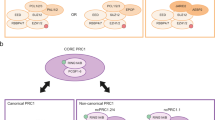
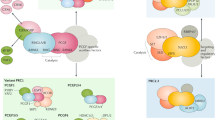
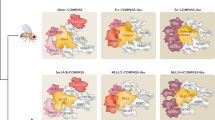
Abstract
More than 80 years ago, the first Polycomb-related phenotype was identified in Drosophila melanogaster. Later, a group of diverse genes collectively called Polycomb group (PcG) genes were identified based on common mutant phenotypes. PcG proteins, which are well-conserved in animals, were originally characterized as negative regulators of gene transcription during development and subsequently shown to function in various biological processes; their deregulation is associated with diverse phenotypes in development and in disease, especially cancer. PcG proteins function on chromatin and can form two distinct complexes with different enzymatic activities: Polycomb repressive complex 1 (PRC1) is a histone ubiquitin ligase and PRC2 is a histone methyltransferase. Recent studies have revealed the existence of various mutually exclusive PRC1 and PRC2 variants. In this Review, we discuss new concepts concerning the biochemical and molecular functions of these new PcG complex variants, and how their epigenetic activities are involved in mammalian development and cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
09 May 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41580-022-00495-6
References
Piunti, A. & Shilatifard, A. Epigenetic balance of gene expression by Polycomb and COMPASS families. Science 352, aad9780 (2016).
Pasini, D., Bracken, A. P., Jensen, M. R., Lazzerini Denchi, E. & Helin, K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 23, 4061–4071 (2004).
Cao, R. & Zhang, Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED–EZH2 complex. Mol. Cell 15, 57–67 (2004).
van Mierlo, G., Veenstra, G. J. C., Vermeulen, M. & Marks, H. The complexity of PRC2 subcomplexes. Trends Cell Biol. 29, 660–671 (2019).
Wang, H. et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 (2004). This study discovers the catalytic activity of PRC1.
Bentley, M. L. et al. Recognition of UbcH5c and the nucleosome by the Bmi1/Ring1b ubiquitin ligase complex. EMBO J. 30, 3285–3297 (2011).
Gao, Z. et al. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell 45, 344–356 (2012). This study systematically identifies biochemically distinct PRC1 variants.
Pengelly, A. R., Copur, O., Jackle, H., Herzig, A. & Muller, J. A histone mutant reproduces the phenotype caused by loss of histone-modifying factor Polycomb. Science 339, 698–699 (2013).
Tamburri, S. et al. Histone H2AK119 mono-ubiquitination is essential for Polycomb-mediated transcriptional repression. Mol. Cell 77, 840–856.e5 (2020).
Blackledge, N. P. et al. PRC1 catalytic activity is central to Polycomb system function. Mol. Cell 77, 857–874.e9 (2020).
Hansen, K. H. et al. A model for transmission of the H3K27me3 epigenetic mark. Nat. Cell Biol. 10, 1291–1300 (2008).
Pengelly, A. R., Kalb, R., Finkl, K. & Muller, J. Transcriptional repression by PRC1 in the absence of H2A monoubiquitylation. Genes Dev. 29, 1487–1492 (2015).
Francis, N. J., Kingston, R. E. & Woodcock, C. L. Chromatin compaction by a Polycomb group protein complex. Science 306, 1574–1577 (2004).
Eskeland, R. et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol. Cell 38, 452–464 (2010).
Shao, Z. et al. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98, 37–46 (1999).
Lagarou, A. et al. dKDM2 couples histone H2A ubiquitylation to histone H3 demethylation during Polycomb group silencing. Genes Dev. 22, 2799–2810 (2008).
Tavares, L. et al. RYBP–PRC1 complexes mediate H2A ubiquitylation at Polycomb target sites independently of PRC2 and H3K27me3. Cell 148, 664–678 (2012).
Laugesen, A., Hojfeldt, J. W. & Helin, K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol. Cell 74, 8–18 (2019).
Wang, R. et al. Polycomb group targeting through different binding partners of RING1B C-terminal domain. Structure 18, 966–975 (2010).
Messmer, S., Franke, A. & Paro, R. Analysis of the functional role of the Polycomb chromo domain in Drosophila melanogaster. Genes Dev. 6, 1241–1254 (1992).
Cao, R. et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002).
Czermin, B. et al. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111, 185–196 (2002).
Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of zeste protein. Genes Dev. 16, 2893–2905 (2002).
Kaustov, L. et al. Recognition and specificity determinants of the human Cbx chromodomains. J. Biol. Chem. 286, 521–529 (2011).
Zhen, C. Y. et al. Live-cell single-molecule tracking reveals co-recognition of H3K27me3 and DNA targets Polycomb Cbx7–PRC1 to chromatin. eLife 5, e17667 (2016).
Bernstein, E. et al. Mouse Polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol. Cell Biol. 26, 2560–2569 (2006).
Plys, A. J. et al. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 33, 799–813 (2019).
O’Loghlen, A. et al. MicroRNA regulation of Cbx7 mediates a switch of Polycomb orthologs during ESC differentiation. Cell Stem Cell 10, 33–46 (2012).
Morey, L. et al. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell 10, 47–62 (2012).
Pemberton, H. et al. Genome-wide co-localization of Polycomb orthologs and their effects on gene expression in human fibroblasts. Genome Biol. 15, R23 (2014).
Vandamme, J., Volkel, P., Rosnoblet, C., Le Faou, P. & Angrand, P. O. Interaction proteomics analysis of Polycomb proteins defines distinct PRC1 complexes in mammalian cells. Mol. Cell Proteom. 10, M110 002642 (2011).
Levine, S. S. et al. The core of the Polycomb repressive complex is compositionally and functionally conserved in flies and humans. Mol. Cell Biol. 22, 6070–6078 (2002).
Peterson, A. J. et al. A domain shared by the Polycomb group proteins Scm and ph mediates heterotypic and homotypic interactions. Mol. Cell Biol. 17, 6683–6692 (1997).
Kim, C. A., Gingery, M., Pilpa, R. M. & Bowie, J. U. The SAM domain of polyhomeotic forms a helical polymer. Nat. Struct. Biol. 9, 453–457 (2002).
Pachano, T., Crispatzu, G. & Rada-Iglesias, A. Polycomb proteins as organizers of 3D genome architecture in embryonic stem cells. Brief Funct. Genomics 18, 358–366 (2019).
Scelfo, A. et al. Functional landscape of PCGF proteins reveals both RING1A/B-dependent-and RING1A/B-independent-specific activities. Mol. Cell 74, 1037–1052.e7 (2019).
Fursova, N. A. et al. Synergy between variant PRC1 complexes defines Polycomb-mediated gene repression. Mol. Cell 74, 1020–1036.e8 (2019).
Cao, R., Tsukada, Y. & Zhang, Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20, 845–854 (2005).
Blackledge, N. P. et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and Polycomb domain formation. Cell 157, 1445–1459 (2014).
Endoh, M. et al. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development 135, 1513–1524 (2008).
Connelly, K. E. & Dykhuizen, E. C. Compositional and functional diversity of canonical PRC1 complexes in mammals. Biochim. Biophys. Acta Gene Regul. Mech. 1860, 233–245 (2017).
Garcia, E., Marcos-Gutierrez, C., del Mar Lorente, M., Moreno, J. C. & Vidal, M. RYBP, a new repressor protein that interacts with components of the mammalian Polycomb complex, and with the transcription factor YY1. EMBO J. 18, 3404–3418 (1999).
Arrigoni, R. et al. The Polycomb-associated protein Rybp is a ubiquitin binding protein. FEBS Lett. 580, 6233–6241 (2006).
Boyer, L. A. et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 (2006). This study is one of the earliest to identify PcG target genes in mammals.
Morey, L., Aloia, L., Cozzuto, L., Benitah, S. A. & Di Croce, L. RYBP and Cbx7 define specific biological functions of Polycomb complexes in mouse embryonic stem cells. Cell Rep. 3, 60–69 (2013).
Rose, N. R. et al. RYBP stimulates PRC1 to shape chromatin-based communication between Polycomb repressive complexes. eLife 5, e18591 (2016).
Farcas, A. M. et al. KDM2B links the Polycomb repressive complex 1 (PRC1) to recognition of CpG islands. eLife 1, e00205 (2012).
He, J. et al. Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nat. Cell Biol. 15, 373–384 (2013).
Wu, X., Johansen, J. V. & Helin, K. Fbxl10/Kdm2b recruits Polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol. Cell 49, 1134–1146 (2013).
Boulard, M., Edwards, J. R. & Bestor, T. H. FBXL10 protects Polycomb-bound genes from hypermethylation. Nat. Genet. 47, 479–485 (2015).
Hauri, S. et al. A high-density map for navigating the human Polycomb complexome. Cell Rep. 17, 583–595 (2016).
Gao, Z. et al. An AUTS2–Polycomb complex activates gene expression in the CNS. Nature 516, 349–354 (2014).
Almeida, M. et al. PCGF3/5-PRC1 initiates Polycomb recruitment in X chromosome inactivation. Science 356, 1081–1084 (2017).
Hurlin, P. J., Steingrimsson, E., Copeland, N. G., Jenkins, N. A. & Eisenman, R. N. Mga, a dual-specificity transcription factor that interacts with Max and contains a T-domain DNA-binding motif. EMBO J. 18, 7019–7028 (1999).
Endoh, M. et al. PCGF6–PRC1 suppresses premature differentiation of mouse embryonic stem cells by regulating germ cell-related genes. eLife 6, e21064 (2017).
Trojer, P. et al. L3MBTL2 protein acts in concert with PcG protein-mediated monoubiquitination of H2A to establish a repressive chromatin structure. Mol. Cell 42, 438–450 (2011).
Zhao, W. et al. Essential role for Polycomb group protein Pcgf6 in embryonic stem cell maintenance and a noncanonical Polycomb repressive complex 1 (PRC1) integrity. J. Biol. Chem. 292, 2773–2784 (2017).
Qin, J. et al. The Polycomb group protein L3mbtl2 assembles an atypical PRC1-family complex that is essential in pluripotent stem cells and early development. Cell Stem Cell 11, 319–332 (2012).
Zdzieblo, D. et al. Pcgf6, a Polycomb group protein, regulates mesodermal lineage differentiation in murine ESCs and functions in iPS reprogramming. Stem Cell 32, 3112–3125 (2014).
Muller, J. et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111, 197–208 (2002). Together with Cao et al. (2002), Czermin et al. (2002) and Kuzmichev et al. (2002), this paper discovers the catalytic activity of PRC2.
Alekseyenko, A. A., Gorchakov, A. A., Kharchenko, P. V. & Kuroda, M. I. Reciprocal interactions of human C10orf12 and C17orf96 with PRC2 revealed by BioTAP-XL cross-linking and affinity purification. Proc. Natl Acad. Sci. USA 111, 2488–2493 (2014).
Grijzenhout, A. et al. Functional analysis of AEBP2, a PRC2 Polycomb protein, reveals a Trithorax phenotype in embryonic development and in ESCs. Development 143, 2716–2723 (2016).
Pajtler, K. W. et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 136, 211–226 (2018). This study identifies a catalytically inhibitory PRC2 subunit.
Piunti, A. et al. CATACOMB: an endogenous inducible gene that antagonizes H3K27 methylation activity of Polycomb repressive complex 2 via an H3K27M-like mechanism. Sci. Adv. 5, eaax2887 (2019).
Ragazzini, R. et al. EZHIP constrains Polycomb repressive complex 2 activity in germ cells. Nat. Commun. 10, 3858 (2019).
Jain, S. U. et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat. Commun. 10, 2146 (2019).
Han, J. et al. Elevated CXorf67 expression in PFA ependymomas suppresses DNA repair and sensitizes to PARP inhibitors. Cancer Cell 38, 844–856.e7 (2020).
Brien, G. L. et al. Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation. Nat. Struct. Mol. Biol. 19, 1273–1281 (2012).
Ballare, C. et al. Phf19 links methylated Lys36 of histone H3 to regulation of Polycomb activity. Nat. Struct. Mol. Biol. 19, 1257–1265 (2012).
Musselman, C. A. et al. Molecular basis for H3K36me3 recognition by the Tudor domain of PHF1. Nat. Struct. Mol. Biol. 19, 1266–1272 (2012).
Cai, L. et al. An H3K36 methylation-engaging Tudor motif of Polycomb-like proteins mediates PRC2 complex targeting. Mol. Cell 49, 571–582 (2013).
Gatchalian, J., Kingsley, M. C., Moslet, S. D., Rosas Ospina, R. D. & Kutateladze, T. G. An aromatic cage is required but not sufficient for binding of Tudor domains of the Polycomblike protein family to H3K36me3. Epigenetics 10, 467–473 (2015).
Ferrari, K. J. et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol. Cell 53, 49–62 (2014).
Healy, E. et al. PRC2.1 and PRC2.2 synergize to coordinate H3K27 trimethylation. Mol. Cell 76, 437–452.e6 (2019).
Li, H. et al. Polycomb-like proteins link the PRC2 complex to CpG islands. Nature 549, 287–291 (2017).
Conway, E. et al. A family of vertebrate-specific Polycombs encoded by the LCOR/LCORL genes balance PRC2 subtype activities. Mol. Cell 70, 408–421.e8 (2018).
Beringer, M. et al. EPOP functionally links Elongin and Polycomb in pluripotent stem cells. Mol. Cell 64, 645–658 (2016).
Zhang, Z. et al. PRC2 complexes with JARID2, MTF2, and esPRC2p48 in ES cells to modulate ES cell pluripotency and somatic cell reprogramming. Stem Cell 29, 229–240 (2011).
Chen, S., Jiao, L., Liu, X., Yang, X. & Liu, X. A dimeric structural scaffold for PRC2–PCL targeting to CpG island chromatin. Mol. Cell 77, 1265–1278.e7 (2020).
Li, G. et al. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 24, 368–380 (2010).
Landeira, D. et al. Jarid2 is a PRC2 component in embryonic stem cells required for multi-lineage differentiation and recruitment of PRC1 and RNA polymerase II to developmental regulators. Nat. Cell Biol. 12, 618–624 (2010).
Liefke, R., Karwacki-Neisius, V. & Shi, Y. EPOP interacts with elongin BC and USP7 to modulate the chromatin landscape. Mol. Cell 64, 659–672 (2016).
Conaway, J. W., Kamura, T. & Conaway, R. C. The elongin B.C. complex and the von Hippel–Lindau tumor suppressor protein. Biochim. Biophys. Acta 1377, M49–M54 (1998).
Duan, D. R. et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science 269, 1402–1406 (1995).
Ardehali, M. B. et al. Polycomb repressive complex 2 methylates elongin A to regulate transcription. Mol. Cell 68, 872–884.e6 (2017).
Bartke, T. et al. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell 143, 470–484 (2010).
Gehani, S. S. et al. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol. Cell 39, 886–900 (2010).
Zhang, Q. et al. RNA exploits an exposed regulatory site to inhibit the enzymatic activity of PRC2. Nat. Struct. Mol. Biol. 26, 237–247 (2019).
Hojfeldt, J. W. et al. Non-core subunits of the PRC2 complex are collectively required for its target-site specificity. Mol. Cell 76, 423–436.e3 (2019).
Kasinath, V. et al. Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science 359, 940–944 (2018).
Sanulli, S. et al. Jarid2 methylation via the PRC2 complex regulates H3K27me3 deposition during cell differentiation. Mol. Cell 57, 769–783 (2015).
Son, J., Shen, S. S., Margueron, R. & Reinberg, D. Nucleosome-binding activities within JARID2 and EZH1 regulate the function of PRC2 on chromatin. Genes Dev. 27, 2663–2677 (2013).
Schmitges, F. W. et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol. Cell 42, 330–341 (2011).
Lee, C. H. et al. Distinct stimulatory mechanisms regulate the catalytic activity of Polycomb repressive complex 2. Mol. Cell 70, 435–448.e5 (2018).
He, G. P., Kim, S. & Ro, H. S. Cloning and characterization of a novel zinc finger transcriptional repressor. A direct role of the zinc finger motif in repression. J. Biol. Chem. 274, 14678–14684 (1999).
Kim, H., Ekram, M. B., Bakshi, A. & Kim, J. AEBP2 as a transcriptional activator and its role in cell migration. Genomics 105, 108–115 (2015).
Pasini, D. et al. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 464, 306–310 (2010).
Shen, X. et al. Jumonji modulates Polycomb activity and self-renewal versus differentiation of stem cells. Cell 139, 1303–1314 (2009).
Peng, J. C. et al. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139, 1290–1302 (2009).
Cooper, S. et al. Jarid2 binds mono-ubiquitylated H2A lysine 119 to mediate crosstalk between Polycomb complexes PRC1 and PRC2. Nat. Commun. 7, 13661 (2016).
Kalb, R. et al. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat. Struct. Mol. Biol. 21, 569–571 (2014).
Hubner, J. M. et al. EZHIP/CXorf67 mimics K27M mutated oncohistones and functions as an intrinsic inhibitor of PRC2 function in aggressive posterior fossa ependymoma. Neuro Oncol. 21, 878–889 (2019).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Jain, S. U. et al. H3 K27M and EZHIP impede H3K27-methylation spreading by inhibiting allosterically stimulated PRC2. Mol. Cell 80, 726–735.e7 (2020).
Voncken, J. W. et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc. Natl Acad. Sci. USA 100, 2468–2473 (2003).
Posfai, E. et al. Polycomb function during oogenesis is required for mouse embryonic development. Genes Dev. 26, 920–932 (2012).
del Mar Lorente, M. et al. Loss- and gain-of-function mutations show a Polycomb group function for Ring1A in mice. Development 127, 5093–5100 (2000).
Buchwald, G. et al. Structure and E3-ligase activity of the ring-ring complex of Polycomb proteins Bmi1 and Ring1b. EMBO J. 25, 2465–2474 (2006).
Illingworth, R. S. et al. The E3 ubiquitin ligase activity of RING1B is not essential for early mouse development. Genes Dev. 29, 1897–1902 (2015).
Katoh-Fukui, Y. et al. Male-to-female sex reversal in M33 mutant mice. Nature 393, 688–692 (1998).
Dickinson, M. E. et al. High-throughput discovery of novel developmental phenotypes. Nature 537, 508–514 (2016).
Forzati, F. et al. CBX7 is a tumor suppressor in mice and humans. J. Clin. Invest. 122, 612–623 (2012).
Isono, K. et al. Mammalian polyhomeotic homologues Phc2 and Phc1 act in synergy to mediate Polycomb repression of Hox genes. Mol. Cell Biol. 25, 6694–6706 (2005).
van der Lugt, N. M. et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8, 757–769 (1994).
Alkema, M. J., van der Lugt, N. M., Bobeldijk, R. C., Berns, A. & van Lohuizen, M. Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature 374, 724–727 (1995).
Akasaka, T. et al. A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122, 1513–1522 (1996).
Akasaka, T. et al. Mice doubly deficient for the Polycomb group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development 128, 1587–1597 (2001).
Pirity, M. K., Locker, J. & Schreiber-Agus, N. Rybp/DEDAF is required for early postimplantation and for central nervous system development. Mol. Cell Biol. 25, 7193–7202 (2005).
Brown, J. P. et al. HP1γ function is required for male germ cell survival and spermatogenesis. Epigenetics Chromatin 3, 9 (2010).
Abe, K. et al. Loss of heterochromatin protein 1γ reduces the number of primordial germ cells via impaired cell cycle progression in mice. Biol. Reprod. 85, 1013–1024 (2011).
Boulet, A. M. & Capecchi, M. R. Signaling by FGF4 and FGF8 is required for axial elongation of the mouse embryo. Dev. Biol. 371, 235–245 (2012).
Faust, C., Schumacher, A., Holdener, B. & Magnuson, T. The eed mutation disrupts anterior mesoderm production in mice. Development 121, 273–285 (1995).
Shumacher, A., Faust, C. & Magnuson, T. Positional cloning of a global regulator of anterior–posterior patterning in mice. Nature 383, 250–253 (1996).
O’Carroll, D. et al. The Polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell Biol. 21, 4330–4336 (2001).
Piunti, A. et al. Polycomb proteins control proliferation and transformation independently of cell cycle checkpoints by regulating DNA replication. Nat. Commun. 5, 3649 (2014).
Grosswendt, S. et al. Epigenetic regulator function through mouse gastrulation. Nature 584, 102–108 (2020).
Kim, H., Kang, K., Ekram, M. B., Roh, T. Y. & Kim, J. Aebp2 as an epigenetic regulator for neural crest cells. PLoS ONE 6, e25174 (2011).
Takeuchi, T. et al. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 9, 1211–1222 (1995).
Koscielny, G. et al. The international mouse phenotyping consortium web portal, a unified point of access for knockout mice and related phenotyping data. Nucleic Acids Res. 42, D802–809 (2014).
Li, X. et al. Mammalian Polycomb-like Pcl2/Mtf2 is a novel regulatory component of PRC2 that can differentially modulate Polycomb activity both at the Hox gene cluster and at Cdkn2a genes. Mol. Cell Biol. 31, 351–364 (2011).
van Lohuizen, M. et al. Identification of cooperating oncogenes in Eμ-myc transgenic mice by provirus tagging. Cell 65, 737–752 (1991). This study is one of the first to show pro-oncogenic activity of a PcG protein.
Varambally, S. et al. The Polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002). This study is one of the first to show pro-oncogenic activity of EZH2 and to identify it as a suitable therapeutic target.
Bracken, A. P. et al. EZH2 is downstream of the pRB–E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 22, 5323–5335 (2003).
Kim, K. H. & Roberts, C. W. Targeting EZH2 in cancer. Nat. Med. 22, 128–134 (2016). This study is one of the first to describe a potential mechanism of PcG control of cellular proliferation.
Jacobs, J. J., Kieboom, K., Marino, S., DePinho, R. A. & van Lohuizen, M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397, 164–168 (1999).
Bracken, A. P. et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 21, 525–530 (2007).
Piunti, A. & Pasini, D. Epigenetic factors in cancer development: Polycomb group proteins. Future Oncol. 7, 57–75 (2011).
Piunti, A. et al. Therapeutic targeting of Polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat. Med. 23, 493–500 (2017).
Chiacchiera, F. et al. Polycomb complex PRC1 preserves intestinal stem cell identity by sustaining Wnt/β-catenin transcriptional activity. Cell Stem Cell 18, 91–103 (2016).
Mohammad, F. et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat. Med. 23, 483–492 (2017).
Bruggeman, S. W. et al. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell 12, 328–341 (2007).
Morin, R. D. et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 42, 181–185 (2010). This study identifies one of the first oncogenic mutations of a PRC subunit.
Sneeringer, C. J. et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc. Natl Acad. Sci. USA 107, 20980–20985 (2010).
Pasqualucci, L. & Dalla-Favera, R. Genetics of diffuse large B-cell lymphoma. Blood 131, 2307–2319 (2018).
McCabe, M. T. et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108–112 (2012).
Knutson, S. K. et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat. Chem. Biol. 8, 890–896 (2012). This study generates one of the first EZH2-specific catalytic inhibitors.
Wilson, B. G. et al. Epigenetic antagonism between Polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 18, 316–328 (2010).
Kim, K. H. et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat. Med. 21, 1491–1496 (2015).
Wang, L. et al. Resetting the epigenetic balance of Polycomb and COMPASS function at enhancers for cancer therapy. Nat. Med. 24, 758–769 (2018).
LaFave, L. M. et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat. Med. 21, 1344–1349 (2015).
Mohammad, F. & Helin, K. Oncohistones: drivers of pediatric cancers. Genes Dev. 31, 2313–2324 (2017).
Pratt, D. et al. Diffuse intrinsic pontine glioma-like tumor with EZHIP expression and molecular features of PFA ependymoma. Acta Neuropathol. Commun. 8, 37 (2020).
Dewaele, B. et al. Identification of a novel, recurrent MBTD1–CXorf67 fusion in low-grade endometrial stromal sarcoma. Int. J. Cancer 134, 1112–1122 (2014).
Bayliss, J. et al. Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci. Transl Med. 8, 366ra161 (2016).
Bender, S. et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24, 660–672 (2013).
Michealraj, K. A. et al. Metabolic regulation of the epigenome drives lethal infantile ependymoma. Cell 181, 1329–1345.e24 (2020).
Nikoloski, G. et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat. Genet. 42, 665–667 (2010).
Ernst, T. et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat. Genet. 42, 722–726 (2010). Together with Nikoloski et al. (2010), this paper provides early evidence for a tumour-suppressive role of EZH2.
Ntziachristos, P. et al. Genetic inactivation of the Polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat. Med. 18, 298–301 (2012).
Simon, C. et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. 26, 651–656 (2012).
Oguro, H. et al. Lethal myelofibrosis induced by Bmi1-deficient hematopoietic cells unveils a tumor suppressor function of the Polycomb group genes. J. Exp. Med. 209, 445–454 (2012).
Mochizuki-Kashio, M. et al. Ezh2 loss in hematopoietic stem cells predisposes mice to develop heterogeneous malignancies in an Ezh1-dependent manner. Blood 126, 1172–1183 (2015).
Suresh, K., Kliot, T., Piunti, A. & Kliot, M. Epigenetic mechanisms drive the progression of neurofibromas to malignant peripheral nerve sheath tumors. Surg. Neurol. Int. 7, S797–S800 (2016).
Marchione, D. M. et al. Histone H3K27 dimethyl loss is highly specific for malignant peripheral nerve sheath tumor and distinguishes true PRC2 loss from isolated H3K27 trimethyl loss. Mod. Pathol. 32, 1434–1446 (2019).
Lee, W. et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat. Genet. 46, 1227–1232 (2014).
Wassef, M. et al. EZH1/2 function mostly within canonical PRC2 and exhibit proliferation-dependent redundancy that shapes mutational signatures in cancer. Proc. Natl Acad. Sci. USA 116, 6075–6080 (2019).
Tseng, C. K. et al. Synthesis of 3-deazaneplanocin A, a powerful inhibitor of S-adenosylhomocysteine hydrolase with potent and selective in vitro and in vivo antiviral activities. J. Med. Chem. 32, 1442–1446 (1989).
Tan, J. et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 21, 1050–1063 (2007).
Miranda, T. B. et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol. Cancer Ther. 8, 1579–1588 (2009).
Beguelin, W. et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23, 677–692 (2013).
Souroullas, G. P. et al. An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation. Nat. Med. 22, 632–640 (2016).
Kim, W. et al. Targeted disruption of the EZH2–EED complex inhibits EZH2-dependent cancer. Nat. Chem. Biol. 9, 643–650 (2013).
He, Y. et al. The EED protein–protein interaction inhibitor A-395 inactivates the PRC2 complex. Nat. Chem. Biol. 13, 389–395 (2017).
Kreso, A. et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat. Med. 20, 29–36 (2014).
Dey, A. et al. Evaluating the mechanism and therapeutic potential of PTC-028, a novel inhibitor of BMI-1 function in ovarian cancer. Mol. Cancer Ther. 17, 39–49 (2018).
Deevy, O. & Bracken, A. P. PRC2 functions in development and congenital disorders. Development 146, dev.181354 (2019).
Shen, X. et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 32, 491–502 (2008).
Ezhkova, E. et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 25, 485–498 (2011).
Margueron, R. et al. Role of the Polycomb protein EED in the propagation of repressive histone marks. Nature 461, 762–767 (2009).
Jiao, L. & Liu, X. Structural basis of histone H3K27 trimethylation by an active Polycomb repressive complex 2. Science 350, aac4383 (2015).
Qi, W. et al. An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED. Nat. Chem. Biol. 13, 381–388 (2017).
Koontz, J. I. et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc. Natl Acad. Sci. USA 98, 6348–6353 (2001).
Zhang, M. et al. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat. Genet. 46, 1170–1172 (2014).
Zhang, J. et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481, 157–163 (2012).
Murzina, N. V. et al. Structural basis for the recognition of histone H4 by the histone-chaperone RbAp46. Structure 16, 1077–1085 (2008).
Yeh, H. H. et al. Ras induces experimental lung metastasis through up-regulation of RbAp46 to suppress RECK promoter activity. BMC Cancer 15, 172 (2015).
Wang, J. et al. HNF1B-mediated repression of SLUG is suppressed by EZH2 in aggressive prostate cancer. Oncogene 39, 1335–1346 (2020).
Zhang, Q. et al. Convergent evolution between PALI1 and JARID2 for the allosteric activation of PRC2. Preprint at bioRxiv https://doi.org/10.1101/2020.05.28.122556 (2020).
Celik, H. et al. JARID2 functions as a tumor suppressor in myeloid neoplasms by repressing self-renewal in hematopoietic progenitor cells. Cancer Cell 34, 741–756.e8 (2018).
Gebre-Medhin, S. et al. Recurrent rearrangement of the PHF1 gene in ossifying fibromyxoid tumors. Am. J. Pathol. 181, 1069–1077 (2012).
Chiang, S. et al. Frequency of known gene rearrangements in endometrial stromal tumors. Am. J. Surg. Pathol. 35, 1364–1372 (2011).
Rothberg, J. L. M. et al. Mtf2–PRC2 control of canonical Wnt signaling is required for definitive erythropoiesis. Cell Discov. 4, 21 (2018).
Maganti, H. B. et al. Targeting the MTF2–MDM2 axis sensitizes refractory acute myeloid leukemia to chemotherapy. Cancer Discov. 8, 1376–1389 (2018).
Jain, P., Ballare, C., Blanco, E., Vizan, P. & Di Croce, L. PHF19 mediated regulation of proliferation and invasiveness in prostate cancer cells. eLife 9, e.51373 (2020).
Ren, Z. et al. PHF19 promotes multiple myeloma tumorigenicity through PRC2 activation and broad H3K27me3 domain formation. Blood 134, 1176–1189 (2019).
Wheeler, L. J. et al. CBX2 identified as driver of anoikis escape and dissemination in high grade serous ovarian cancer. Oncogenesis 7, 92 (2018).
Wang, X. et al. Targeting the CK1α/CBX4 axis for metastasis in osteosarcoma. Nat. Commun. 11, 1141 (2020).
Deng, H. et al. CBX6 is negatively regulated by EZH2 and plays a potential tumor suppressor role in breast cancer. Sci. Rep. 9, 197 (2019).
Scott, C. L. et al. Role of the chromobox protein CBX7 in lymphomagenesis. Proc. Natl Acad. Sci. USA 104, 5389–5394 (2007).
Klauke, K. et al. Polycomb Cbx family members mediate the balance between haematopoietic stem cell self-renewal and differentiation. Nat. Cell Biol. 15, 353–362 (2013).
Tan, J. et al. CBX8, a Polycomb group protein, is essential for MLL-AF9-induced leukemogenesis. Cancer Cell 20, 563–575 (2011).
Guo, W. J. et al. Mel-18 acts as a tumor suppressor by repressing Bmi-1 expression and down-regulating Akt activity in breast cancer cells. Cancer Res. 67, 5083–5089 (2007).
Lee, J. Y. et al. Mel-18 negatively regulates INK4a/ARF-independent cell cycle progression via Akt inactivation in breast cancer. Cancer Res. 68, 4201–4209 (2008).
Haupt, Y., Bath, M. L., Harris, A. W. & Adams, J. M. Bmi-1 transgene induces lymphomas and collaborates with myc in tumorigenesis. Oncogene 8, 3161–3164 (1993).
Jacobs, J. J. et al. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 13, 2678–2690 (1999).
Lessard, J. & Sauvageau, G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423, 255–260 (2003).
Rai, K. et al. Dual roles of RNF2 in melanoma progression. Cancer Discov. 5, 1314–1327 (2015).
van den Boom, V. et al. Non-canonical PRC1.1 targets active genes independent of H3K27me3 and is essential for leukemogenesis. Cell Rep. 14, 332–346 (2016).
Danen-van Oorschot, A. A. et al. Human death effector domain-associated factor interacts with the viral apoptosis agonist Apoptin and exerts tumor-preferential cell killing. Cell Death Differ. 11, 564–573 (2004).
Andricovich, J., Kai, Y., Peng, W., Foudi, A. & Tzatsos, A. Histone demethylase KDM2B regulates lineage commitment in normal and malignant hematopoiesis. J. Clin. Invest. 126, 905–920 (2016).
Tzatsos, A. et al. KDM2B promotes pancreatic cancer via Polycomb-dependent and -independent transcriptional programs. J. Clin. Invest. 123, 727–739 (2013).
Coyaud, E. et al. PAX5–AUTS2 fusion resulting from t(7;9)(q11.2;p13.2) can now be classified as recurrent in B cell acute lymphoblastic leukemia. Leuk. Res. 34, e323–e325 (2010).
Denk, D. et al. PAX5–AUTS2: a recurrent fusion gene in childhood B-cell precursor acute lymphoblastic leukemia. Leuk. Res. 36, e178–e181 (2012).
Zhao, S. P. et al. CBX3 promotes glioma U87 cell proliferation and predicts an unfavorable prognosis. J. Neurooncol. 145, 35–48 (2019).
Zhong, X. et al. CBX3/HP1γ promotes tumor proliferation and predicts poor survival in hepatocellular carcinoma. Aging 11, 5483–5497 (2019).
Ma, C. et al. CBX3 predicts an unfavorable prognosis and promotes tumorigenesis in osteosarcoma. Mol. Med. Rep. 19, 4205–4212 (2019).
Slifer, E. H. A mutant stock of Drosophila with extra sex-combs. J. Exp. Zool. 90, 31–40 (1942). This study discovers the first Polycomb phenotype.
Hannah, A. M. & Stromnaes, O. Extra sex comb mutants in Drosophila melanogaster. Drosoph. Inf. Serv. 29, 121–123 (1955).
Lewis, P. H. [New mutants report]. Drosoph. Inf. Serv. 21, 69 (1947).
Lewis, E. B. A gene complex controlling segmentation in Drosophila. Nature 276, 565–570 (1978). This study identifies the first Polycomb gene.
Maeda, R. K. & Karch, F. The ABC of the BX-C: the bithorax complex explained. Development 133, 1413–1422 (2006).
Orlando, V., Jane, E. P., Chinwalla, V., Harte, P. J. & Paro, R. Binding of trithorax and Polycomb proteins to the bithorax complex: dynamic changes during early Drosophila embryogenesis. EMBO J. 17, 5141–5150 (1998).
Cenik, B. K. & Shilatifard, A. COMPASS and SWI/SNF complexes in development and disease. Nat. Rev. Genet. 22, 38–58 (2021).
Ingham, P. W. & Whittle, R. Trithorax: a new homeotic mutation of Drosophila melanogaster causing transformations of abdominal and thoracic imaginal segments. Mol. Gen. Genet. 179, 607–614 (1980).
Ingham, P. W. Differential expression of bithorax complex genes in the absence of the extra sex combs and trithorax genes. Nature 306, 591–593 (1983).
Acknowledgements
The authors thank the members of the Shilatifard laboratory for insightful discussions and comments. In particular, they thank N. Ethen for the original illustrations, E. Smith for critical review of the manuscript and M. Morgan for editing and scientific suggestions. The work of A.P. is supported by the transition to independence grant K99CA234434. Complex of proteins associated with Set1 (COMPASS)-related and Polycomb group (PcG)-related studies in the Shilatifard laboratory are supported by generous funding from the National Cancer Institute through Outstanding Investigator Award R35CA197569 to A.S.
Author information
Authors and Affiliations
Contributions
A.P. researched data for the article, substantially contributed to discussion of content, wrote the manuscript and edited it before submission. A.S. substantially contributed to discussion of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks Judith Kassis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Facultative heterochromatin
-
Locus-specific and cell type-specific dense and transcriptionally silenced chromatin.
- Complex variants
-
Multi-protein complexes that share core subunits, but include defining subunits that distinguish one variant from the other.
- Subcomplexes
-
Protein complexes that lack one or more subunits that define the full complex.
- Mutually exclusive
-
Refers to proteins that, even if they interact with a common set of proteins, cannot be found interacting in immunoprecipitation assays followed by western blotting and/or mass spectrometry protein identification.
- Constitutive heterochromatin
-
Very dense and transcriptionally silenced chromatin that is consistently formed (for example, in centromeres) in many cell types.
- CpG islands
-
DNA regions, often corresponding with gene promoters, of several hundred base pairs that contain more than 50% G or C nucleotides and an observed over expected ratio of CpG dinucleotides higher than 0.6.
- Reduced-representation bisulfite sequencing
-
A technique for detecting methylated (or an oxidized form of methylated) cytidine residues. The ‘reduced representation’ allows obtaining information for a limited subset of genomic regions.
- Primitive streak
-
A structure formed in the blastula in the caudal part of the embryo, which is the precursor of the mesoderm.
Rights and permissions
About this article
Cite this article
Piunti, A., Shilatifard, A. The roles of Polycomb repressive complexes in mammalian development and cancer. Nat Rev Mol Cell Biol 22, 326–345 (2021). https://doi.org/10.1038/s41580-021-00341-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-021-00341-1
This article is cited by
-
Polycomb repressive complex 2 and its core component EZH2: potential targeted therapeutic strategies for head and neck squamous cell carcinoma
Clinical Epigenetics (2024)
-
Correlation between large rearrangements and patient phenotypes in NF1 deletion syndrome: an update and review
BMC Medical Genomics (2024)
-
Chromatin profiling reveals TFAP4 as a critical transcriptional regulator of bovine satellite cell differentiation
BMC Genomics (2024)
-
Combination of EZH2 and ATM inhibition in BAP1-deficient mesothelioma
British Journal of Cancer (2024)
-
Retinoids and EZH2 inhibitors cooperate to orchestrate anti-oncogenic effects on bladder cancer cells
Cancer Gene Therapy (2024)