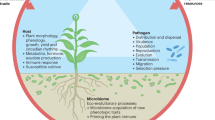
Abstract
The plant extracellular space, referred to as the apoplast, is inhabited by a variety of microorganisms. Reflecting the crucial nature of this compartment, both plants and microorganisms seek to control, exploit and respond to its composition. Upon sensing the apoplastic environment, pathogens activate virulence programmes, including the delivery of effectors with well-established roles in suppressing plant immunity. We posit that another key and foundational role of effectors is niche establishment — specifically, the manipulation of plant physiological processes to enrich the apoplast in water and nutritive metabolites. Facets of plant immunity counteract niche establishment by restricting water, nutrients and signals for virulence activation. The complex competition to control and, in the case of pathogens, exploit the apoplast provides remarkable insights into the nature of virulence, host susceptibility, host defence and, ultimately, the origin of phytopathogenesis. This novel framework focuses on the ecology of a microbial niche and highlights areas of future research on plant–microorganism interactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jones, J. D. G. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Toruño, T. Y., Stergiopoulos, I. & Coaker, G. Plant-pathogen effectors: cellular probes interfering with plant defenses in spatial and temporal manners. Annu. Rev. Phytopathol. 54, 419–441 (2016).
Ngou, B. P. M., Ding, P. & Jones, J. D. G. Thirty years of resistance: zig-zag through the plant immune system. Plant Cell 34, 1447–1478 (2022).
Lovelace, A. H. & Ma, W. How do bacteria transform plants into their oasis? Cell Host Microbe 30, 412–414 (2022).
Cai, J. et al. Manipulation of plant metabolism by pathogen effectors: more than just food. FEMS Microbiol. Rev. 47, fuad007 (2023).
Roussin-Léveillée, C. et al. Evolutionarily conserved bacterial effectors hijack abscisic acid signaling to induce an aqueous environment in the apoplast. Cell Host Microbe 30, 489–501.e4 (2022).
Hu, Y. et al. Bacterial effectors manipulate plant abscisic acid signaling for creation of an aqueous apoplast. Cell Host Microbe 30, 518–529.e6 (2022).
Gentzel, I. et al. Dynamic nutrient acquisition from a hydrated apoplast supports biotrophic proliferation of a bacterial pathogen of maize. Cell Host Microbe 30, 502–517.e4 (2022).
Takahashi, F. et al. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556, 235–238 (2018).
Melotto, M., Underwood, W., Koczan, J., Nomura, K. & He, S. Y. Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980 (2006).
Melotto, M., Zhang, L., Oblessuc, P. R. & He, S. Y. Stomatal defense a decade later. Plant Physiol. 174, 561–571 (2017).
Wu, J. & Liu, Y. Stomata–pathogen interactions: over a century of research. Trends Plant Sci. 27, 964–967 (2022).
Freeman, B. C. & Beattie, G. A. Bacterial growth restriction during host resistance to Pseudomonas syringae is associated with leaf water loss and localized cessation of vascular activity in Arabidopsis thaliana. Mol. Plant Microbe Interact. 22, 857–867 (2009).
Xin, X.-F. et al. Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539, 524–529 (2016).
Jin, L. et al. Direct and indirect targeting of PP2A by conserved bacterial type-III effector proteins. PLoS Pathog. 12, e1005609 (2016).
Waadt, R. et al. Identification of open stomata1-interacting proteins reveals interactions with sucrose non-fermenting1-related protein kinases2 and with type 2A protein phosphatases that function in abscisic acid responses. Plant Physiol. 169, 760–779 (2015).
Lajeunesse, G. et al. Light prevents pathogen-induced aqueous microenvironments via potentiation of salicylic acid signaling. Nat. Commun. 14, 713 (2023).
Wu, J. et al. CURLY LEAF modulates apoplast liquid water status in Arabidopsis leaves. Plant Physiol. 193, 792–808 (2023).
Lievens, L., Pollier, J., Goossens, A., Beyaert, R. & Staal, J. Abscisic acid as pathogen effector and immune regulator. Front. Plant Sci. 8, 597 (2017).
Zhang, D., Tian, C., Yin, K., Wang, W. & Qiu, J.-L. Postinvasive bacterial resistance conferred by open stomata in rice. Mol. Plant Microbe Interact. 32, 255–266 (2019).
Peng, Z. et al. Xanthomonas translucens commandeers the host rate-limiting step in ABA biosynthesis for disease susceptibility. Proc. Natl Acad. Sci. USA 116, 20938–20946 (2019).
You, Y. et al. The eINTACT system dissects bacterial exploitation of plant osmosignalling to enhance virulence. Nat. Plants 9, 128–141 (2022).
Dong, H.-P. et al. The ABI2-dependent abscisic acid signalling controls HrpN-induced drought tolerance in Arabidopsis. Planta 221, 313–327 (2005).
Liu, Y., Mahmud, Md. R., Xu, N. & Liu, J. The Pseudomonas syringae effector AvrPtoB targets abscisic acid signaling pathway to promote its virulence in Arabidopsis. Phytopathol. Res. 4, 5 (2022).
Siewers, V., Kokkelink, L., Smedsgaard, J. & Tudzynski, P. Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Appl. Env. Microbiol. 72, 4619–4626 (2006).
Spence, C. A., Lakshmanan, V., Donofrio, N. & Bais, H. P. Crucial roles of abscisic acid biogenesis in virulence of rice blast fungus Magnaporthe oryzae. Front. Plant Sci. 6, 1082 (2015).
Dörffling, K., Petersen, W., Sprecher, E., Urbasch, I. & Hanssen, H.-P. Abscisic acid in phytopathogenic fungi of the genera Botrytis, Ceratocystis, Fusarium, and Rhizoctonia. Z. Naturforsch. C 39, 683–684 (1984).
Hernandez, M. N. & Lindow, S. E. Pseudomonas syringae increases water availability in leaf microenvironments via production of hygroscopic syringafactin. Appl. Env. Microbiol. 85, e01014-19 (2019).
Oulghazi, S. et al. Pectobacterium brasiliense: genomics, host range and disease management. Microorganisms 9, 106 (2021).
Ekanayake, G., Gohmann, R. & Mackey, D. A method for quantitation of apoplast hydration in Arabidopsis leaves reveals water-soaking activity of effectors of Pseudomonas syringae during biotrophy. Sci. Rep. 12, 18363 (2022).
Schwartz, A. R., Morbitzer, R., Lahaye, T. & Staskawicz, B. J. TALE-induced bHLH transcription factors that activate a pectate lyase contribute to water soaking in bacterial spot of tomato. Proc. Natl Acad. Sci. USA 114, E897–E903 (2017).
Nomura, K. et al. Bacterial pathogens deliver water- and solute-permeable channels to plant cells. Nature 621, 586–591 (2023).
Oh, H.-S. & Collmer, A. Basal resistance against bacteria in Nicotiana benthamiana leaves is accompanied by reduced vascular staining and suppressed by multiple Pseudomonas syringae type III secretion system effector proteins: vascular staining assay for basal resistance in Nicotiana benthamiana. Plant J. 44, 348–359 (2005).
Gentzel, I., Giese, L., Zhao, W., Alonso, A. P. & Mackey, D. A simple method for measuring apoplast hydration and collecting apoplast contents. Plant Physiol. 179, 1265–1272 (2019).
O’Leary, B. M., Rico, A., McCraw, S., Fones, H. N. & Preston, G. M. The infiltration-centrifugation technique for extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an example.J. Vis. Exp. 94, 52113 (2014).
Nouchi, I. et al. Overcoming the difficulties in collecting apoplastic fluid from rice leaves by the infiltration–centrifugation method. Plant Cell Physiol. 53, 1659–1668 (2012).
Rico, A. & Preston, G. M. Pseudomonas syringae pv. tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol. Plant Microbe Interact. 21, 269–282 (2008).
Zhang, C. & Turgeon, R. Mechanisms of phloem loading. Curr. Opin. Plant Biol. 43, 71–75 (2018).
Kim, J.-Y. et al. Cellular export of sugars and amino acids: role in feeding other cells and organisms. Plant Physiol. 187, 1893–1914 (2021).
Gupta, P. K., Balyan, H. S. & Gautam, T. SWEET genes and TAL effectors for disease resistance in plants: present status and future prospects. Mol. Plant Pathol. 22, 1014–1026 (2021).
Chen, L.-Q. et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468, 527–532 (2010).
Yang, S. et al. Rhizoctonia solani transcriptional activator interacts with rice WRKY53 and grassy tiller 1 to activate SWEET transporters for nutrition. J. Adv. Res. 50, 1–12 (2022).
Sonawala, U., Dinkeloo, K., Danna, C. H., McDowell, J. M. & Pilot, G. Review: functional linkages between amino acid transporters and plant responses to pathogens. Plant Sci. 277, 79–88 (2018).
Rudrappa, T., Czymmek, K. J., Paré, P. W. & Bais, H. P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 148, 1547–1556 (2008).
Wen, T. et al. Enrichment of beneficial cucumber rhizosphere microbes mediated by organic acid secretion. Hortic. Res. 7, 154 (2020).
Macias-Benitez, S. et al. Rhizospheric organic acids as biostimulants: monitoring feedbacks on soil microorganisms and biochemical properties. Front. Plant Sci. 11, 633 (2020).
Guo, W. et al. Ketoglutarate transport protein KgtP is secreted through the type III secretion system and contributes to virulence in Xanthomonas oryzae pv. oryzae. Appl. Env. Microbiol. 78, 5672–5681 (2012).
Wang, W., Liu, J., Mishra, B., Mukhtar, M. S. & McDowell, J. M. Sparking a sulfur war between plants and pathogens. Trends Plant Sci. 27, 1253–1265 (2022).
Fatima, U. & Senthil-Kumar, M. Plant and pathogen nutrient acquisition strategies. Front. Plant Sci. 6, 750 (2015).
Herlihy, J. H., Long, T. A. & McDowell, J. M. Iron homeostasis and plant immune responses: recent insights and translational implications. J. Biol. Chem. 295, 13444–13457 (2020).
Firmin, J. L. & Fenwick, G. R. Agropine — a major new plasmid-determined metabolite in crown gall tumours. Nature 276, 842–844 (1978).
Doehlemann, G. et al. Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis: plant response to U. maydis infection. Plant J. 56, 181–195 (2008).
Kretschmer, M. et al. Organic acids and glucose prime late-stage fungal biotrophy in maize. Science 376, 1187–1191 (2022).
Horst, R. J. et al. Ustilago maydis infection strongly alters organic nitrogen allocation in maize and stimulates productivity of systemic source leaves. Plant Physiol. 152, 293–308 (2009).
McIntyre, K. E., Bush, D. R. & Argueso, C. T. Cytokinin regulation of source-sink relationships in plant-pathogen interactions. Front. Plant Sci. 12, 677585 (2021).
Voll, L. Common motifs in the response of cereal primary metabolism to fungal pathogens are not based on similar transcriptional reprogramming. Front. Plant Sci. 2, 39 (2011).
Rodenburg, S. Y. A. et al. Metabolic model of the Phytophthora infestans-tomato interaction reveals metabolic switches during host colonization. mBio 10, e00454–19 (2019).
Xu, Y. et al. Phytophthora sojae apoplastic effector AEP1 mediates sugar uptake by mutarotation of extracellular aldose and is recognized as a MAMP. Plant Physiol. 187, 321–335 (2021).
Xian, L., Yu, G. & Macho, A. P. The GABA transaminase GabT is required for full virulence of Ralstonia solanacearum in tomato.MicroPubl. Biol. https://doi.org/10.17912/micropub.biology.000478 (2021).
Xian, L. et al. A bacterial effector protein hijacks plant metabolism to support pathogen nutrition. Cell Host Microbe 28, 548–557.e7 (2020).
Ward, J. L. et al. The metabolic transition during disease following infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato: the metabolic transition during disease following infection of Arabidopsis. Plant J. 63, 443–457 (2010).
Asselin, J. A. E. et al. Perturbation of maize phenylpropanoid metabolism by an AvrE family type III effector from Pantoea stewartii. Plant Physiol. 167, 1117–1135 (2015).
Yoshida, T., Christmann, A., Yamaguchi-Shinozaki, K., Grill, E. & Fernie, A. R. Revisiting the basal role of ABA — roles outside of stress. Trends Plant Sci. 24, 625–635 (2019).
Velásquez, A. C., Castroverde, C. D. M. & He, S. Y. Plant–pathogen warfare under changing climate conditions. Curr. Biol. 28, R619–R634 (2018).
Deb, D., Mackey, D., Opiyo, S. O. & McDowell, J. M. Application of alignment-free bioinformatics methods to identify an oomycete protein with structural and functional similarity to the bacterial AvrE effector protein. PLoS ONE 13, e0195559 (2018).
Siamer, S. et al. Expression of the bacterial type III effector DspA/E in Saccharomyces cerevisiae down-regulates the sphingolipid biosynthetic pathway leading to growth arrest. J. Biol. Chem. 289, 18466–18477 (2014).
Asselbergh, B., Achuo, A. E., Höfte, M. & Van Gijsegem, F. Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol. Plant Pathol. 9, 11–24 (2008).
Mackey, D. & McFall, A. J. MAMPs and MIMPs: proposed classifications for inducers of innate immunity. Mol. Microbiol. 61, 1365–1371 (2006).
Liu, Z. et al. Phytocytokine signalling reopens stomata in plant immunity and water loss. Nature 605, 332–339 (2022).
Wright, C. A. & Beattie, G. A. Pseudomonas syringae pv. tomato cells encounter inhibitory levels of water stress during the hypersensitive response of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 101, 3269–3274 (2004).
Schenstnyi, K. et al. The tomato resistance gene Bs4 suppresses leaf watersoaking phenotypes induced by AvrHah1, a transcription activator‐like effector from tomato‐pathogenic xanthomonads. New Phytol. 236, 1856–1870 (2022).
Zhou, F. et al. High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J. 39, 920–932 (2004).
Qiu, J. et al. Dual impact of ambient humidity on the virulence of Magnaporthe oryzae and basal resistance in rice. Plant Cell Environ. 45, 3399–3411 (2022).
Ngou, B. P. M., Ahn, H.-K., Ding, P. & Jones, J. D. G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115 (2021).
Yuan, M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109 (2021).
Pruitt, R. N. et al. The EDS1–PAD4–ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature 598, 495–499 (2021).
Tian, H. et al. Activation of TIR signalling boosts pattern-triggered immunity. Nature 598, 500–503 (2021).
Anderson, J. C. et al. Decreased abundance of type III secretion system-inducing signals in Arabidopsis mkp1 enhances resistance against Pseudomonas syringae. Proc. Natl Acad. Sci. USA 111, 6846–6851 (2014).
Yan, Q., Rogan, C. J. & Anderson, J. C. Development of a Pseudomonas syringae — Arabidopsis suspension cell infection system for investigating host metabolite-dependent regulation of type III secretion and pattern-triggered immunity. Mol. Plant Microbe Interact. 32, 527–539 (2019).
Yamada, K., Saijo, Y., Nakagami, H. & Takano, Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 354, 1427–1430 (2016).
Tubergen, P. J. et al. A computational model of Pseudomonas syringae metabolism unveils the role of branched-chain amino acids in virulence expression at the early stages of Arabidopsis colonization. Preprint at:bioRxiv https://doi.org/10.1101/2022.12.16.520825 (2022).
Zhang, X. et al. MAMP-elicited changes in amino acid transport activity contribute to restricting bacterial growth. Plant Physiol. 189, 2315–2331 (2022).
Zhang, X. et al. Elicitor-induced plant immunity relies on amino acids accumulation to delay the onset of bacterial virulence. Plant Physiol. 192, 601–615 (2023).
Geilfus, C.-M. The pH of the apoplast: dynamic factor with functional impact under stress. Mol. Plant 10, 1371–1386 (2017).
Mittler, R., Zandalinas, S. I., Fichman, Y. & Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 23, 663–679 (2022).
Ma, Z. et al. A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 27, 2057–2072 (2015).
Ma, Z. et al. A paralogous decoy protects Phytophthora sojae apoplastic effector PsXEG1 from a host inhibitor. Science 355, 710–714 (2017).
Xia, Y. et al. N-glycosylation shields Phytophthora sojae apoplastic effector PsXEG1 from a specific host aspartic protease. Proc. Natl Acad. Sci. USA 117, 27685–27693 (2020).
Figueroa, M., Ortiz, D. & Henningsen, E. C. Tactics of host manipulation by intracellular effectors from plant pathogenic fungi. Curr. Opin. Plant Biol. 62, 102054 (2021).
Xing, Y. et al. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization. Plant Cell 33, 2015–2031 (2021).
Taguchi, F. et al. The siderophore pyoverdine of Pseudomonas syringae pv. tabaci 6605 is an intrinsic virulence factor in host tobacco infection. J. Bacteriol. 192, 117–126 (2010).
Nomura, K. et al. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science 313, 220–223 (2006).
McDowell, J. M. et al. Downy mildew (Peronospora parasitica) resistance genes in Arabidopsis vary in functional requirements for NDR1, EDS1, NPR1 and salicylic acid accumulation. Plant J. 22, 523–529 (2000).
Muskett, P. R. et al. Arabidopsis RAR1 exerts rate-limiting control of R gene-mediated defenses against multiple pathogens. Plant Cell 14, 979–992 (2002).
Kim, M. G. et al. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121, 749–759 (2005).
Redditt, T. J. et al. AvrRpm1 functions as an ADP-ribosyl transferase to modify NOI domain-containing proteins, including Arabidopsis and soybean RPM1-interacting protein4. Plant Cell 31, 2664–2681 (2019).
Mackey, D., Holt, B. F., Wiig, A. & Dangl, J. L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108, 743–754 (2002).
Mackey, D., Belkhadir, Y., Alonso, J. M., Ecker, J. R. & Dangl, J. L. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112, 379–389 (2003).
Tian, D., Traw, M. B., Chen, J. Q., Kreitman, M. & Bergelson, J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423, 74–77 (2003).
Mauricio, R. et al. Natural selection for polymorphism in the disease resistance gene Rps2 of Arabidopsis thaliana. Genetics 163, 735–746 (2003).
Zhou, J. et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 82, 632–643 (2015).
Strauß, T. et al. RNA-seq pinpoints a Xanthomonas TAL-effector activated resistance gene in a large-crop genome. Proc. Natl Acad. Sci. USA 109, 19480–19485 (2012).
Ji, C. et al. Xa1 allelic R genes activate rice blight resistance suppressed by interfering TAL effectors. Plant Commun. 1, 100087 (2020).
Nowack, M. K., Holmes, D. R. & Lahaye, T. TALE-induced cell death executors: an origin outside immunity? Trends Plant Sci. 27, 536–548 (2022).
Oliva, R. et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 37, 1344–1350 (2019).
Rosebrock, T. R. et al. A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448, 370–374 (2007).
Zhang, J. et al. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1, 175–185 (2007).
Mine, A. et al. Pathogen exploitation of an abscisic acid- and jasmonate-inducible MAPK phosphatase and its interception by Arabidopsis immunity. Proc. Natl Acad. Sci. USA 114, 7456–7461 (2017).
Badel, J. L., Shimizu, R., Oh, H.-S. & Collmer, A. A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol. Plant Microbe Interact. 19, 99–111 (2006).
DebRoy, S., Thilmony, R., Kwack, Y.-B., Nomura, K. & He, S. Y. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc. Natl Acad. Sci. USA 101, 9927–9932 (2004).
Xin, X.-F. et al. Pseudomonas syringae effector avirulence protein E localizes to the host plasma membrane and down-regulates the expression of the NONRACE-SPECIFIC DISEASE RESISTANCE1/HARPIN-INDUCED1-LIKE13 gene required for antibacterial immunity in Arabidopsis. Plant Physiol. 169, 793–802 (2015).
Gangadharan, A., Sreerekha, M.-V., Whitehill, J., Ham, J. H. & Mackey, D. The Pseudomonas syringae pv. tomato type III effector HopM1 suppresses Arabidopsis defenses independent of suppressing salicylic acid signaling and of targeting AtMIN7. PLoS ONE 8, e82032 (2013).
Ham, J. H., Majerczak, D. R., Arroyo-Rodriguez, A. S., Mackey, D. M. & Coplin, D. L. WtsE, an AvrE-family effector protein from Pantoea stewartii subsp. stewartii, causes disease-associated cell death in corn and requires a chaperone protein for stability. Mol. Plant Microbe Interact. 19, 1092–1102 (2006).
Guo, M., Tian, F., Wamboldt, Y. & Alfano, J. R. The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol. Plant Microbe Interact. 22, 1069–1080 (2009).
Chen, H. et al. A bacterial type III effector targets the master regulator of salicylic acid signaling, NPR1, to subvert plant immunity. Cell Host Microbe 22, 777–788.e7 (2017).
Faris, J. D. et al. A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc. Natl Acad. Sci. USA 107, 13544–13549 (2010).
Shao, D., Smith, D. L., Kabbage, M. & Roth, M. G. Effectors of plant necrotrophic fungi. Front. Plant Sci. 12, 687713 (2021).
Malvestiti, M. C. et al. Analysis of plant cell death-inducing proteins of the necrotrophic fungal pathogens Botrytis squamosa and Botrytis elliptica. Front. Plant. Sci. 13, 993325 (2022).
Zhu, J. et al. Single-cell profiling of Arabidopsis leaves to Pseudomonas syringae infection. Cell Rep. 42, 112676 (2023).
Shi, J., Wang, X. & Wang, E. Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annu. Rev. Plant Biol. 74, 569–607 (2023).
Chen, T. et al. A plant genetic network for preventing dysbiosis in the phyllosphere. Nature 580, 653–657 (2020).
Helmann, T. C., Deutschbauer, A. M. & Lindow, S. E. Distinctiveness of genes contributing to growth of Pseudomonas syringae in diverse host plant species. PLoS ONE 15, e0239998 (2020).
Nobori, T. et al. Multidimensional gene regulatory landscape of a bacterial pathogen in plants. Nat. Plants 6, 883–896 (2020).
Murdoch, C. C. & Skaar, E. P. Nutritional immunity: the battle for nutrient metals at the host–pathogen interface. Nat. Rev. Microbiol. 20, 657–670 (2022).
Marchi, S., Morroni, G., Pinton, P. & Galluzzi, L. Control of host mitochondria by bacterial pathogens. Trends Microbiol. 30, 452–465 (2022).
Shen, Q., Ray, S. C., Evans, H. M., Deepe, G. S. & Rappleye, C. A. Metabolism of gluconeogenic substrates by an intracellular fungal pathogen circumvents nutritional limitations within macrophages. mBio 11, e02712-19 (2020).
Acknowledgements
Research in the Moffett laboratory is supported by funding from the Natural Sciences and Engineering Research Council (NSERC) of Canada, from the Fonds de Recherche Québécois — Nature et Technologies, and C.R-L. is supported by an NSERC doctoral fellowship. Support for the Mackey laboratory is provided by the National Science Foundation (Division of Integrative Organismal Systems, grant no. 1953509). The authors are grateful to members of the research group of Sebastian Schornack (Sainsbury Laboratory, University of Cambridge) for suggestions to improve the visual models.
Author information
Authors and Affiliations
Contributions
C.R.-L. and D.M. contributed equally to the conceptualization, research and writing of this manuscript. C.R.-L. and D.M. drafted the figures. R.G., G.E. and P.M. contributed to the research and to writing and reviewing of this manuscript. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Yuanchao Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roussin-Léveillée, C., Mackey, D., Ekanayake, G. et al. Extracellular niche establishment by plant pathogens. Nat Rev Microbiol (2024). https://doi.org/10.1038/s41579-023-00999-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41579-023-00999-8