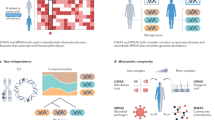
Abstract
Inter-individual human variability, driven by various genetic and environmental factors, complicates the ability to develop effective population-based early disease detection, treatment and prognostic assessment. The microbiome, consisting of diverse microorganism communities including viruses, bacteria, fungi and eukaryotes colonizing human body surfaces, has recently been identified as a contributor to inter-individual variation, through its person-specific signatures. As such, the microbiome may modulate disease manifestations, even among individuals with similar genetic disease susceptibility risks. Information stored within microbiomes may therefore enable early detection and prognostic assessment of disease in at-risk populations, whereas microbiome modulation may constitute an effective and safe treatment tailored to the individual. In this Review, we explore recent advances in the application of microbiome data in precision medicine across a growing number of human diseases. We also discuss the challenges, limitations and prospects of analysing microbiome data for personalized patient care.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M. & Owen, L. J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, https://doi.org/10.3402/mehd.v26.26191 (2015).
Stewart, C. J. et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562, 583–588 (2018).
Chen, L. et al. The long-term genetic stability and individual specificity of the human gut microbiome. Cell 184, 2302–2315.e12 (2021).
Valles-Colomer, M. et al. The person-to-person transmission landscape of the gut and oral microbiomes. Nature 614, 125–135 (2023).
Schupack, D. A., Mars, R. A. T., Voelker, D. H., Abeykoon, J. P. & Kashyap, P. C. The promise of the gut microbiome as part of individualized treatment strategies. Nat. Rev. Gastroenterol. Hepatol. 19, 7–25 (2022).
Owens, D. K. et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US preventive services task force recommendation statement. JAMA 322, 652–665 (2019).
Marchesi, J. R. et al. The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339 (2016).
Oh, J., Byrd, A. L., Park, M., Kong, H. H. & Segre, J. A. Temporal stability of the human skin microbiome. Cell 165, 854–866 (2016).
Kumari, P., Prakash, P., Yadav, S. & Saran, V. Microbiome analysis: an emerging forensic investigative tool. Forensic Sci. Int. 340, 111462 (2022).
Reitmeier, S. et al. Arrhythmic gut microbiome signatures predict risk of type 2 diabetes. Cell Host Microbe 28, 258–272.e6 (2020).
Ferreiro, A. L. et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med. 15, eabo2984 (2023).
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Liu, Y. et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 31, 77–91.e5 (2020).
Manor, O. et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 11, 5206 (2020).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Thingholm, L. B. et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe 26, 252–264.e10 (2019).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
Maifeld, A. et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat. Commun. 12, 1970 (2021).
Guo, F., Zhou, J., Li, Z., Yu, Z. & Ouyang, D. The association between trimethylamine N-oxide and its predecessors choline, l-carnitine, and betaine with coronary artery disease and artery stenosis. Cardiol. Res. Pract. 2020, 1–10 (2020).
Chen, T. et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 6, 20594 (2016).
Wu, H. et al. The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab. 32, 379–390.e3 (2020).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Ghaemi, F. et al. Intestinal microbiota composition in Iranian diabetic, pre-diabetic and healthy individuals. J. Diabetes Metab. Disord. 19, 1199–1203 (2020).
Thaiss, C. A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014).
Thaiss, C. A. et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167, 1495–1510.e12 (2016).
Zheng, D., Ratiner, K. & Elinav, E. Circadian influences of diet on the microbiome and immunity. Trends Immunol. 41, 512–530 (2020).
Ratiner, K., Shapiro, H., Goldenberg, K. & Elinav, E. Time‐limited diets and the gut microbiota in cardiometabolic disease. J. Diabetes 14, 377–393 (2022).
Cosnes, J. Smoking, physical activity, nutrition and lifestyle: environmental factors and their impact on IBD. Dig. Dis. 28, 411–417 (2010).
Rippe, J. M. Lifestyle strategies for risk factor reduction, prevention, and treatment of cardiovascular disease. Am. J. Lifestyle Med. 13, 204–212 (2019).
Schulze, M. B. & Hu, F. B. Primary prevention of diabetes: what can be done and how much can be prevented? Annu. Rev. Public Health 26, 445–467 (2005).
Wu, Q., Gao, Z. J., Yu, X. & Wang, P. Dietary regulation in health and disease. Signal Transduct. Target. Ther. 7, 252 (2022).
Ludwig, D. S. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287, 2414–2423 (2002).
Leshem, A., Segal, E. & Elinav, E. The gut microbiome and individual-specific responses to diet. mSystems 5, e00665-20 (2020).
Korem, T. et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 25, 1243–1253.e5 (2017).
Vega-López, S., Ausman, L. M., Griffith, J. L. & Lichtenstein, A. H. Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 30, 1412–1417 (2007).
Vrolix, R. & Mensink, R. P. Variability of the glycemic response to single food products in healthy subjects. Contemp. Clin. Trials 31, 5–11 (2010).
Ben-Yacov, O. et al. Personalized postprandial glucose response-targeting diet versus Mediterranean diet for glycemic control in prediabetes. Diabetes Care 44, 1980–1991 (2021).
Rein, M. et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: a randomized dietary intervention pilot trial. BMC Med. 20, 56 (2022).
Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27, 321–332 (2021).
Thaiss, C. A. et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540, 544–551 (2016).
Suez, J. et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 185, 3307–3328.e19 (2022).
Otaru, N. et al. GABA production by human intestinal Bacteroides spp.: prevalence, regulation, and role in acid stress tolerance. Front. Microbiol. 12, 656895 (2021).
Harris, K. K., Zopey, M. & Friedman, T. C. Metabolic effects of smoking cessation. Nat. Rev. Endocrinol. 12, 299–308 (2016).
Biedermann, L. et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE 8, e59260 (2013).
Biedermann, L. et al. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel Dis. 20, 1496–1501 (2014).
Fluhr, L. et al. Gut microbiota modulates weight gain in mice after discontinued smoke exposure. Nature 600, 713–719 (2021).
Schloss, P. D. Identifying and overcoming threats to reproducibility, replicability, robustness, and generalizability in microbiome research. mBio 9, e00525-18 (2018).
Allaband, C. et al. Time of sample collection critical for microbiome replicability. Preprint at bioRxiv https://doi.org/10.1101/2022.10.26.513817 (2022).
Mirzayi, C. et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat. Med. 27, 1885–1892 (2021).
Yu, J. et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66, 70–78 (2017).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019).
Thomas, A. M. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 25, 667–678 (2019).
Zeller, G. et al. Potential of fecal microbiota for early‐stage detection of colorectal cancer. Mol. Syst. Biol. 10, 766 (2014).
Liu, N. N. et al. Multi-kingdom microbiota analyses identify bacterial–fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol. 7, 238–250 (2022).
Yachida, S. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976 (2019).
Loomba, R. et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 25, 1054–1062.e5 (2017).
Oh, T. G. et al. A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab. 32, 878–888.e6 (2020).
Chen, F. et al. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut 71, 1315–1325 (2022).
Serrano-Villar, S. et al. Microbiome-derived cobalamin and succinyl-CoA as biomarkers for improved screening of anal cancer. Nat. Med. 29, 1738–1749 (2023).
Zeng, H., Umar, S., Rust, B., Lazarova, D. & Bordonaro, M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int. J. Mol. Sci. 20, 1214 (2019).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016).
Li, R., Andreu-Sánchez, S., Kuipers, F. & Fu, J. Gut microbiome and bile acids in obesity-related diseases. Best. Pract. Res. Clin. Endocrinol. Metab. 35, 101493 (2021).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Pushalkar, S. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416 (2018).
Poore, G. D. et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574 (2020).
Narunsky-Haziza, L. et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 185, 3789–3806.e17 (2022).
Aykut, B. et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019).
Gihawi, A. et al. Major data analysis errors invalidate cancer microbiome findings. mBio 14, e0160723 (2023).
Offord, C. Key study of cancer microbiomes challenged. Science 381, 590–591 (2023).
Lang, S. & Schnabl, B. Microbiota and fatty liver disease — the known, the unknown, and the future. Cell Host Microbe 28, 233–244 (2020).
Hill, C. et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
O’Toole, P. W., Marchesi, J. R. & Hill, C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2, 17057 (2017).
Charbonneau, M. R., Isabella, V. M., Li, N. & Kurtz, C. B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738 (2020).
Gibson, G. R. et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Junca, H., Pieper, D. H. & Medina, E. The emerging potential of microbiome transplantation on human health interventions. Comput. Struct. Biotechnol. J. 20, 615–627 (2022).
Federici, S., Nobs, S. P. & Elinav, E. Phages and their potential to modulate the microbiome and immunity. Cell Mol. Immunol. 18, https://doi.org/10.1038/s41423-020-00532-4 (2021).
Salminen, S. et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667 (2021).
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Khan, M. T. et al. Synergy and oxygen adaptation for development of next-generation probiotics. Nature 620, 381–385 (2023).
Singh, T. P. & Natraj, B. H. Next-generation probiotics: a promising approach towards designing personalized medicine. Crit. Rev. Microbiol. 47, 479–498 (2021).
Szajewska, H. et al. Probiotics for the management of pediatric gastrointestinal disorders: position paper of the ESPGHAN special interest group on gut microbiota and modifications. J. Pediatr. Gastroenterol. Nutr. 76, 232–247 (2023).
Savarino, E. et al. Functional bowel disorders with diarrhoea: clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 10, 556–284 (2022).
Su, G. L. et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 159, 697–705 (2020).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Aron-Wisnewsky, J. et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 17, 279–297 (2020).
Zhou, L. et al. Faecalibacterium prausnitzii produces butyrate to maintain TH17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis. 24, 1926–1940 (2018).
Munukka, E. et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 11, 1667–1679 (2017).
Miquel, S. et al. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 16, 255–261 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05542355 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04938843 (2021).
Ansaldo, E. et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364, 1179–1184 (2019).
Blacher, E. et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480 (2019).
Li, J., Lin, S., Vanhoutte, P. M., Woo, C. W. & Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe−/− mice. Circulation 133, 2434–2446 (2016).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016).
Heintz-Buschart, A. et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 33, 88–98 (2018).
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353.e21 (2016).
Sorbara, M. T. & Pamer, E. G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 20, 365–380 (2022).
Dewulf, E. M. et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62, 1112–1121 (2013).
Kovatcheva-Datchary, P. et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 22, 971–982 (2015).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021).
Mehta, R. S. et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 3, 921–927 (2017).
Gurry, T. et al. Predictability and persistence of prebiotic dietary supplementation in a healthy human cohort. Sci. Rep. 8, 12699 (2018).
Korpela, K. et al. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE 9, e90702 (2014).
Nguyen, N. K. et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 8, https://doi.org/10.7939/r3-9yab-g811 (2020).
Hall, H. et al. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 16, e2005143 (2018).
Mendes-Soares, H. et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw. Open 2, e188102 (2019).
Popp, C. J. et al. Effect of a personalized diet to reduce postprandial glycemic response vs a low-fat diet on weight loss in adults with abnormal glucose metabolism and obesity: a randomized clinical trial. JAMA Netw. Open. 5, E2233760 (2022).
Hwang, I. Y. & Chang, M. W. Engineering commensal bacteria to rewire host–microbiome interactions. Curr. Opin. Biotechnol. 62, 116–122 (2020).
Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 11, eaau7975 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03447730 (2021).
Russell, B. J. et al. Intestinal transgene delivery with native E. coli chassis allows persistent physiological changes. Cell 185, 3263–3277.e15 (2022).
Funabashi, M. et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 582, 566–570 (2020).
Ashraf, M. F., Tageldin, O., Nassar, Y. & Batool, A. Fecal microbiota transplantation in patients with recurrent Clostridium difficile infection: a four-year single-center retrospective review. Gastroenterol. Res. 14, 237–243 (2021).
Bibbò, S. et al. Fecal microbiota transplantation: screening and selection to choose the optimal donor. J. Clin. Med. 9, 1757 (2020).
Baruch, E. N., Gaglani, T. & Wargo, J. A. Fecal microbiota transplantation as a mean of overcoming immunotherapy-resistant cancers—hype or hope? Ther. Adv. Med. Oncol. 13, 17588359211045853 (2021).
Jensen, C., Antonsen, M. F. & Lied, G. A. Gut microbiota and fecal microbiota transplantation in patients with food allergies: a systematic review. Microorganisms 10, 1904 (2022).
Bajaj, J. S. et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology 73, 1688–1700 (2021).
Lavelle, A. & Sokol, H. Understanding and predicting the efficacy of FMT. Nat. Med. 28, 1759–1760 (2022).
Schmidt, T. S. B. et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat. Med. 28, 1902–1912 (2022).
Li, S. S. et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 352, 586–589 (2016).
Ianiro, G. et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat. Med. 28, 1913–1923 (2022).
Louie, T. et al. VE303, a defined bacterial consortium, for prevention of recurrent Clostridioides difficile infection. JAMA 329, 1356 (2023).
DeFilipp, Z. et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2, 745–753 (2018).
Taur, Y. et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 10, eaap9489 (2018).
Goeser, F. et al. Fecal microbiota transfer for refractory intestinal graft-versus-host disease—experience from two German tertiary centers. Eur. J. Haematol. 107, 229–245 (2021).
Stein-Thoeringer, C. K. et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 366, 1143–1149 (2019).
Lev-Sagie, A. et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25, 1500–1504 (2019).
Nath, S. et al. Development and characterization of an oral microbiome transplant among Australians for the treatment of dental caries and periodontal disease: a study protocol. PLoS ONE 16, e0260433 (2021).
Duan, Y. et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 575, 505–511 (2019).
Yuan, J. et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 30, 675–688.e7 (2019).
Chassaing, B. et al. Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J. Clin. Invest. 121, 966–975 (2011).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
Huh, J. W. & Roh, T. Y. Opportunistic detection of Fusobacterium nucleatum as a marker for the early gut microbial dysbiosis. BMC Microbiol. 20, 208 (2020).
Ventola, C. L. The antibiotics resistance crisis: part 1: causes and threats. P. T. 40, 277–283 (2015).
Stern, A. & Sorek, R. The phage–host arms race: shaping the evolution of microbes. BioEssays 33, 43–51 (2011).
Federici, S. et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898.e24 (2022).
Gan, L. et al. Bacteriophage targeting microbiota alleviates non-alcoholic fatty liver disease induced by high alcohol-producing Klebsiella pneumoniae. Nat. Commun. 14, 3215 (2023).
Ichikawa, M. et al. Bacteriophage therapy against pathological Klebsiella pneumoniae ameliorates the course of primary sclerosing cholangitis. Nat. Commun. 14, 3261 (2023).
Gagliardi, A. et al. Rebuilding the gut microbiota ecosystem. Int. J. Environ. Res. Public Health 15, 1679 (2018).
Hobley, L. et al. Dual predation by bacteriophage and Bdellovibrio bacteriovorus can eradicate Escherichia coli prey in situations where single predation cannot. J. Bacteriol. 202, e00629-19 (2020).
Citorik, R. J., Mimee, M. & Lu, T. K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 32, 1141–1145 (2014).
Krautkramer, K. A., Fan, J. & Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 19, 77–94 (2021).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Anachad, O., Taouil, A., Taha, W., Bennis, F. & Chegdani, F. The implication of short-chain fatty acids in obesity and diabetes. Microbiol. Insights 16, 117863612311627 (2023).
Kim, C. H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 20, 341–350 (2023).
O’Keefe, S. J. D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 13, 691–706 (2016).
Silva, Y. P., Bernardi, A. & Frozza, R. L. The role of short-chain fatty acids from gut microbiota in gut–brain communication. Front. Endocrinol. 11, 25 (2020).
Psichas, A. et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39, 424–429 (2015).
Zhao, L. et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 1151–1156 (2018).
Bouter, K. E. C. et al. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects article. Clin. Transl. Gastroenterol. 9, 155 (2018).
Cherta-Murillo, A. et al. The effects of SCFAs on glycemic control in humans: a systematic review and meta-analysis. Am. J. Clin. Nutr. 116, 335–361 (2022).
Kowdley, K. V. et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 67, 1890–1902 (2018).
Kowdley, K. V. et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J. Hepatol. 73, 94–101 (2020).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Loomba, R. et al. Factors associated with histologic response in adult patients with nonalcoholic steatohepatitis. Gastroenterology 156, 88–95.e5 (2019).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Roberts, A. B. et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 24, 1407–1417 (2018).
Natividad, J. M. et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 28, 737–749.e4 (2018).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Wrzosek, L. et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 70, 1299–1308 (2021).
Naganuma, M. et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154, 935–947 (2018).
Alexander, J. L. et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 14, 356–365 (2017).
Yoo, J. Y., Groer, M., Dutra, S. V. O., Sarkar, A. & McSkimming, D. I. Gut microbiota and immune system interactions. Microorganisms 8, 1587 (2020).
Stein-Thoeringer, C. K. et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19–CAR-T cell cancer immunotherapy. Nat. Med. 29, 906–916 (2023).
Muñiz Pedrogo, D. A. et al. Gut microbial carbohydrate metabolism hinders weight loss in overweight adults undergoing lifestyle intervention with a volumetric diet. Mayo Clin. Proc. 93, 1104–1110 (2018).
Artacho, A. et al. The pretreatment gut microbiome is associated with lack of response to methotrexate in new-onset rheumatoid arthritis. Arthritis Rheumatol. 73, 934–946 (2021).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Tintelnot, J. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615, 163–174 (2023).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Derosa, L. et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 29, 1437–1444 (2018).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
Smith, M. et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 28, 713–723 (2022).
Haiser, H. J., Seim, K. L., Balskus, E. P. & Turnbaugh, P. J. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes 5, 233–238 (2014).
Kaddurah-Daouk, R. et al. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS ONE 6, e25482 (2011).
Liu, Y. et al. Gut microbiome associates with lipid-lowering effect of rosuvastatin in vivo. Front. Microbiol. 9, 530 (2018).
Cammarota, G. et al. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 17, 635–648 (2020).
Ferreira, M. R. et al. Microbiota- and radiotherapy-induced gastrointestinal side-effects (MARS) study: a large pilot study of the microbiome in acute and late-radiation enteropathy. Clin. Cancer Res. 25, 6487–6500 (2019).
Manichanh, C. et al. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 103, 1754–1761 (2008).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
LoGuidice, A., Wallace, B. D., Bendel, L., Redinbo, M. R. & Boelsterli, U. A. Pharmacologic targeting of bacterial β-glucuronidase alleviates nonsteroidal anti-inflammatory drug-induced enteropathy in mice. J. Pharmacol. Exp. Ther. 341, 447–454 (2012).
Stein, A., Voigt, W. & Jordan, K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther. Adv. Med. Oncol. 2, 51–63 (2010).
Mego, M. et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther. Med. 23, 356–362 (2015).
Bhatt, A. P. et al. Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl Acad. Sci. USA 117, 7374–7381 (2020).
Cheng, K. W. et al. Pharmacological inhibition of bacterial β-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor efficacy in vivo. Pharmacol. Res. 139, 41–49 (2019).
Ianiro, G. et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat. Commun. 11, 4333 (2020).
Heinken, A. et al. Genome-scale metabolic reconstruction of 7,302 human microorganisms for personalized medicine. Nat. Biotechnol. 41, 1320–1331 (2023).
Liwinski, T., Leshem, A. & Elinav, E. Breakthroughs and bottlenecks in microbiome research. Trends Mol. Med. 27, 298–301 (2021).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Almeida, A. et al. A new genomic blueprint of the human gut microbiota. Nature 568, 499–504 (2019).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Gupta, V. K., Paul, S. & Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 8, 1162 (2017).
Porras, A. M. et al. Geographic differences in gut microbiota composition impact susceptibility to enteric infection. Cell Rep. 36, 109457 (2021).
de Jong, S. E., Olin, A. & Pulendran, B. The impact of the microbiome on immunity to vaccination in humans. Cell Host Microbe 28, 169–179 (2020).
Kennedy, K. M. et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 613, 639–649 (2023).
Suez, J. et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174, 1406–1423.e16 (2018).
Zmora, N. et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174, 1388–1405.e21 (2018).
Montassier, E. et al. Probiotics impact the antibiotic resistance gene reservoir along the human GI tract in a person-specific and antibiotic-dependent manner. Nat. Microbiol. 6, 1043–1054 (2021).
Veiga, P., Suez, J., Derrien, M. & Elinav, E. Moving from probiotics to precision probiotics. Nat. Microbiol. 5, 878–880 (2020).
van der Lelie, D. et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat. Commun. 12, 3105 (2021).
Shalon, D. et al. Profiling the human intestinal environment under physiological conditions. Nature 617, 581–591 (2023).
Park, S. et al. A mountable toilet system for personalized health monitoring via the analysis of excreta. Nat. Biomed. Eng. 4, 624–635 (2020).
Ge, T. J. et al. Passive monitoring by smart toilets for precision health. Sci. Transl. Med. 15, eabk3489 (2023).
Lin, X., Waring, K., Tyson, J. & Ziels, M. R. High-accuracy meets high-throughput for microbiome profiling with near full-length 16S rRNA amplicon sequencing on the Nanopore platform. Preprint at BioRxiv https://doi.org/10.1101/2023.06.19.544637 (2023).
Puschhof, J., Pleguezuelos-Manzano, C. & Clevers, H. Organoids and organs-on-chips: insights into human gut–microbe interactions. Cell Host Microbe 29, 867–878 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03999853 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05218850 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05576597 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04566679 (2021).
Nevens, F. et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 375, 631–643 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05576038 (2023).
Lopera-Maya, E. A. et al. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat. Genet. 54, 143–151 (2022).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Schroeder, B. O. et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23, 27–40.e7 (2018).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 12374339 (2013).
Kundu, P., Blacher, E., Elinav, E. & Pettersson, S. Our gut microbiome: the evolving inner self. Cell 171, 1481–1493 (2017).
Acknowledgements
K.R. is supported by The Nehemia Levtzion Scholarship for Outstanding Doctoral Students. D.C. received postdoctoral fellowship funding from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement No 101068352. S.K.A. is supported by the Israeli Ministry of Science and Technology Zvi Yanai Fellowship. E.E. is supported by the Leona M. and Harry B. Helmsley Charitable Trust, Adelis Foundation, Ben B. and Joyce E. Eisenberg Foundation, Estate of Bernard Bishin for the WIS-Clalit Program, Jeanne and Joseph Nissim Center for Life Sciences Research, Miel de Botton, Swiss Society Institute for Cancer Prevention Research, Belle S. and Irving E. Meller Center for the Biology of Aging, Sagol Institute for Longevity Research, Sagol Weizmann-MIT Bridge Program, Norman E. Alexander Family M Foundation Coronavirus Research Fund, Mike and Valeria Rosenbloom Foundation, Daniel Morris Trust, Isidore and Penny Myers Foundation and Vainboim Family, and by grants funded by the European Research Council, Israel Science Foundation, Israel Ministry of Science and Technology, Israel Ministry of Health, German–Israeli Helmholtz International Research School: Cancer-TRAX (HIRS-0003), Helmholtz Association’s Initiative and Networking Fund, Minerva Foundation, Garvan Institute, European Crohn’s and Colitis Organization, DeutschIsraelische Projektkooperation, IDSA Foundation, WIS-MIT grant, Emulate, Charlie Teo Foundation, Mark Foundation for Cancer Research and Welcome Trust; is the incumbent of the Sir Marc and Lady Tania Feldmann Professorial Chair of immunology; is a senior fellow at the Canadian Institute of Advanced Research; and is an international scholar at the The Bill & Melinda Gates Foundation and Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
E.E. is a scientific founder of Daytwo and BiomX, and a paid consultant to Igen, PurposeBio, Aposense and Zoe. The other authors declare no competing interests.
Peer review
Peer review information
Nature Review Microbiology thanks Marcel van den Brink and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Dysbiosis
-
An altered microbiome configuration, characterized by imbalances in the microbial community structure such as composition, metabolic activities or distribution, that can be associated with disease.
- Next-generation probiotics
-
Microbial taxa, experimentally evaluated as therapeutic living microorganisms, that would be optimized for human colonization, while featuring a reproducible safety and efficacy in promoting health.
- Personalized nutrition
-
An experimental predictive approach that incorporates multi-faceted information, including data derived from the microbiome, to optimize the precision and efficacy of tailored dietary recommendations.
- Postbiotics
-
Small molecules — metabolites — of varying chemical composition that are produced, degraded or modified by commensal microorganisms, or released following bacterial lysis.
- Prebiotics
-
Nutritional supplements, such as fibres, that are selectively utilized by host microorganisms, conferring a health benefit by promoting the growth of commensal microorganisms.
- Probiotics
-
Living microorganisms that are consumed to confer a health benefit to the host; despite widespread popularity, no probiotic preparations were approved to date by the US and European regulatory agencies as medical treatment or prevention.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ratiner, K., Ciocan, D., Abdeen, S.K. et al. Utilization of the microbiome in personalized medicine. Nat Rev Microbiol 22, 291–308 (2024). https://doi.org/10.1038/s41579-023-00998-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-023-00998-9