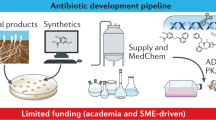
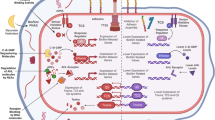
Abstract
Resistance threatens to render antibiotics — which are essential for modern medicine — ineffective, thus posing a threat to human health. The discovery of novel classes of antibiotics able to overcome resistance has been stalled for decades, with the developmental pipeline relying almost entirely on variations of existing chemical scaffolds. Unfortunately, this approach has been unable to keep pace with resistance evolution, necessitating new therapeutic strategies. In this Review, we highlight recent efforts to discover non-traditional antimicrobials, specifically describing the advantages and limitations of antimicrobial peptides and macrocycles, antibodies, bacteriophages and antisense oligonucleotides. These approaches have the potential to stem the tide of resistance by expanding the physicochemical property space and target spectrum occupied by currently approved antibiotics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Genilloud, O. Natural products discovery and potential for new antibiotics. Curr. Opin. Microbiol. 51, 81–87 (2019).
Hutchings, M., Truman, A. & Wilkinson, B. Antibiotics: past, present and future. Curr. Opin. Microbiol. 51, 72–80 (2019).
Lepore, C., Silver, L., Theuretzbacher, U., Thomas, J. & Visi, D. The small-molecule antibiotics pipeline: 2014–2018. Nat. Rev. Drug Discov. 18, 739–739 (2019).
Theuretzbacher, U., Outterson, K., Engel, A. & Karlamp, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 18, 275–285 (2020).
Sorbara, M. T. & Pamer, E. G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 20, 365–380 (2022).
Hotinger, J. A., Morris, S. T. & May, A. E. The case against antibiotics and for anti-virulence therapeutics. Microorganisms 9, 2049 (2021).
Tyers, M. & Wright, G. D. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 17, 141–155 (2019).
Micoli, F., Bagnoli, F., Rappuoli, R. & Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 19, 287–302 (2021).
Jun, L. et al. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 11, 3919–3931 (2019).
Baltzer, S. A. & Brown, M. H. Antimicrobial peptides — promising alternatives to conventional antibiotics. J. Mol. Microb. Biotech. 20, 228–235 (2011).
Kumar, P., Kizhakkedathu, J. N. & Straus, S. K. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 8, 4 (2018).
Tommasi, R., Brown, D. G., Walkup, G. K., Manchester, J. I. & Miller, A. A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 14, 529–542 (2015).
Chen, C. H. & Lu, T. K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 9, 24 (2020).
Kang, S.-J., Park, S. J., Mishig-Ochir, T. & Lee, B.-J. Antimicrobial peptides: therapeutic potentials. Expert Rev. Anti Infect.Ther. 12, 1477–1486 (2014).
Durand, G. A., Raoult, D. & Dubourg, G. Antibiotic discovery: history, methods and perspectives. Int. J. Antimicrob. Agents. 53, 371–382 (2019).
Torres, M. D. T., Sothiselvam, S., Lu, T. K. & de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 431, 3547–3567 (2019).
Lee, P., Chu, C., Tsai, Y., Chuang, Y. & Lung, F. Design, synthesis, and antimicrobial activities of novel functional peptides against Gram‐positive and Gram‐negative bacteria. Chem. Biol. Drug Des. 94, 1537–1544 (2019).
Edwards, I. A., Elliott, A. G., Kavanagh, A. M., Blaskovich, M. A. T. & Cooper, M. A. Structure–activity and −toxicity relationships of the antimicrobial peptide tachyplesin-1. ACS Infect. Dis. 3, 917–926 (2017).
Torres, M. D. T. et al. Structure-function-guided exploration of the antimicrobial peptide polybia-CP identifies activity determinants and generates synthetic therapeutic candidates. Commun. Biol. 1, 221 (2018).
Cardoso, M. H. et al. Computer-aided design of antimicrobial peptides: are we generating effective drug candidates? Front. Microbiol. 10, 3097 (2020).
Fjell, C. D., Hiss, J. A., Hancock, R. E. W. & Schneider, G. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11, 37–51 (2012).
Bhadra, P., Yan, J., Li, J., Fong, S. & Siu, S. W. I. AmPEP: sequence-based prediction of antimicrobial peptides using distribution patterns of amino acid properties and random forest. Sci. Rep. 8, 1697 (2018).
Lee, E. Y., Lee, M. W., Fulan, B. M., Ferguson, A. L. & Wong, G. C. L. What can machine learning do for antimicrobial peptides, and what can antimicrobial peptides do for machine learning? Interface Focus 7, 20160153 (2017).
Spohn, R. et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 10, 4538 (2019).
Martin-Loeches, I., Dale, G. E. & Torres, A. Murepavadin: a new antibiotic class in the pipeline. Expert Rev. Anti Infect. Ther. 16, 259–268 (2018).
Polyphor, A. G. Polyphor temporarily halts enrollment in the Phase III studies of murepavadin for the treatment of patients with nosocomial pneumonia. https://spexisbio.com/news/corporate-news-details/?newsid=1775911 (2019).
Hartzell, J. D. et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 48, 1724–1728 (2009).
Gai, Z., Samodelov, S. L., Kullak-Ublick, G. A. & Visentin, M. Molecular mechanisms of colistin-induced nephrotoxicity. Molecules 24, 653 (2019).
Vaara, M. New polymyxin derivatives that display improved efficacy in animal infection models as compared to polymyxin B and colistin. Med. Res. Rev. 38, 1661–1673 (2018).
Zurawski, D. V. et al. SPR741, an antibiotic adjuvant, potentiates the in vitro and in vivo activity of rifampin against clinically relevant extensively drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 61, e01239-17 (2017).
Brown, P. & Dawson, M. J. Development of new polymyxin derivatives for multi-drug resistant Gram-negative infections. J. Antibiotics 70, 386–394 (2017).
Quale, J. et al. Activity of polymyxin B and the novel polymyxin analogue CB-182,804 against contemporary gram-negative pathogens in New York City. Microb. Drug Resist. 18, 132–136 (2011).
Brown, P. et al. Design of next generation polymyxins with lower toxicity: the discovery of SPR206. ACS Infect. Dis. 5, 1645–1656 (2019).
Bruss, J. et al. Single- and multiple-ascending-dose study of the safety, tolerability, and pharmacokinetics of the polymyxin derivative SPR206. Antimicrob. Agents Chemother. 65, e00739-21 (2021).
Roberts, K. D. et al. A synthetic lipopeptide targeting top-priority multidrug-resistant Gram-negative pathogens. Nat. Commun. 13, 1625 (2022).
Du, H., Chen, L., Tang, Y. W. & Kreiswirth, B. N. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect. Dis. 16, 287–288 (2016).
Wang, Z. et al. A naturally inspired antibiotic to target multidrug-resistant pathogens. Nature 601, 606–611 (2022).
Tavassoli, A. & Benkovic, S. J. Split-intein mediated circular ligation used in the synthesis of cyclic peptide libraries in E. coli. Nat. Protoc. 2, 1126–1133 (2007).
Lam, K. S., Lebl, M. & Krchňák, V. The “One-Bead-One-Compound” combinatorial library method. Chem. Rev. 97, 411–448 (1997).
Ullman, C. G., Frigotto, L. & Cooley, R. N. In vitro methods for peptide display and their applications. Brief. Funct. Genomics 10, 125–134 (2011).
Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 20, 309–325 (2021).
Goto, Y., Katoh, T. & Suga, H. Flexizymes for genetic code reprogramming. Nat. Protoc. 6, 779–790 (2011).
Davies, J. S. The cyclization of peptides and depsipeptides. J. Pept. Sci. 9, 471–501 (2003).
Kale, S. S. et al. Cyclization of peptides with two chemical bridges affords large scaffold diversities. Nat. Chem. 10, 715–723 (2018).
Tucker, A. T. et al. Discovery of next-generation antimicrobials through bacterial self-screening of surface-displayed peptide libraries. Cell 172, 618–628.e13 (2018).
Randall, J. R. et al. Synthetic antibacterial discovery of symbah-1, a macrocyclic β-hairpin peptide antibiotic. iScience 25, 103611 (2022).
Sivertsen, A. et al. Synthetic cationic antimicrobial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct. Biol. 14, 4 (2014).
Jangir, P. K. et al. The evolution of colistin resistance increases bacterial resistance to host antimicrobial peptides and virulence. eLife 12, e84395 (2023).
Nagy, E., Nagy, G., Power, C. A., Badarau, A. & Szijártó, V. Anti-bacterial monoclonal antibodies. Adv. Exp. Med. Biol. 1053, 119–153 (2017).
Niebecker, R. & Kloft, C. Safety of therapeutic monoclonal antibodies. Curr. Drug Saf. 5, 275–286 (2010).
Kollef, M. H. & Betthauser, K. D. Monoclonal antibodies as antibacterial therapies: thinking outside of the box. Lancet Infect. Dis. 21, 1201–1202 (2021).
Motley, M. P., Banerjee, K. & Fries, B. C. Monoclonal antibody-based therapies for bacterial infections. Curr. Opin. Infect. Dis. 32, 210–216 (2019).
Singh, S. et al. Monoclonal antibodies: a review. Curr. Clin. Pharmacol. 13, 85–99 (2018).
Vij, R. et al. A targeted boost-and-sort immunization strategy using Escherichia coli BamA identifies rare growth inhibitory antibodies. Sci. Rep. 8, 7136 (2018).
Storek, K. M. et al. Massive antibody discovery used to probe structure-function relationships of the essential outer membrane protein LptD. eLife 8, 3002 (2019).
LaRocca, T. J. et al. The bactericidal effect of a complement-independent antibody is osmolytic and specific to Borrelia. Proc. Natl Acad. Sci. USA 106, 10752–10757 (2009).
Migone, T.-S. et al. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361, 135–144 (2009).
Greig, S. L. Obiltoxaximab: first global approval. Drugs 76, 823–830 (2016).
Wilcox, M. H. et al. Bezlotoxumab for prevention of recurrent clostridium difficile infection. N. Engl. J. Med. 376, 305–317 (2017).
François, B. et al. Efficacy and safety of suvratoxumab for prevention of Staphylococcus aureus ventilator-associated pneumonia (SAATELLITE): a multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 2 pilot trial. Lancet Infect. Dis. 21, 1313–1323 (2021).
Wang, H., Chen, D. & Lu, H. Anti-bacterial monoclonal antibodies: next generation therapy against superbugs. Appl. Microbiol. Biotechnol. 106, 3957–3972 (2022).
Zurawski, D. V. & McLendon, M. K. Monoclonal antibodies as an antibacterial approach against bacterial pathogens. Antibiotics 9, 155 (2020).
López, E. L. et al. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against shiga-like toxin 2 in healthy adults and in pediatric patients infected with shiga-like toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 54, 239–243 (2010).
Mohawk, K. L., Melton-Celsa, A. R., Robinson, C. M. & O’Brien, A. D. Neutralizing antibodies to Shiga toxin type 2 (Stx2) reduce colonization of mice by Stx2-expressing Escherichia coli O157:H7. Vaccine 28, 4777–4785 (2010).
Cheng, L. W., Henderson, T. D., Patfield, S., Stanker, L. H. & He, X. Mouse in vivo neutralization of Escherichia coli Shiga toxin 2 with monoclonal antibodies. Toxins 5, 1845–1858 (2013).
Yamagami, S. et al. Efficacy of postinfection treatment with anti-shiga toxin (Stx) 2 humanized monoclonal antibody TMA-15 in mice lethally challenged with stx-producing Escherichia coli. J. Infect. Dis. 184, 738–742 (2001).
Hotinger, J. A. & May, A. E. Antibodies inhibiting the type III secretion system of gram-negative pathogenic bacteria. Antibodies 9, 35 (2020).
Yu, S. et al. Identification of a novel linear epitope on EspA from enterohemorrhagic E. coli using a neutralizing and protective monoclonal antibody. Clin. Immunol. 138, 77–84 (2011).
Ruano-Gallego, D. et al. A nanobody targeting the translocated intimin receptor inhibits the attachment of enterohemorrhagic E. coli to human colonic mucosa. PLoS Pathog. 15, e1008031 (2019).
Mishra, M. et al. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 14, 95–106 (2012).
Howell, H. A., Logan, L. K. & Hauser, A. R. Type III secretion of ExoU is critical during early Pseudomonas aeruginosa pneumonia. mBio 4, e00032-13 (2013).
DiGiandomenico, A. et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 6, 262ra155 (2014).
DiGiandomenico, A. et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 209, 1273–1287 (2012).
Ray, V. A. et al. Anti-Psl targeting of Pseudomonas aeruginosa biofilms for neutrophil-mediated disruption. Sci. Rep. 7, 16065 (2018).
Thanabalasuriar, A. et al. Bispecific antibody targets multiple Pseudomonas aeruginosa evasion mechanisms in the lung vasculature. J. Clin. Invest. 127, 2249–2261 (2017).
Ali, S. O. et al. Phase 1 study of MEDI3902, an investigational anti-Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 25, 629.e1–629.e6 (2019).
Chastre, J. et al. 635. Efficacy, pharmacokinetics (PK), and safety profile of MEDI3902, an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody in mechanically ventilated intensive care unit patients; results of the phase 2 EVADE study conducted by the public-private COMBACTE-MAGNET consortium in the Innovative Medicines Initiative (IMI) program. Open Forum Infect. Dis. 7, S377–S378 (2020).
Que, Y.-A. et al. Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1861–1867 (2014).
Secher, T. et al. The anti-Pseudomonas aeruginosa antibody panobacumab is efficacious on acute pneumonia in neutropenic mice and has additive effects with meropenem. PLoS One 8, e73396–12 (2013).
Cao, J. et al. Targeting the gram-negative bacteria peptidoglycan synthase MraY as a new approach for monoclonal antibody anti-bacterial activity. Hum. Vaccin. Immunother. 13, 2086–2091 (2017).
Storek, K. M. et al. Monoclonal antibody targeting the β-barrel assembly machine of Escherichia coli is bactericidal. Proc. Natl Acad. Sci. USA 115, 3692–3697 (2018).
Deveuve, Q., Lajoie, L., Barrault, B. & Thibault, G. The proteolytic cleavage of therapeutic monoclonal antibody hinge region: more than a matter of subclass. Front. Immunol. 11, 168 (2020).
Prokešová, L. et al. Cleavage of human immunoglobulins by serine proteinase from Staphylococcus aureus. Immunol. Lett. 31, 259–265 (1992).
Storek, K. M. et al. The Escherichia coli β-barrel assembly machinery is sensitized to perturbations under high membrane fluidity. J. Bacteriol. 201, e00517-18 (2018).
Mariathasan, S. & Tan, M. W. Antibody-antibiotic conjugates: a novel therapeutic platform against bacterial infections. Trends Mol. Med. 23, 135–149 (2017).
Lehar, S. M. et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 527, 323–328 (2015).
Chari, R. V. J., Miller, M. L. & Widdison, W. C. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew. Chem. Int. Ed. Engl. 53, 3796–3827 (2014).
Zhou, C. et al. Pharmacokinetics and pharmacodynamics of DSTA4637A: a novel THIOMABTM antibody antibiotic conjugate against Staphylococcus aureus in mice. mAbs 8, 1612–1619 (2016).
Peck, M. et al. A phase 1, randomized, single-ascending-dose study to investigate the safety, tolerability, and pharmacokinetics of DSTA4637S, an anti-Staphylococcus aureus thiomab antibody-antibiotic conjugate, in healthy volunteers. Antimicrob. Agents Chemother. 63, e02588-18 (2019).
Rymut, S. M. et al. 1305. Comparison of pharmacokinetics of DSTA4637S, a novel THIOMABTM antibody-antibiotic conjugate, in patients with Staphylococcus aureus bacteremia receiving standard-of-care antibiotics with pharmacokinetics in healthy volunteers. Open Forum Infect. Dis. 7, S666–S667 (2020).
Cai, H. et al. Characterization of tissue distribution, catabolism and elimination of an anti-Staphylococcus aureus THIOMABTM antibody-antibiotic conjugate in rats. Drug Metab. Dispos. 48, 1161–1168 (2020).
Deng, R. et al. Preclinical and translational pharmacokinetics of a novel THIOMABTM antibody-antibiotic conjugate against Staphylococcus aureus. mAbs 11, 1162–1174 (2019).
Stagg, N. J. et al. Nonclinical toxicology development of a novel antibody antibiotic conjugate for treating invasive Staphylococcus aureus infections. Toxicol. Appl. Pharm. 435, 115811 (2022).
Zacharias, N. et al. A homogeneous high-DAR antibody–drug conjugate platform combining THIOMAB antibodies and XTEN polypeptides. Chem. Sci. 13, 3147–3160 (2022).
McCallin, S., Sacher, J. C., Zheng, J. & Chan, B. K. Current state of compassionate phage therapy. Viruses 11, 343 (2019).
Hyman, P. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals 12, 35 (2019).
Eliava Institute https://eliava-institute.org/?lang=en.
Żaczek, M., Weber-Dąbrowska, B., Międzybrodzki, R., Łusiak-Szelachowska, M. & Górski, A. Phage therapy in Poland — a centennial journey to the first ethically approved treatment facility in Europe. Front. Microbiol. 11, 1056 (2020).
Dedrick, R. M. et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733 (2019).
Nick, J. A. et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 185, 1860–1874.e12 (2022).
LaVergne, S. et al. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect. Dis. 5, ofy064 (2018).
Jault, P. et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45 (2019).
Sarker, S. A. et al. Oral application of Escherichia coli bacteriophage: safety tests in healthy and diarrheal children from Bangladesh. Env. Microbiol. 19, 237–250 (2017).
Sarker, S. A. et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. Ebiomedicine 4, 124–137 (2016).
Leitner, L. et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 21, 427–436 (2020).
Górski, A., Borysowski, J. & Międzybrodzki, R. Phage therapy: towards a successful clinical trial. Antibiotics 9, 827 (2020).
Melo, L. D. R., Oliveira, H., Pires, D. P., Dabrowska, K. & Azeredo, J. Phage therapy efficacy: a review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 46, 78–99 (2020).
Oechslin, F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 10, 351 (2018).
Rodríguez-Rubio, L., Jofre, J. & Muniesa, M. Is genetic mobilization considered when using bacteriophages in antimicrobial therapy? Antibiotics 6, 32 (2017).
Pires, D. P., Costa, A. R., Pinto, G., Meneses, L. & Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 44, 684–700 (2020).
Nagel, T. et al. Phage banks as potential tools to rapidly and cost-effectively manage antimicrobial resistance in the developing world. Curr. Opin. Virol. 53, 101208 (2022).
Chan, B. K., Abedon, S. T. & Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 8, 769–783 (2013).
Lehman, S. M. et al. Design and preclinical development of a phage product for the treatment of antibiotic-resistant Staphylococcus aureus infections. Viruses 11, 88 (2019).
Molina, F. et al. A new pipeline for designing phage cocktails based on phage-bacteria infection networks. Front. Microbiol. 12, 564532 (2021).
Barbu, E. M., Cady, K. C. & Hubby, B. Phage therapy in the era of synthetic biology. Cold Spring Harb. Perspect. Biol. 8, a023879 (2016).
Lenneman, B. R., Fernbach, J., Loessner, M. J., Lu, T. K. & Kilcher, S. Enhancing phage therapy through synthetic biology and genome engineering. Curr. Opin. Biotech. 68, 151–159 (2021).
Egido, J. E., Costa, A. R., Aparicio-Maldonado, C., Haas, P.-J. & Brouns, S. J. J. Mechanisms and clinical importance of bacteriophage resistance. FEMS Microbiol. Rev. 46, fuab048 (2021).
Chen, M. et al. Alterations in gp37 expand the host range of a T4-like phage. Appl. Environ. Microb. 83, e01576-17 (2017).
Mahichi, F., Synnott, A. J., Yamamichi, K., Osada, T. & Tanji, Y. Site‐specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol. Lett. 295, 211–217 (2009).
Yoichi, M., Abe, M., Miyanaga, K., Unno, H. & Tanji, Y. Alteration of tail fiber protein gp38 enables T2 phage to infect Escherichia coli O157:H7. J. Biotechnol. 115, 101–107 (2005).
Burrowes, B. H., Molineux, I. J. & Fralick, J. A. Directed in vitro evolution of therapeutic bacteriophages: the Appelmans protocol. Viruses 11, 241 (2019).
Yehl, K. et al. Engineering phage host-range and suppressing bacterial resistance through phage tail fiber mutagenesis. Cell 179, 459–469.e9 (2019).
Li, B., Niu, Y., Ji, W. & Dong, Y. Strategies for the CRISPR-based therapeutics. Trends Pharmacol. Sci. 41, 55–65 (2020).
Makarova, K. S. et al. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Microbiol. 9, 467–477 (2011).
Pawluk, A., Davidson, A. R. & Maxwell, K. L. Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol. 16, 12–17 (2018).
Bikard, D. et al. Development of sequence-specific antimicrobials based on programmable CRISPR-Cas nucleases. Nat. Biotechnol. 32, 1146–1150 (2014).
Citorik, R. J., Mimee, M. & Lu, T. K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 32, 1141–1145 (2014).
Goren, M., Yosef, I. & Qimron, U. Sensitizing pathogens to antibiotics using the CRISPR-Cas system. Drug Resist. Updat. 30, 1–6 (2017).
Yosef, I., Manor, M., Kiro, R. & Qimron, U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc. Natl Acad. Sci. USA 112, 7267–7272 (2015).
Wang, B., Guo, F., Dong, S.-H. & Zhao, H. Activation of silent biosynthetic gene clusters using transcription factor decoys. Nat. Chem. Biol. 15, 111–114 (2019).
Murray, E., Draper, L. A., Ross, R. P. & Hill, C. The advantages and challenges of using endolysins in a clinical setting. Viruses 13, 680 (2021).
Schuch, R. et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J. Infect. Dis. 209, 1469–1478 (2014).
Fowler, V. G. Jr. et al. Exebacase for Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Invest. 130, 3750–3760 (2020).
Gontijo, M. T. P., Jorge, G. P. & Brocchi, M. Current status of endolysin-based treatments against gram-negative bacteria. Antibiotics 10, 1143 (2021).
Shavrina, M. S. et al. In vitro study of the antibacterial effect of the bacteriophage T5 thermostable endolysin on Escherichia coli cells. J. Appl. Microbiol. 121, 1282–1290 (2016).
Ma, Q. et al. Enhancement of the direct antimicrobial activity of Lysep3 against Escherichia coli by inserting cationic peptides into its C terminus. Antonie Van Leeuwenhoek 110, 347–355 (2017).
Yang, H., Wang, M., Yu, J. & Wei, H. Antibacterial activity of a novel peptide-modified lysin against Acinetobacter baumannii and Pseudomonas aeruginosa. Front. Microbiol. 6, 1471 (2015).
Defraine, V. et al. Efficacy of artilysin art-175 against resistant and persistent Acinetobacter baumannii. Antimicrob. Agents Chemother. 60, 3480–3488 (2016).
Yang, H. et al. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 5, 17257 (2015).
Blázquez, B., Fresco-Taboada, A., Iglesias-Bexiga, M., Menéndez, M. & García, P. PL3 amidase, a tailor-made lysin constructed by domain shuffling with potent killing activity against pneumococci and related species. Front. Microbiol. 7, 1156 (2016).
Crooke, S. T., Witztum, J. L., Bennett, C. F. & Baker, B. F. RNA-targeted therapeutics. Cell Metab. 27, 714–739 (2018).
Crooke, S. T., Liang, X., Crooke, R. M., Baker, B. F. & Geary, R. S. Antisense drug discovery and development technology considered in a pharmacological context. Biochem. Pharmacol. 189, 114196 (2020).
Khvorova, A. & Watts, J. K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 35, 238–248 (2017).
Cirak, S. et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 378, 595–605 (2011).
Santos, R. D. et al. Mipomersen, an antisense oligonucleotide to apolipoprotein b-100, reduces lipoprotein(a) in various populations with hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 35, 689–699 (2015).
Bai, H. et al. Antisense antibiotics: a brief review of novel target discovery and delivery. Curr. Drug Discov. Technol. 7, 76–85 (2010).
Good, L., Sandberg, R., Larsson, O., Nielsen, P. E. & Wahlestedt, C. Antisense PNA effects in Escherichia coli are limited by the outer-membrane LPS layer. Microbiology 146, 2665–2670 (2000).
O’Shea, R. & Moser, H. E. Physicochemical properties of antibacterial compounds: implications for drug discovery. J. Med. Chem. 51, 2871–2878 (2008).
Geller, B. L. et al. Inhibition of gene expression in Escherichia coli by antisense phosphorodiamidate morpholino oligomers. Antimicrob. Agents Chemother. 47, 3233–3239 (2003).
Bennett, C. F., Chiang, M. Y., Chan, H., Shoemaker, J. E. & Mirabelli, C. K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides.Mol. Pharmacol. 41, 1023–1033 (1992).
Chirila, T. V., Rakoczy, P. E., Garrett, K. L., Lou, X. & Constable, I. J. The use of synthetic polymers for delivery of therapeutic antisense oligodeoxynucleotides. Biomaterials 23, 321–342 (2002).
Falzarano, M. S., Passarelli, C. & Ferlini, A. Nanoparticle delivery of antisense oligonucleotides and their application in the exon skipping strategy for Duchenne muscular dystrophy. Nucleic Acid. Ther. 24, 87–100 (2014).
McClorey, G. & Banerjee, S. Cell-penetrating peptides to enhance delivery of oligonucleotide-based therapeutics. Biomedicines 6, 51 (2018).
Ramsey, J. D. & Flynn, N. H. Cell-penetrating peptides transport therapeutics into cells. Pharmacol. Ther. 154, 78–86 (2015).
Foged, C. & Nielsen, H. M. Cell-penetrating peptides for drug delivery across membrane barriers. Expert Opin. Drug Del. 5, 105–117 (2007).
Lee, H.-M. et al. Identification of efficient prokaryotic cell-penetrating peptides with applications in bacterial biotechnology. Commun. Biol. 4, 205 (2021).
Wesolowski, D., Alonso, D. & Altman, S. Combined effect of a peptide–morpholino oligonucleotide conjugate and a cell-penetrating peptide as an antibiotic. Proc. Natl Acad. Sci. USA 110, 8686–8689 (2013).
Geller, B. L. et al. Gene-silencing antisense oligomers inhibit Acinetobacter growth in vitro and in vivo. J. Infect. Dis. 208, 1553–1560 (2013).
Ghosal, A. & Nielsen, P. E. Potent antibacterial antisense peptide–peptide nucleic acid conjugates against Pseudomonas aeruginosa. Nucleic Acid. Ther. 22, 323–334 (2012).
Kurupati, P., Tan, K. S. W., Kumarasinghe, G. & Poh, C. L. Inhibition of gene expression and growth by antisense peptide nucleic acids in a multiresistant β-lactamase-producing Klebsiella pneumoniae strain. Antimicrob. Agents Chemother. 51, 805–811 (2007).
Hansen, A. M. et al. Antibacterial peptide nucleic acid–antimicrobial peptide (PNA–AMP) conjugates: antisense targeting of fatty acid biosynthesis. Bioconjug. Chem. 27, 863–867 (2016).
Ghosal, A., Vitali, A., Stach, J. E. M. & Nielsen, P. E. Role of SbmA in the uptake of peptide nucleic acid (PNA)-peptide conjugates in E. coli. ACS Chem. Biol. 8, 360–367 (2013).
Fischer, D., Li, Y., Ahlemeyer, B., Krieglstein, J. & Kissel, T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 24, 1121–1131 (2003).
García, V. et al. Genome-wide analysis of fitness-factors in uropathogenic Escherichia coli during growth in laboratory media and during urinary tract infections. Microb. Genom. 7, 000719 (2021).
Subashchandrabose, S. et al. Acinetobacter baumannii genes required for bacterial survival during bloodstream infection. mSphere 1, e00013-15 (2015).
Skurnik, D. et al. A comprehensive analysis of in vitro and in vivo genetic fitness of pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog. 9, e1003582 (2013).
Bai, H. et al. Antisense inhibition of gene expression and growth in gram-negative bacteria by cell-penetrating peptide conjugates of peptide nucleic acids targeted to rpoD gene. Biomaterials 33, 659–667 (2012).
Goh, S., Boberek, J. M., Nakashima, N., Stach, J. & Good, L. Concurrent growth rate and transcript analyses reveal essential gene stringency in Escherichia coli. PLoS One 4, e6061 (2009).
Popella, L. et al. Comprehensive analysis of PNA-based antisense antibiotics targeting various essential genes in uropathogenic Escherichia coli. Nucleic Acids Res. 50, gkac362 (2022).
Zhang, Y. & Cronan, J. E. Transcriptional analysis of essential genes of the Escherichia coli fatty acid biosynthesis gene cluster by functional replacement with the analogous Salmonella typhimurium gene cluster. J. Bacteriol. 180, 3295–3303 (1998).
Li, G.-W., Burkhardt, D., Gross, C. & Weissman, J. S. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 157, 624–635 (2014).
Tilley, L. D. et al. Antisense peptide-phosphorodiamidate morpholino oligomer conjugate: dose–response in mice infected with Escherichia coli. J. Antimicrob. Chemother. 59, 66–73 (2007).
Moustafa, D. A. et al. Peptide-conjugated phosphorodiamidate morpholino oligomers retain activity against multidrug-resistant Pseudomonas aeruginosa in vitro and in vivo. mBio 12, e02411-20 (2021).
Geller, B. L. et al. Morpholino oligomers tested in vitro, in biofilm and in vivo against multidrug-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 73, 1611–1619 (2018).
Silvis, M. R. et al. Morphological and transcriptional responses to CRISPRi knockdown of essential genes in Escherichia coli. mBio 12, e02561-21 (2021).
Greenberg, D. E. et al. Antisense phosphorodiamidate morpholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex. J. Infect. Dis. 201, 1822–1830 (2010).
Good, L. & Nielsen, P. E. Inhibition of translation and bacterial growth by peptide nucleic acid targeted to ribosomal RNA. Proc. Natl Acad. Sci. USA 95, 2073–2076 (1998).
Lopez, C., Arivett, B. A., Actis, L. A. & Tolmasky, M. E. Inhibition of AAC(6′)-Ib-mediated resistance to amikacin in Acinetobacter baumannii by an antisense peptide-conjugated 2′,4′-bridged nucleic Acid-NC-DNA hybrid oligomer. Antimicrob. Agents Chemother. 59, 5798–5803 (2015).
Goh, S., Loeffler, A., Lloyd, D. H., Nair, S. P. & Good, L. Oxacillin sensitization of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius by antisense peptide nucleic acids in vitro. BMC Microbiol. 15, 262 (2015).
Sully, E. K. et al. Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) restores carbapenem susceptibility to NDM-1-positive pathogens in vitro and in vivo. J. Antimicrob. Chemother. 72, 782–790 (2016).
Daly, S. M., Sturge, C. R., Felder-Scott, C. F., Geller, B. L. & Greenberg, D. E. MCR-1 inhibition with peptide-conjugated phosphorodiamidate morpholino oligomers restores sensitivity to polymyxin in Escherichia coli. mBio 8, e01315-17 (2017).
Jani, S., Ramirez, M. S. & Tolmasky, M. E. Silencing antibiotic resistance with antisense oligonucleotides. Biomedicines 9, 416 (2021).
Wang, H. et al. oprM as a new target for reversion of multidrug resistance in Pseudomonas aeruginosa by antisense phosphorothioate oligodeoxynucleotides. FEMS Immunol. Med. Microbiol. 60, 275–282 (2010).
Brown, E. D. & Wright, G. D. Antibacterial drug discovery in the resistance era. Nature 529, 336–343 (2016).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Parker, E. N. et al. Implementation of permeation rules leads to a FabI inhibitor with activity against Gram-negative pathogens. Nat. Microbiol. 5, 67–75 (2019).
Lamut, A., Mašič, L. P., Kikelj, D. & Tomašič, T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 39, 2460–2504 (2019).
MacNair, C. R. & Brown, E. D. Outer membrane disruption overcomes intrinsic, acquired, and spontaneous antibiotic resistance. mBio 11, e01615-20 (2020).
Reinisch, W. et al. Safety, pharmacokinetic, and pharmacodynamic study of sibofimloc, a novel FimH blocker in patients with active Crohn’s disease. J. Gastroenterol. Hepatol. 37, 832–840 (2022).
Wunderink, R. G. et al. Effect and safety of meropenem–vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect. Dis. Ther. 7, 439–455 (2018).
DeFilipp, Z. et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 381, 2043–2050 (2019).
Dsouza, M. et al. Colonization of the live biotherapeutic product VE303 and modulation of the microbiota and metabolites in healthy volunteers. Cell Host Microbe 30, 583–598.e8 (2022).
Feuerstadt, P., Allegretti, J. R. & Khanna, S. Practical use of rebyota for the prevention of recurrent Clostridioides difficile infection. Am. J. Gastroenterol. 118, 1303–1306 (2023).
Hurley, D. et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin. Infect. Dis. 73, ciaa1045 (2020).
Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 12, 371–387 (2013).
Emmerson, A. M. The quinolones: decades of development and use. J. Antimicrob. Chemother. 51, 13–20 (2003).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6, 29–40 (2007).
Brown, D. G., May-Dracka, T. L., Gagnon, M. M. & Tommasi, R. Trends and exceptions of physical properties on antibacterial activity for Gram-positive and Gram-negative pathogens. J. Med. Chem. 57, 10144–10161 (2014).
Nikaido, H. & Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49, 1–32 (1985).
Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003).
Kamio, Y. & Nikaido, H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase C and cyanogen bromide activated dextran in the external medium. Biochemistry 15, 2561–2570 (1976).
Funahara, Y. & Nikaido, H. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhimurium. J. Bacteriol. 141, 1463–1465 (1980).
Nikaido, H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 33, 1831–1836 (1989).
Acknowledgements
The authors thank C. Tsai for critically reading the manuscript and providing helpful comments. We also thank members of the Infectious Diseases department for their constructive discussions.
Author information
Authors and Affiliations
Contributions
C.R.M. and S.T.R. wrote the manuscript with input from M.-W.T.
Corresponding author
Ethics declarations
Competing interests
The authors are employees of Genentech Inc., which is a member of the Roche Group.
Peer review
Peer review information
Nature Reviews Microbiology thanks Ursula Theuretzbacher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
MacNair, C.R., Rutherford, S.T. & Tan, MW. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nat Rev Microbiol 22, 262–275 (2024). https://doi.org/10.1038/s41579-023-00993-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-023-00993-0
This article is cited by
-
Diverse and abundant phages exploit conjugative plasmids
Nature Communications (2024)