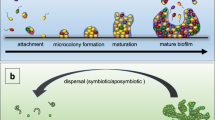
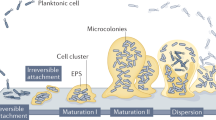
Abstract
Many microorganisms live in the form of a biofilm. Although they are feared in the medical sector, biofilms that are composed of non-pathogenic organisms can be highly beneficial in many applications, including the production of bulk and fine chemicals. Biofilm systems are natural retentostats in which the biocatalysts can adapt and optimize their metabolism to different conditions over time. The adherent nature of biofilms allows them to be used in continuous systems in which the hydraulic retention time is much shorter than the doubling time of the biocatalysts. Moreover, the resilience of organisms growing in biofilms, together with the potential of uncoupling growth from catalytic activity, offers a wide range of opportunities. The ability to work with continuous systems using a potentially self-advancing whole-cell biocatalyst is attracting interest from a range of disciplines, from applied microbiology to materials science and from bioengineering to process engineering. The field of beneficial biofilms is rapidly evolving, with an increasing number of applications being explored, and the surge in demand for sustainable and biobased solutions and processes is accelerating advances in the field. This Review provides an overview of the research topics, challenges, applications and future directions in beneficial and applied biofilm research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Muffler, K. & Ulber, R. Productive biofilms. Adv. Biochem. Eng. Biotechnol. 146, 264 (2014).
Jo, J., Price-Whelan, A. & Dietrich, L. E. P. Gradients and consequences of heterogeneity in biofilms. Nat. Rev. Microbiol. 20, 593–607 (2022).
Halan, B., Buehler, K. & Schmid, A. Biofilms as living catalysts in continuous chemical syntheses. Trends Biotechnol. 30, 453–465 (2012).
Schmeckebier, A., Zayed, A. & Ulber, R. Productive biofilms: from prokaryotic to eukaryotic systems. J. Chem. Technol. Biotechnol. 97, 3049–3064 (2022).
Rosche, B., Li, X. Z., Hauer, B., Schmid, A. & Buehler, K. Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol. 27, 636–643 (2009).
Alvarez, A. L., Weyers, S. L., Goemann, H. M., Peyton, B. M. & Gardner, R. D. Microalgae, soil and plants: a critical review of microalgae as renewable resources for agriculture. Algal Res. 54, 102200 (2021).
Nguyen, P. Q., Botyanszki, Z., Tay, P. K. R. & Joshi, N. S. Programmable biofilm-based materials from engineered curli nanofibres. Nat. Commun. 5, 4945 (2014).
Park, H., Schwartzman, A. F., Tang, T.-C., Wang, L. & Lu, T. K. Ultra-lightweight living structural material for enhanced stiffness and environmental sensing. Mater. Today Bio 18, 100504 (2023).
Karygianni, L., Ren, Z., Koo, H. & Thurnheer, T. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 28, 668–681 (2020).
Flemming, H. C. et al. The biofilm matrix: multitasking in a shared space. Nat. Rev. Microbiol. 21, 70–86 (2022).
Edwards, S. J. & Kjellerup, B. V. Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl. Microbiol. Biotechnol. 97, 9909–9921 (2013).
Härrer, D., Elreedy, A., Ali, R., Hille-Reichel, A. & Gescher, J. Probing the robustness of Geobacter sulfurreducens against fermentation hydrolysate for uses in bioelectrochemical systems. Bioresour. Technol. 369, 128363 (2023).
Morgan-Sagastume, F. Biofilm development, activity and the modification of carrier material surface properties in moving-bed biofilm reactors (MBBRs) for wastewater treatment. Crit. Rev. Env. Sci. Technol. 48, 439–470 (2018).
Grießmeier, V., Wienhöfer, J., Horn, H. & Gescher, J. Assessing and modeling biocatalysis in field denitrification beds reveals key influencing factors for future constructions. Water Res. 188, 116467 (2021).
Lepine, C., Christianson, L., Davidson, J. & Summerfelt, S. Woodchip bioreactors as treatment for recirculating aquaculture systems’ wastewater: a cost assessment of nitrogen removal. Aquacult. Eng. 83, 85–92 (2018).
Rittmann, B. E. Biofilms, active substrata, and me. Water Res. 132, 135–145 (2018).
Bruin, L. M. M., de, Kreuk, M. K., de, Roest, H. F. R., van der, Uijterlinde, C. & van Loosdrecht, M. C. M. Aerobic granular sludge technology: an alternative to activated sludge? Water Sci. Technol. 49, 1–7 (2004).
Tang, C. et al. Performance of high-loaded ANAMMOX UASB reactors containing granular sludge. Water Res. 45, 135–144 (2011).
van de Graaf, A. A. et al. Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 61, 1246–1251 (1995).
Liu, C. et al. Rapid formation of granules coupling n-DAMO and anammox microorganisms to remove nitrogen. Water Res. 194, 116963 (2021).
Dorias, B., Hauber, G. & Baumann, P. in Biotechnology: Environmental Processes I Vol. 11, 2nd edn, Ch. 16 (eds Rehm, H.-J. & Reed, G.) (Wiley, 1999)
Wang, J., Liang, J., Ning, D., Zhang, T. & Wang, M. A review of biomass immobilization in anammox and partial nitrification/anammox systems: advances, issues, and future perspectives. Sci. Total. Environ. 821, 152792 (2022).
Ebner, H., Sellmer, S. & Follmann, H. in Biotechnology: Products of Primary Metabolism 2nd edn, Ch. 12 (eds Rehm, H.‐J. & Reed, G.) 381–401 (Wiley, 1996).
König, H. in Biotechnology Set 2nd edn (eds Rehm, H.‐J. & Reed, G.) 249–264 (Wiley, 2001).
Yuan, Q., Jia, Z., Roots, P. & Wells, G. A strategy for fast anammox biofilm formation under mainstream conditions. Chemosphere 318, 137955 (2023).
Riesenberg, D. & Guthke, R. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 51, 422–430 (1999).
Shiloach, J. & Fass, R. Growing E. coli to high cell density—a historical perspective on method development. Biotechnol. Adv. 23, 345–357 (2005).
Shukla, S. K., Manobala, T., Rao, T. S., Shukla, S. K. & Rao, T. S. in Immobilization Strategies (eds Tripathi, A. & Melo, J. S.) 535–555 (Springer, 2021).
Morgan-Sagastume, J. M. & Noyola, A. Evaluation of an aerobic submerged filter packed with volcanic scoria. Bioresour. Technol. 99, 2528–2536 (2008).
Cuny, L. et al. Evaluation of productive biofilms for continuous lactic acid production. Biotechnol. Bioeng. 116, 2687–2697 (2019).
Zhang, Q. et al. Mechanical resilience of biofilms toward environmental perturbations mediated by extracellular matrix. Adv. Funct. Mater. 32, 2110699 (2022).
Ciofu, O., Moser, C., Jensen, P. Ø. & Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 20, 621–635 (2022).
Halan, B., Schmid, A. & Buehler, K. Real-time solvent tolerance analysis of Pseudomonas sp. strain VLB120ΔC catalytic biofilms. Appl. Environ. Microb. 77, 1563–1571 (2011).
Mishra, S. et al. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 294, 133609 (2022).
Darmon, E. & Leach, D. R. F. Bacterial genome instability. Microbiol. Mol. Biol. Rev. 78, 1–39 (2014).
Fraser, C., Alm, E. J., Polz, M. F., Spratt, B. G. & Hanage, W. P. The bacterial species challenge: making sense of genetic and ecological diversity. Science 323, 741–746 (2009).
Tenaillon, O. et al. Tempo and mode of genome evolution in a 50,000-generation experiment. Nature 536, 165–170 (2016).
Renda, B. A., Hammerling, M. J. & Barrick, J. E. Engineering reduced evolutionary potential for synthetic biology. Mol. BioSyst. 10, 1668–1678 (2014).
Akeno, Y., Ying, B. W., Tsuru, S. & Yomo, T. A reduced genome decreases the host carrying capacity for foreign DNA. Microb. Cell Fact. 13, 49 (2014).
Lieder, S., Nikel, P. I., Lorenzo, Vde & Takors, R. Genome reduction boosts heterologous gene expression in Pseudomonas putida. Microb. Cell Fact. 14, 23 (2015).
Umenhoffer, K. et al. Reduced evolvability of Escherichia coli MDS42, an IS-less cellular chassis for molecular and synthetic biology applications. Microb. Cell Fact. 9, 38 (2010).
Csörgo, B., Fehér, T., Tímár, E., Blattner, F. R. & Pósfai, G. Low-mutation-rate, reduced-genome Escherichia coli: an improved host for faithful maintenance of engineered genetic constructs. Microb. Cell Fact. 11, 11 (2012).
Xia, Y. et al. Coupled CFD‐DEM modeling to predict how EPS affects bacterial biofilm deformation, recovery and detachment under flow conditions. Biotechnol. Bioeng. 119, 2551 (2022).
Lewandowski, Z. & Beyenal, H. Fundamentals of Biofilm Research (CRC Press, 2017).
Waharte, F., Steenkeste, K., Briandet, R. & Fontaine-Aupart, M. P. Diffusion measurements inside biofilms by image-based fluorescence recovery after photobleaching (FRAP) analysis with a commercial confocal laser scanning microscope. Appl. Environ. Microbiol. 76, 5860–5869 (2010).
Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E. & Webb, W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 (1976).
Hauth, J., Chodorski, J., Wirsen, A. & Ulber, R. Improved FRAP measurements on biofilms. Biophys. J. 118, 2354–2365 (2020).
van den Berg, L., van Loosdrecht, M. C. M. & de Kreuk, M. K. How to measure diffusion coefficients in biofilms: a critical analysis. Biotechnol. Bioeng. 118, 1273–1285 (2021).
Kumar, A. et al. Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol. 28, 371–380 (2010).
Lee, S. H. et al. Higher biomass productivity of microalgae in an attached growth system, using wastewater. J. Microbiol. Biotechnol. 24, 1566–1573 (2014).
Scherer, K., Stiefelmaier, J., Strieth, D., Wahl, M. & Ulber, R. Development of a lightweight multi-skin sheet photobioreactor for future cultivation of phototrophic biofilms on facades. J. Biotechnol. 320, 28–35 (2020).
Al-Kaidy, H. et al. Biotechnology and bioprocess engineering – from the first Ullmann’s article to recent trends. ChemBioEng Rev. 2, 175–184 (2015).
Kashid, M. N., Harshe, Y. M. & Agar, D. W. Liquid–liquid slug flow in a capillary: an alternative to suspended drop or film contactors. Ind. Eng. Chem. Res. 46, 8420–8430 (2007).
Karande, R., Halan, B., Schmid, A. & Buehler, K. Segmented flow is controlling growth of catalytic biofilms in continuous multiphase microreactors. Biotechnol. Bioeng. 111, 1831–1840 (2014).
Gross, R., Buehler, K. & Schmid, A. Engineered catalytic biofilms for continuous large scale production of n-octanol and (S)-styrene oxide. Biotechnol. Bioeng. 110, 424–436 (2013).
Hoschek, A. et al. Mixed-species biofilms for high-cell-density application of Synechocystis sp. PCC 6803 in capillary reactors for continuous cyclohexane oxidation to cyclohexanol. Bioresour. Technol. 282, 171–178 (2019).
Dong, H., Zhang, W., Zhou, S., Ying, H. & Wang, P. Rational design of artificial biofilms as sustainable supports for whole-cell catalysis through integrating extra- and intracellular catalysis. ChemSusChem 15, e202200850 (2022).
Gunes, B. A critical review on biofilm-based reactor systems for enhanced syngas fermentation processes. Renew. Sustain. Energy Rev. 143, 110950 (2021).
Sun, X., Atiyeh, H. K., Huhnke, R. L. & Tanner, R. S. Syngas fermentation process development for production of biofuels and chemicals: a review. Bioresour. Technol. Rep. 7, 100279 (2019).
Stoll, I. K. et al. The complex way to sustainability: petroleum-based processes versus biosynthetic pathways in the formation of c4 chemicals from syngas. Ind. Eng. Chem. Res. 58, 15863–15871 (2019).
Zhang, F. et al. Fatty acids production from hydrogen and carbon dioxide by mixed culture in the membrane biofilm reactor. Water Res. 47, 6122–6129 (2013).
Mohammadi, M. et al. Bioconversion of synthesis gas to second generation biofuels: a review. Renew. Sustain. Energy Rev. 15, 4255–4273 (2011).
Riegler, P. et al. Continuous conversion of CO2/H2 with Clostridium aceticum in biofilm reactors. Bioresour. Technol. 291, 121760 (2019).
Ning, X. et al. Emerging bioelectrochemical technologies for biogas production and upgrading in cascading circular bioenergy systems. iScience 24, 102998 (2021).
Chavez, B. A., Raghavan, V. & Tartakovsky, B. A comparative analysis of biopolymer production by microbial and bioelectrochemical technologies. RSC Adv. 12, 16105–16118 (2022).
Ahmad, A., Priyadarshani, M., Das, S. & Ghangrekar, M. M. Role of bioelectrochemical systems for the remediation of emerging contaminants from wastewater: a review. J. Basic. Microbiol. 62, 201–222 (2021).
Roy, M., Aryal, N., Zhang, Y., Patil, S. A. & Pant, D. Technological progress and readiness level of microbial electrosynthesis and electrofermentation for carbon dioxide and organic wastes valorization. Curr. Opin. Green. Sustain. Chem. 35, 100605 (2022).
Conners, E. M., Rengasamy, K. & Bose, A. Electroactive biofilms: how microbial electron transfer enables bioelectrochemical applications. J. Ind. Microbiol. Biotechnol. 49, 12 (2022).
Hackbarth, M. et al. Monitoring and quantification of bioelectrochemical Kyrpidia spormannii biofilm development in a novel flow cell setup. Chem. Eng. J. 390, 124604 (2020).
Renslow, R. S. et al. Metabolic spatial variability in electrode-respiring Geobacter sulfurreducens biofilms. Energy Environ. Sci. 6, 1827–1836 (2013).
Lichtervelde, A. C. L., de, Heijne, A., ter, Hamelers, H. V. M., Biesheuvel, P. M. & Dykstra, J. E. Theory of ion and electron transport coupled with biochemical conversions in an electroactive biofilm. Phys. Rev. Appl. 12, 014018 (2019).
Fujikawa, T. et al. Unexpected genomic features of high current density-producing Geobacter sulfurreducens strain YM18. Fems. Microbiol. Lett. 368, fnab119 (2021).
Jiang, X. et al. Probing single- to multi-cell level charge transport in Geobacter sulfurreducens DL-1. Nat. Commun. 4, 2751 (2013).
Liu, X., Walker, D. J. F., Nonnenmann, S. S., Sun, D. & Lovley, D. R. Direct observation of electrically conductive pili emanating from Geobacter sulfurreducens. mBio 12, e02209–e02221 (2021).
Gu, Y. et al. Structure of Geobacter pili reveals secretory rather than nanowire behaviour. Nature 597, 430–434 (2021).
Philipp, L.-A., Edel, M. & Gescher, J. Chapter one genetic engineering for enhanced productivity in bioelectrochemical systems. Adv. Appl. Microbiol. 111, 1–31 (2020).
Lovley, D. R. Powering microbes with electricity: direct electron transfer from electrodes to microbes. Env. Microbiol. Rep. 3, 27–35 (2011).
Edel, M., Horn, H. & Gescher, J. Biofilm systems as tools in biotechnological production. Appl. Microbiol. Biotechnol. 103, 5095–5103 (2019).
Jourdin, L., Sousa, J., van Stralen, N. & Strik, D. P. B. T. B. Techno-economic assessment of microbial electrosynthesis from CO2 and/or organics: an interdisciplinary roadmap towards future research and application. Appl. Energ. 279, 115775 (2020).
Prévoteau, A., Carvajal-Arroyo, J. M., Ganigué, R. & Rabaey, K. Microbial electrosynthesis from CO2: forever a promise? Curr. Opin. Biotech. 62, 48–57 (2020).
Walter, X. A. et al. From the lab to the field: self-stratifying microbial fuel cells stacks directly powering lights. Appl. Energ. 277, 115514 (2020).
Cao, B. et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 373, 1336–1340 (2021).
Wang, D. et al. Surface modification of Shewanella oneidensis MR-1 with polypyrrole-dopamine coating for improvement of power generation in microbial fuel cells. J. Power Sources 483, 229220 (2021).
Knoll, M. T., Fuderer, E. & Gescher, J. Sprayable biofilm—agarose hydrogels as 3D matrix for enhanced productivity in bioelectrochemical systems. Biofilm 4, 100077 (2022).
Krieg, T., Sydow, A., Schröder, U., Schrader, J. & Holtmann, D. Reactor concepts for bioelectrochemical syntheses and energy conversion. Trends Biotechnol. 32, 645–655 (2014).
Kerzenmacher, S. Engineering of microbial electrodes. Adv. Biochem. Eng. Biotechnol. 167, 135–180 (2017).
Asensio, Y. et al. Upgrading fluidized bed bioelectrochemical reactors for treating brewery wastewater by using a fluid-like electrode. Chem. Eng. J. 406, 127103 (2021).
Hackbarth, M., Gescher, J., Horn, H. & Reiner, J. E. A scalable, rotating disc bioelectrochemical reactor (RDBER) suitable for the cultivation of both cathodic and anodic biofilms. Bioresour. Technol. Rep. 21, 101357 (2023).
Nerenberg, R. The membrane-biofilm reactor (MBfR) as a counter-diffusional biofilm process. Curr. Opin. Biotech. 38, 131–136 (2016).
Martin, K. J. & Nerenberg, R. The membrane biofilm reactor (MBfR) for water and wastewater treatment: principles, applications, and recent developments. Bioresour. Technol. 122, 83–94 (2012).
Elisiário, M. P., Wever, H. D., Hecke, W. V., Noorman, H. & Straathof, A. J. J. Membrane bioreactors for syngas permeation and fermentation. Crit. Rev. Biotechnol. 42, 856–872 (2022).
Yasin, M. et al. Microbial synthesis gas utilization and ways to resolve kinetic and mass-transfer limitations. Bioresour. Technol. 177, 361–374 (2015).
Asimakopoulos, K., Gavala, H. N. & Skiadas, I. V. Reactor systems for syngas fermentation processes: a review. Chem. Eng. J. 348, 732–744 (2018).
Dong, K. et al. Nitrogen removal from nitrate-containing wastewaters in hydrogen-based membrane biofilm reactors via hydrogen autotrophic denitrification: biofilm structure, microbial community and optimization strategies. Front. Microbiol. 13, 924084 (2022).
Nangle, S. N. et al. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 62, 207–220 (2020).
Windhorst, C. & Gescher, J. Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16. Biotechnol. Biofuels 12, 163 (2019).
Stiefelmaier, J. et al. Characterization of terrestrial phototrophic biofilms of cyanobacterial species. Algal Res. 50, 101996 (2020).
Bolhuis, H., Cretoiu, M. S. & Stal, L. J. Molecular ecology of microbial mats. FEMS Microbiol. Ecol. 90, 335–350 (2014).
Angermayr, S. A., Rovira, A. G. & Hellingwerf, K. J. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 33, 352–361 (2015).
Fisher, M. L., Allen, R., Luo, Y. & Curtiss, R. Export of extracellular polysaccharides modulates adherence of the cyanobacterium Synechocystis. PLoS One 8, e74514 (2013).
Agostoni, M., Waters, C. M. & Montgomery, B. L. Regulation of biofilm formation and cellular buoyancy through modulating intracellular cyclic di-GMP levels in engineered cyanobacteria. Biotechnol. Bioeng. 113, 311–319 (2016).
Rosenbaum, M., He, Z. & Angenent, L. T. Light energy to bioelectricity: photosynthetic microbial fuel cells. Curr. Opin. Biotechnol. 21, 259–264 (2010).
Obileke, K. C., Onyeaka, H., Meyer, E. L. & Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: a mini-review. Electrochem. Commun. 125, 107003 (2021).
Tschörtner, J., Lai, B. & Krömer, J. O. Biophotovoltaics: green power generation from sunlight and water. Front. Microbiol. 10, 866 (2019).
McCormick, A. J. et al. Photosynthetic biofilms in pure culture harness solar energy in a mediatorless bio-photovoltaic cell (BPV) system. Energy Environ. Sci. 4, 4699–4709 (2011).
Miranda, A. F. et al. Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol. Biofuels 10, 1–23 (2017).
Kollmen, J. & Strieth, D. The beneficial effects of cyanobacterial co-culture on plant growth. Life 12, 223 (2022).
Mutale-Joan, C., Sbabou, L. & Hicham, E. A. Microalgae and cyanobacteria: how exploiting these microbial resources can address the underlying challenges related to food sources and sustainable agriculture: a review. J. Plant. Growth Regul. 42, 1–20 (2022).
Stirk, W. A., Ördög, V., Staden, J. V. & Jäger, K. Cytokinin- and auxin-like activity in Cyanophyta and microalgae. J. Appl. Phycol. 14, 215–221 (2002).
Han, X., Zeng, H., Bartocci, P., Fantozzi, F. & Yan, Y. Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation 4, 25 (2018).
Shariatmadari, Z., Riahi, H., Hashtroudi, M. S., Ghassempour, A. R. & Aghashariatmadary, Z. Plant growth promoting cyanobacteria and their distribution in terrestrial habitats of Iran. Soil. Sci. Plant. Nutr. 59, 535–547 (2013).
Kumar, G., Teli, B., Mukherjee, A., Bajpai, R. & Sarma, B. K. in Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms (eds Singh, H., Keswani, C., Reddy, M., Sansinenea, E. & García-Estrada, C.) 239–252 (Springer, 2019).
Irisarri, P., Gonnet, S. & Monza, J. Cyanobacteria in Uruguayan rice fields: diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. J. Biotechnol. 91, 95–103 (2001).
Prasanna, R., Joshi, M., Rana, A., Shivay, Y. S. & Nain, L. Influence of co-inoculation of bacteria-cyanobacteria on crop yield and C–N sequestration in soil under rice crop. World J. Microbiol. Biotechnol. 28, 1223–1235 (2012).
Roeselers, G., Loosdrecht, M. C. M. V. & Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 20, 227–235 (2008).
Fischer, S. E., Fischer, S. I., Magris, S. & Mori, G. B. Isolation and characterization of bacteria from the rhizosphere of wheat. World J. Microbiol. Biotechnol. 23, 895–903 (2007).
Karthikeyan, N., Prasanna, R., Nain, L. & Kaushik, B. D. Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur. J. Soil. Biol. 43, 23–30 (2007).
Grzesik, M., Romanowska-Duda, Z. & Kalaji, H. M. Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 55, 510–521 (2017).
Saadatnia, H. & Riahi, H. Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant. Soil. Env. 55, 207–212 (2009).
Coppens, J. et al. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 28, 2367–2377 (2016).
Maqubela, M. P., Mnkeni, P. N. S., Issa, O. M., Pardo, M. T. & D’Acqui, L. P. Nostoc cyanobacterial inoculation in South African agricultural soils enhances soil structure, fertility, and maize growth. Plant. Soil. 315, 79–92 (2009).
Prasanna, R. et al. Cyanobacteria-based bioinoculants influence growth and yields by modulating the microbial communities favourably in the rhizospheres of maize hybrids. Eur. J. Soil. Biol. 75, 15–23 (2016).
Fedeson, D. T. & Ducat, D. C. Cyanobacterial surface display system mediates engineered interspecies and abiotic binding. ACS Synth. Biol. 6, 367–374 (2017).
Cengic, I., Uhlén, M. & Hudson, E. P. Surface display of small affinity proteins on Synechocystis sp. strain PCC 6803 mediated by fusion to the major type IV pilin PilA1. J. Bacteriol. 200, e00270–e00318 (2018).
Quoc, B. N. et al. An investigation into the optimal granular sludge size for simultaneous nitrogen and phosphate removal. Water Res. 198, 117119 (2021).
Bathe, S., Kreuk, M. de, McSwain, B. & Schwarzenbeck, N. (eds) Aerobic Granular Sludge (IWA Publishing, 2015).
Ghosh, S. & Chakraborty, S. Production of polyhydroxyalkanoates (PHA) from aerobic granules of refinery sludge and Micrococcus aloeverae strain SG002 cultivated in oily wastewater. Int. Biodeterior. Biodegrad. 155, 105091 (2020).
Bahgat, N. T., Wilfert, P., Korving, L. & Loosdrecht, Mvan Integrated resource recovery from aerobic granular sludge plants. Water Res. 234, 119819 (2023).
Nancharaiah, Y. V. & Reddy, G. K. K. Aerobic granular sludge technology: mechanisms of granulation and biotechnological applications. Bioresour. Technol. 247, 1128–1143 (2018).
Jiang, Q. et al. Current progress, challenges and perspectives in the microalgal-bacterial aerobic granular sludge process: a review. Int. J. Environ. Res. Publ. Health 19, 13950 (2022).
Chen, A. Y. et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nat. Mater. 13, 515–523 (2014).
Cheng, K.-C., Catchmark, J. M. & Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 3, 12 (2009).
Lee, Y. S. & Park, W. Current challenges and future directions for bacterial self-healing concrete. Appl. Microbiol. Biotechnol. 102, 3059–3070 (2018).
Dhami, N. K., Reddy, S. M. & Mukherjee, A. in Advanced Topics in Biomineralization (ed. Seto, J.) 137–164 (InTechOpen, 2012).
Saracho, A. C. et al. Controlling the calcium carbonate microstructure of engineered living building materials. J. Mater. Chem. A. 9, 24438–24451 (2021).
Little, B. J., Hinks, J. & Blackwood, D. J. Microbially influenced corrosion: towards an interdisciplinary perspective on mechanisms. Int. Biodeter. Biodegr. 154, 105062 (2020).
Zuo, R., Kus, E., Mansfeld, F. & Wood, T. K. The importance of live biofilms in corrosion protection. Corros. Sci. 47, 279–287 (2005).
Liu, T. et al. Marine bacteria provide lasting anticorrosion activity for steel via biofilm-induced mineralization. ACS Appl. Mater. Inter. 10, 40317–40327 (2018).
Li, Z. et al. Marine biofilms with significant corrosion inhibition performance by secreting extracellular polymeric substances. ACS Appl. Mater. Inter. 13, 47272–47282 (2021).
Karande, R. et al. Continuous cyclohexane oxidation to cyclohexanol using a novel cytochrome P450 monooxygenase from Acidovorax sp. CHX100 in recombinant P. taiwanensis VLB120 biofilms. Biotechnol. Bioeng. 113, 52–61 (2016).
Gross, R., Hauer, B., Otto, K. & Schmid, A. Microbial biofilms: new catalysts for maximizing productivity of long‐term biotransformations. Biotechnol. Bioeng. 98, 1123–1134 (2007).
Yang, G., Mai, Q., Zhuang, Z. & Zhuang, L. Buffer capacity regulates the stratification of anode-respiring biofilm during brewery wastewater treatment. Environ. Res. 201, 111572 (2021).
Flemming, H. C. & Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 17, 247–260 (2019).
Penesyan, A., Paulsen, I. T., Kjelleberg, S. & Gillings, M. R. Three faces of biofilms: a microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. npj Biofilms Microbiomes 7, 80 (2021).
Sauer, K. et al. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 20, 608–620 (2022).
Cai, Y. M. Non-surface attached bacterial aggregates: a ubiquitous third lifestyle. Front. Microbiol. 11, 3106 (2020).
Valentini, M. & Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 291, 12547–12555 (2016).
Baker, A. E. et al. Flagellar stators stimulate c-di-GMP production by Pseudomonas aeruginosa. J. Bacteriol. 201, e00741–e00818 (2019).
Webster, S. S., Lee, C. K., Schmidt, W. C., Wong, G. C. L. & O’Toole, G. A. Interaction between the type 4 pili machinery and a diguanylate cyclase fine-tune c-di-GMP levels during early biofilm formation. Proc. Natl Acad. Sci. USA 118, e2105566118 (2021).
Baker, A. E. et al. PilZ domain protein FlgZ mediates cyclic di-GMP-dependent swarming motility control in Pseudomonas aeruginosa. J. Bacteriol. 198, 1837–1846 (2016).
Thormann, K. M. Dynamic hybrid flagellar motors—fuel switch and more. Front. Microbiol. 13, 867 (2022).
Gerven, N. V., Klein, R. D., Hultgren, S. J. & Remaut, H. Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 23, 693–706 (2015).
Mukherjee, S. & Bassler, B. L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 17, 371–382 (2019).
Paula, A. J., Hwang, G. & Koo, H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat. Commun. 11, 1354 (2020).
Blauert, F., Horn, H. & Wagner, M. Time‐resolved biofilm deformation measurements using optical coherence tomography. Biotechnol. Bioeng. 112, 1893–1905 (2015).
Rumbaugh, K. P. & Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 18, 571–586 (2020).
Moore-Ott, J. A., Chiu, S., Amchin, D. B., Bhattacharjee, T. & Datta, S. S. A biophysical threshold for biofilm formation. eLife 11, e76380 (2022).
Acknowledgements
The authors thank J. Kollmen, D. Strieth, J. Stiefelmaier and A. Schmeckebier (funded by the DFG (German Research Foundation), Collaborative Research Center 926, project C03 – Project-ID 172116086) as well as R. Karande and M. Bozan for providing research data and illustrations on the topic of phototropic biofilms and reactor configurations, respectively.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Kai Bester and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Philipp, LA., Bühler, K., Ulber, R. et al. Beneficial applications of biofilms. Nat Rev Microbiol 22, 276–290 (2024). https://doi.org/10.1038/s41579-023-00985-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-023-00985-0