Rep. 35, 109263 (2021).
Lee, E. S. et al. Redox-dependent structural switch and CBF activation confer freezing tolerance in plants. Nat. Plants 7, 914–922 (2021).
Charbonnel, C. et al. The siRNA suppressor RTL1 is redox-regulated through glutathionylation of a conserved cysteine in the double-stranded-RNA-binding domain. Nucleic Acids Res. 45, 11891–11907 (2017).
Garcia-Mata, C. et al. A minimal cysteine motif required to activate the SKOR K+channel of Arabidopsis by the reactive oxygen species H2O2. J. Biol. Chem. 285, 29286–29294 (2010).
Tian, Y. et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 9, 1063 (2018).
Wu, F. et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578, 577–581 (2020). This report identifies HPCA1 as a leucine-rich repeat sensor for H2O2.
Laohavisit, A. et al. Quinone perception in plants via leucine-rich-repeat receptor-like kinases. Nature 587, 92–97 (2020).
Chae, H. B. et al. Redox sensor QSOX1 regulates plant immunity by targeting GSNOR to modulate ROS generation. Mol. Plant 14, 1312–1327 (2021).
Green, M. N. et al. Structure of the Arabidopsis thaliana glutamate receptor-like channel GLR3.4. Mol. Cell 81, 3216–3226.e8 (2021).
Hao, H. et al. Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 26, 1729–1745 (2014).
Nagano, M. et al. Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice. Plant Cell 28, 1966–1983 (2016).
Martinière, A. et al. Osmotic stress activates two reactive oxygen species pathways with distinct effects on protein nanodomains and diffusion. Plant Physiol. 179, 1581–1593 (2019).
Nühse, T. S., Bottrill, A. R., Jones, A. M. E. & Peck, S. C. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 51, 931–940 (2007).
Ogasawara, Y. et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 283, 8885–8892 (2008).
Kimura, S. et al. CRK2 and C-terminal phosphorylation of NADPH oxidase RBOHD regulate reactive oxygen species production in Arabidopsis. Plant Cell 32, 1063–1080 (2020).
Yu, H. et al. Suppression of innate immunity mediated by the CDPK-Rboh complex is required for rhizobial colonization in Medicago truncatula nodules. New Phytol. 220, 425–434 (2018).
Lee, J., Nguyen, H. H., Park, Y., Lin, J. & Hwang, I. Spatial regulation of RBOHD via AtECA4-mediated recycling and clathrin-mediated endocytosis contributes to ROS accumulation upon salt stress response but not flg22-induced immune response. Plant J. 109, 816–830 (2022).
Shen, J. et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 32, 1000–1017 (2020).
Lee, D. H. et al. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 11, 1838 (2020).
Zhang, Y. et al. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21, 2357–2377 (2009).
Yun, B.-W. et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478, 264–268 (2011). This study finds that RBOH is regulated by S-nitrosylation.
Dubiella, U. et al. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl Acad. Sci. USA 110, 8744–8749 (2013).
Kadota, Y. et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54, 43–55 (2014).
Chen, D. et al. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat. Commun. 8, 2265 (2017).
Zhang, M. et al. The MAP4 kinase SIK1 ensures robust extracellular ROS burst and antibacterial immunity in plants. Cell Host Microbe 24, 379–391 (2018).
Han, J. P. et al. Fine-tuning of RBOHF activity is achieved by differential phosphorylation and Ca2+binding. New Phytol. 221, 1935–1949 (2019).
Wang, P. et al. Mapping proteome-wide targets of protein kinases in plant stress responses. Proc. Natl Acad. Sci. USA 117, 3270–3280 (2020).
Foreman, J. et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 422–446 (2003). This study links RBOH function to plant development.
Kaya, H. et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26, 1069–1080 (2014).
Miller, G. et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2, ra45 (2009). This article reports the discovery of the ROS wave.
Mangano, S., Juárez, S. P. D. & Estevez, J. M. ROS regulation of polar growth in plant cells. Plant Physiol. 171, 1593–1605 (2016).
Voothuluru, P. & Sharp, R. E. Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress. I. Increased levels are specific to the apical region of growth maintenance. J. Exp. Bot. 64, 1223–1233 (2013).
Maurel, C. et al. Aquaporins in plants. Physiol. Rev. 95, 1321–1358 (2015).
Bienert, G. P. et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 (2007).
Törnroth-Horsefield, S. et al. Structural mechanism of plant aquaporin gating. Nature 439, 688–694 (2005).
Qing, D. et al. Quantitative and functional phosphoproteomic analysis reveals that ethylene regulates water transport via the C-terminal phosphorylation of aquaporin PIP2;1 in Arabidopsis. Mol. Plant 9, 158–174 (2016).
Qiu, J., McGaughey, S. A., Groszmann, M., Tyerman, S. D. & Byrt, C. S. Phosphorylation influences water and ion channel function of AtPIP2;1. Plant Cell Environ. 43, 2428–2442 (2020).
Wang, H., Schoebel, S., Schmitz, F., Dong, H. & Hedfalk, K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 1862, 183065 (2020).
Almasalmeh, A., Krenc, D., Wu, B. & Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 281, 647–656 (2014).
Golani, Y. et al. Inositol polyphosphate phosphatidylinositol 5-phosphatase9 (At5PTase9) controls plant salt tolerance by regulating endocytosis. Mol. Plant 6, 1781–1794 (2013).
Davletova, S. et al. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17, 268–281 (2005).
Vanderauwera, S. et al. Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl Acad. Sci. USA 108, 1711–1716 (2011).
Zhu, J. K. Abiotic stress signaling and responses in plants. Cell 167, 313–324 (2016).
Yang, H. et al. S-nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol. 167, 1604–1615 (2015).
Gadjev, I. et al. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 141, 436–445 (2006).
Warren, E. A. K., Netterfield, T. S., Sarkar, S., Kemp, M. L. & Payne, C. K. Spatially-resolved intracellular sensing of hydrogen peroxide in living cells. Sci. Rep. 5, 16929 (2015).
Fu, Z. Q. & Dong, X. Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64, 839–863 (2013).
Giesguth, M., Sahm, A., Simon, S. & Dietz, K.-J. Redox-dependent translocation of the heat shock transcription factor AtHSFA8 from the cytosol to the nucleus in Arabidopsis thaliana. FEBS Lett. 589, 718–725 (2015).
Meng, X. et al. ANAC017 coordinates organellar functions and stress responses by reprogramming retrograde signaling. Plant Physiol. 180, 634–653 (2019).
D’Alessandro, S., Ksas, B. & Havaux, M. Decoding β-cyclocitral-mediated retrograde signaling reveals the role of a detoxification response in plant tolerance to photooxidative stress. Plant Cell 30, 2495–2511 (2018).
Pulido, P., Cazalis, R. & Cejudo, F. J. An antioxidant redox system in the nucleus of wheat seed cells suffering oxidative stress. Plant J. 57, 132–145 (2009).
Marchal, C. et al. NTR/NRX define a new thioredoxin system in the nucleus of Arabidopsis thaliana cells. Mol. Plant 7, 30–44 (2014).
Li, Y., Liu, W., Zhong, H., Zhang, H.-L. & Xia, Y. Redox-sensitive bZIP68 plays a role in balancing stress tolerance with growth in Arabidopsis. Plant J. 100, 768–783 (2019).
Gutsche, N. & Zachgo, S. The N-terminus of the floral Arabidopsis TGA transcription factor PERIANTHIA mediates redox-sensitive DNA-binding. PLoS ONE 11, e0153810 (2016).
Zhou, M. et al. Redox rhythm reinforces the circadian clock to gate immune response. Nature 523, 472–476 (2015).
Sandalio, L. M., Peláez-Vico, M. A., Molina-Moya, E. & Romero-Puertas, M. C. Peroxisomes as redox-signaling nodes in intracellular communication and stress responses. Plant Physiol. 186, 22–35 (2021).
Caplan, J. L. et al. Chloroplast stromules function during innate immunity. Dev. Cell 34, 45–57 (2015).
Brunkard, J. O., Runkel, A. M. & Zambryski, P. C. From the cover: chloroplasts extend stromules independently and in response to internal redox signals. Proc. Natl Acad. Sci. USA 112, 10044–10049 (2015).
Gray, J. C. et al. Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J. 69, 387–398 (2012).
Rodríguez-Serrano, M., Romero-Puertas, M. C., Sanz-Fernández, M., Hu, J. & Sandalio, L. M. Peroxisomes extend peroxules in a fast response to stress via a reactive oxygen species-mediated induction of the peroxin PEX11a. Plant Physiol. 171, 1665–1674 (2016).
Jaipargas, E.-A., Mathur, N., Bou Daher, F., Wasteneys, G. O. & Mathur, J. High light intensity leads to increased peroxule-mitochondria interactions in plants. Front. Cell Dev. Biol. 4, 6 (2016).
Jaipargas, E.-A., Barton, K. A., Mathur, N. & Mathur, J. Mitochondrial pleomorphy in plant cells is driven by contiguous ER dynamics. Front. Plant Sci. 6, 783 (2015).
Schmidt, R., Kunkowska, A. B. & Schippers, J. H. M. Role of reactive oxygen species during cell expansion in leaves. Plant Physiol. 172, 2098–2106 (2016).
Koussevitzky, S. et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719 (2007).
Fichman, Y., Myers, R. J., Grant, D. G. & Mittler, R. Plasmodesmata-localized proteins and ROS orchestrate light-induced rapid systemic signaling in Arabidopsis. Sci. Signal. 14, eabf0322 (2021).
Devireddy, A. R., Liscum, E. & Mittler, R. Phytochrome B is required for systemic stomatal responses and reactive oxygen species signaling during light stress. Plant Physiol. 184, 1563–1572 (2020).
Jung, J. H. et al. Phytochromes function as thermosensors in Arabidopsis. Science 354, 886–889 (2016).
Legris, M. et al. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354, 897–900 (2016).
Finka, A., Cuendet, A. F. H., Maathuis, F. J. M., Saidi, Y. & Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24, 3333–3348 (2012).
Yuan, F. et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514, 367–371 (2014).
Basu, D. & Haswell, E. S. The mechanosensitive ion channel MSL10 potentiates responses to cell swelling in Arabidopsis seedlings. Curr. Biol. 30, 2716–2728.e6 (2020).
Griffin, J. H. C., Prado, K., Sutton, P. & Toledo-Ortiz, G. Coordinating light responses between the nucleus and the chloroplast, a role for plant cryptochromes and phytochromes. Physiol. Plant 169, 515–528 (2020).
Feng, W. et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666–675 (2018).
Xiong, H. et al. Photosynthesis-independent production of reactive oxygen species in the rice bundle sheath during high light is mediated by NADPH oxidase. Proc. Natl Acad. Sci. USA 118, e2022702118 (2021).
Suzuki, N. et al. Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. Plant J. 84, 760–772 (2015).
Choudhury, F. K., Devireddy, A. R., Azad, R. K., Shulaev, V. & Mittler, R. Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol. 178, 1461–1472 (2018).
Kollist, H. et al. Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci. 24, 25–37 (2019).
Zandalinas, S. I., Sengupta, S., Burks, D., Azad, R. K. & Mittler, R. Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J. 98, 126–141 (2019).
Wang, Q. et al. Transcriptome analysis of upland cotton revealed novel pathways to scavenge reactive oxygen species (ROS) responding to Na2SO4 tolerance. Sci. Rep. 11, 8670 (2021).
Ameye, M. et al. Metabolomics reveal induction of ROS production and glycosylation events in wheat upon exposure to the green leaf volatile Z-3-hexenyl acetate. Front. Plant Sci. 11, 596271 (2020).
Glauser, G. et al. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J. Biol. Chem. 284, 34506–34513 (2009).
Yuan, L.-B. et al. Jasmonate regulates plant responses to postsubmergence reoxygenation through transcriptional activation of antioxidant synthesis. Plant Physiol. 173, 1864–1880 (2017).
Suzuki, N. et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25, 3553–3569 (2013).
Yang, C. et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 89, 338–353 (2017).
Karpinski, S. et al. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284, 654–657 (1999). This article reports the discovery of systemic acquired acclimatization in plants.
Locato, V., Cimini, S. & De Gara, L. ROS and redox balance as multifaceted players of cross-tolerance: epigenetic and retrograde control of gene expression. J. Exp. Bot. 69, 3373–3391 (2018).
Koussevitzky, S. et al. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 283, 34197–34203 (2008).
Zandalinas, S. I. et al. The impact of multifactorial stress combination on plant growth and survival. New Phytol. 230, 1034–1048 (2021).
Jagodzik, P., Tajdel-Zielinska, M., Ciesla, A., Marczak, M. & Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 9, 1387 (2018).
Muhlemann, J. K., Younts, T. L. B. & Muday, G. K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl Acad. Sci. USA 115, E11188–E11197 (2018).
Pfeilmeier, S. et al. The plant NADPH oxidase RBOHD is required for microbiota homeostasis in leaves. Nat. Microbiol. 6, 852–864 (2021).
Schmidt, R. et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 25, 2115–2131 (2013).
Pérez-Salamó, I. et al. The heat shock factor A4A confers salt tolerance and is regulated by oxidative stress and the mitogen-activated protein kinases MPK3 and MPK6. Plant Physiol. 165, 319–334 (2014).
De Clercq, I. et al. Integrative inference of transcriptional networks in Arabidopsis yields novel ROS signalling regulators. Nat. Plants 7, 500–513 (2021). This study uses artificial intelligence-based network analysis tools to study ROS signalling.
Ohama, N., Sato, H., Shinozaki, K. & Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 22, 53–65 (2017).
Qiu, J. L. et al. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 27, 2214–2221 (2008).
Meng, X. et al. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 25, 1126–1142 (2013).
Tran, D. et al. Post-transcriptional regulation of GORK channels by superoxide anion contributes to increases in outward-rectifying K+ currents. New Phytol. 198, 1039–1048 (2013).
Iyer, N. J., Jia, X., Sunkar, R., Tang, G. & Mahalingam, R. microRNAs responsive to ozone-induced oxidative stress in Arabidopsis thaliana. Plant Signal. Behav. 7, 484–491 (2012).
He, H. et al. The Arabidopsis mediator complex subunit 8 regulates oxidative stress responses. Plant Cell 33, 2032–2057 (2021).
Mabuchi, K. et al. MYB30 links ROS signaling, root cell elongation, and plant immune responses. Proc. Natl Acad. Sci. USA 115, 4710–4719 (2018).
Fichman, Y. et al. MYB30 orchestrates systemic reactive oxygen signaling and plant acclimation. Plant Physiol. 184, 666–675 (2020).
Srivastava, R., Deng, Y., Shah, S., Rao, A. G. & Howell, S. H. BINDING PROTEIN is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in Arabidopsis. Plant Cell 25, 1416–1429 (2013).
Dickinson, P. J. et al. Chloroplast signaling gates thermotolerance in Arabidopsis. Cell Rep. 22, 1657–1665 (2018).
Daudi, A. et al. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24, 275–287 (2012).
Tada, Y. et al. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 321, 952–956 (2008).
Jayakannan, M. et al. The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. J. Exp. Bot. 66, 1865–1875 (2015).
Davletova, S., Schlauch, K., Coutu, J. & Mittler, R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 139, 847–856 (2005).
Shaikhali, J. et al. The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol. 8, 48 (2008).
Zandalinas, S. I. et al. Systemic signaling during abiotic stress combination in plants. Proc. Natl Acad. Sci. USA 117, 13810–13820 (2020).
Fichman, Y., Miller, G. & Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 12, 1203–1210 (2019). This article reports whole-plant live imaging of ROS.
Marhavý, P. et al. Single-cell damage elicits regional, nematode-restricting ethylene responses in roots. EMBO J. 38, e100972 (2019).
Lew, T. T. S. et al. Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat. Plants 6, 404–415 (2020). This study uses nanosensors to monitor ROS production during stress.
Zandalinas, S. I., Fichman, Y. & Mittler, R. Vascular bundles mediate systemic reactive oxygen signaling during light stress in Arabidopsis. Plant Cell 32, 3425–3435 (2020).
Goh, K. Y. et al. Impaired mitochondrial network excitability in failing guinea-pig cardiomyocytes. Cardiovasc. Res. 109, 79–89 (2016).
Kuznetsov, A. V., Javadov, S., Saks, V., Margreiter, R. & Grimm, M. Synchronism in mitochondrial ROS flashes, membrane depolarization and calcium sparks in human carcinoma cells. Biochim. Biophys. Acta Bioenerg. 1858, 418–431 (2017).
Zhou, L. et al. A reaction-diffusion model of ROS-induced ROS release in a mitochondrial network. PLoS Comput. Biol. 6, e1000657 (2010).
Fichman, Y. & Mittler, R. A systemic whole-plant change in redox levels accompanies the rapid systemic response to wounding. Plant Physiol. 186, 4–8 (2021). This article reports the discovery of the redox wave.
Devireddy, A. R., Zandalinas, S. I., Gómez-Cadenas, A., Blumwald, E. & Mittler, R. Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci. Signal. 11, eaam9514 (2018).
Devireddy, A. R., Arbogast, J. & Mittler, R. Coordinated and rapid whole-plant systemic stomatal responses. New Phytol. 225, 21–25 (2020).
Steinbeck, J. et al. In vivo NADH/NAD+ biosensing reveals the dynamics of cytosolic redox metabolism in plants. Plant Cell 32, 3324–3345 (2020).
Mittler, R., Vanderauwera, S., Gollery, M. & Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498 (2004).
Acknowledgements
The authors apologize to the many authors whose important work could not be referred to. This work was supported by funding from the US National Science Foundation (IOS-2110017, IOS-1353886, MCB-1936590 and IOS-1932639), The US National Institutes of Health (GM111364), the Interdisciplinary Plant Group, University of Missouri, Research Foundation — Flanders (project G0D7914N) and the Excellence of Science Research project 30829584.
Author information
Authors and Affiliations
Contributions
R.M., S.I.Z., Y.F. and F.V.B. wrote the Review, designed the figures and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Christine Foyer, Nicholas Smirnoff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Acclimatization
-
Also referred to as ‘acclimation’, a process by which plants adjust their metabolism, physiology and biochemistry to become accustomed to changes in their growth conditions or environment.
- Aquaporins
-
(AQPs). Transmembrane water channel proteins that allow the diffusion of H2O2 from one side of the membrane to the other in a regulated manner.
- Photosystems I and II
-
Multiprotein complexes that reside on the thylakoid membranes inside chloroplasts and participate in the harvesting of light energy for the purpose of CO2 fixation and sugar biosynthesis.
- Photorespiration
-
A biochemical pathway that results in the accumulation of H2O2 in peroxisomes, triggered when CO2 concentrations are limited in C3 plants.
- C3 plants
-
A large group of plants in which the initial product of the assimilation of CO2 through photosynthesis is 3-phosphoglycerate, which contains three carbon atoms.
- Stomata
-
Specialized pore structures found in the epidermal layer of plants and used for gas exchange with the atmosphere.
- Nucleophilic attack
-
Attack of an electron-rich species (the nucleophile) on an electron-deficient species (the electrophile), forming a new bond between the nucleophile and the electrophile.
- Yap1
-
A redox-regulated transcription factor that is essential for yeast survival under conditions of oxidative stress.
- Type 2C serine/threonine protein phosphatase 2A
-
(PP2A). A family of phosphatases that generally function as negative regulators of different stress responses in plants and are inhibited by reactive oxygen species-induced redox reactions.
- Dicer proteins
-
Endoribonucleases that cleave double-stranded RNA and pre-microRNAs into short double-stranded RNA fragments such as small interfering RNA and microRNA.
- Brassinosteroid
-
Member of a class of polyhydroxysteroids that function as plant hormones involved in many developmental processes and responses to stress.
- Leucine-rich repeat receptor kinase
-
A large protein family in plants composed of a leucine-rich repeat-containing extracellular domain, a transmembrane domain and an intracellular kinase domain, involved in developmental processes and stress responses.
- Quinones
-
A redox-active class of cyclic organic compounds containing two carbonyl groups, involved in many electron transport reactions and signalling processes.
- EF-hand domains
-
Helix–loop–helix structural domains with an E and F structural orientation of the two α-helices, found in many calcium-binding proteins.
- Stromules, peroxules and matrixules
-
Dynamic tubular membrane structures extending from the surface of chloroplasts, peroxisomes and mitochondria, respectively, used for the transport of signals between different organelles and the nucleus.
- Salicylic acid
-
A phytohormone, characterized by an aromatic ring and a hydroxy group, involved in the response of plants to different biotic and abiotic stresses.
- Plasmodesmata
-
Small channels or pores that transverse the plant cell walls connecting the cytoplasm and plasma membrane of neighbouring cells with each other, establishing metabolic and signalling bridges between cells.
- Mediator complex
-
An important component of the eukaryotic transcriptional machinery, linking different transcription factors with RNA polymerase II.
- Unfolded protein response
-
A cellular stress response pathway triggered by the presence of unfolded proteins inside the endoplasmic reticulum.
Rights and permissions
About this article
Cite this article
Mittler, R., Zandalinas, S.I., Fichman, Y. et al. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol 23, 663–679 (2022). https://doi.org/10.1038/s41580-022-00499-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-022-00499-2
This article is cited by
-
Effect of salinity on growth and biochemical responses of brinjal varieties: implications for salt tolerance and antioxidant mechanisms
BMC Plant Biology (2024)
-
Enhancing drought, heat shock, and combined stress tolerance in Myrobalan 29C rootstocks with foliar application of potassium nitrate
BMC Plant Biology (2024)
-
Defense Responses of Different Rice Varieties Affect Growth Performance and Food Utilization of Cnaphalocrocis medinalis Larvae
Rice (2024)
-
Extracellular niche establishment by plant pathogens
Nature Reviews Microbiology (2024)
-
Decoding early stress signaling waves in living plants using nanosensor multiplexing
Nature Communications (2024)