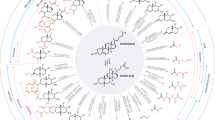
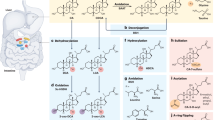
Abstract
Despite decades of bile acid research, diverse biological roles for bile acids have been discovered recently due to developments in understanding the human microbiota. As additional bacterial enzymes are characterized, and the tools used for identifying new bile acids become increasingly more sensitive, the repertoire of bile acids metabolized and/or synthesized by bacteria continues to grow. Additionally, bile acids impact microbiome community structure and function. In this Review, we highlight how the bile acid pool is manipulated by the gut microbiota, how it is dependent on the metabolic capacity of the bacterial community and how external factors, such as antibiotics and diet, shape bile acid composition. It is increasingly important to understand how bile acid signalling networks are affected in distinct organs where the bile acid composition differs, and how these networks impact infectious, metabolic and neoplastic diseases. These advances have enabled the development of therapeutics that target imbalances in microbiota-associated bile acid profiles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Turnbaugh, P. J. et al. The Human Microbiome Project. Nature 449, 804–810 (2007).
Fuchs, C. D. & Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. https://doi.org/10.1038/s41575-021-00566-7 (2022).
Hamilton, J. P. et al. Human cecal bile acids: concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G256–G263 (2007).
Jones, B. V., Begley, M., Hill, C., Gahan, C. G. M. & Marchesi, J. R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl Acad. Sci. USA 105, 13580–13585 (2008).
Hofmann, A. F. The enterohepatic circulation of bile acids in mammals: form and functions. Front. Biosci. 14, 2584–2598 (2009).
Thakare, R., Alamoudi, J. A., Gautam, N., Rodrigues, A. D. & Alnouti, Y. Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol. 38, 1323–1335 (2018).
Wahlström, A. et al. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J. Lipid Res. 58, 412–419 (2017).
Li, J. & Dawson, P. A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta 1865, 895–911 (2019).
Honda, A. et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res. 61, 54–69 (2020).
Guzior, D. V. & Quinn, R. A. Review: microbial transformations of human bile acids. Microbiome 9, 140 (2021).
Foley, M. H. et al. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization. Proc. Natl Acad. Sci. USA 118, e2017709118 (2021).
Eldere, J. V., Celis, P., Pauw, G. D., Lesaffre, E. & Eyssen, H. Tauroconjugation of cholic acid stimulates 7 alpha-dehydroxylation by fecal bacteria. Appl. Environ. Microbiol. 62, 656–661 (1996).
Tanaka, H., Hashiba, H., Kok, J. & Mierau, I. Bile salt hydrolase of Bifidobacterium longum — biochemical and genetic characterization. Appl. Environ. Microbiol. 66, 2502–2512 (2000).
Ridlon, J. M., Kang, D.-J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
White, B. A., Lipsky, R. L., Fricke, R. J. & Hylemon, P. B. Bile acid induction specificity of 7α-dehydroxylase activity in an intestinal Eubacterium species. Steroids 35, 103–109 (1980).
Funabashi, M. et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 582, 566–570 (2020).
Streidl, T. et al. The gut bacterium Extibacter muris produces secondary bile acids and influences liver physiology in gnotobiotic mice. Gut Microbes 13, 1–21 (2021).
Marion, S. et al. Biogeography of microbial bile acid transformations along the murine gut. J. Lipid Res. 61, 1450–1463 (2020).
Wahlström, A., Sayin, S. I., Marschall, H.-U. & Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Devlin, A. S. & Fischbach, M. A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 11, 685–690 (2015).
Van Eldere, J., Robben, J., De Pauw, G., Merckx, R. & Eyssen, H. Isolation and identification of intestinal steroid-desulfating bacteria from rats and humans. Appl. Environ. Microbiol. 54, 2112–2117 (1988).
Korpela, J. T., Fotsis, T. & Adlercreutz, H. Multicomponent analysis of bile acids in faeces by anion exchange and capillary column gas-liquid chromatography: application in oxytetracycline treated subjects. J. Steroid Biochem. 25, 277–284 (1986).
Dawson, P. A. & Karpen, S. J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 56, 1085–1099 (2015).
Quinn, R. A. et al. Global chemical impact of the microbiome includes novel bile acid conjugations. Nature 579, 123–129 (2020).
Hoffmann, M. A. et al. High-confidence structural annotation of metabolites absent from spectral libraries. Nat. Biotechnol. 40, 411–421 (2022).
Gentry, E. et al. A synthesis-based reverse metabolomics approach for the discovery of chemical structures from humans and animals. Res. Sq. https://doi.org/10.21203/rs.3.rs-820302/v1 (2021).
Wang, M. et al. Mass spectrometry searches using MASST. Nat. Biotechnol. 38, 19–26 (2020).
Lucas, L. N. et al. Dominant bacterial phyla from the human gut show widespread ability to transform and conjugate bile acids. mSystems https://doi.org/10.1128/mSystems.00805-21 (2021).
Antunes, L. C. M. et al. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob. Agents Chemother. 55, 1494–1503 (2011).
Swann, J. R. et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl Acad. Sci. USA 108, 4523–4530 (2011).
Theriot, C. M. et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 5, 3114 (2014).
Vrieze, A. et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 60, 824–831 (2014).
Kuno, T., Hirayama-Kurogi, M., Ito, S. & Ohtsuki, S. Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels. Sci. Rep. 8, 1253 (2018).
Dethloff, F. et al. Paroxetine administration affects microbiota and bile acid levels in mice. Front. Psychiatry 11, 518 (2020).
Molina-Molina, E. et al. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur. J. Clin. Invest. 48, e12958 (2018).
Ca, Y., Bj, M. & Jb, W. Chronic physical activity alters hepatobiliary excretory function in rats. J. Pharmacol. Exp. Ther. 265, 321–327 (1993).
Meissner, M. et al. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 218, 323–329 (2011).
Wertheim, B. C. et al. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol. Biomark. Prev. 18, 1591–1598 (2009).
Danese, E. et al. Middle-distance running acutely influences the concentration and composition of serum bile acids: potential implications for cancer risk? Oncotarget 8, 52775–52782 (2017).
Hughes, A. et al. Exercise training reverses gut dysbiosis in patients with biopsy-proven nonalcoholic steatohepatitis: a proof of concept study. Clin. Gastroenterol. Hepatol. 19, 1723–1725 (2021).
Wan, Y. et al. Unconjugated and secondary bile acid profiles in response to higher-fat, lower-carbohydrate diet and associated with related gut microbiota: a 6-month randomized controlled-feeding trial. Clin. Nutr. 39, 395–404 (2020).
Ellegård, L. & Andersson, H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: an ileostomy study. Eur. J. Clin. Nutr. 61, 938–945 (2007).
Ellegård, L., Bosaeus, I. & Andersson, H. Will recommended changes in fat and fibre intake affect cholesterol absorption and sterol excretion? An ileostomy study. Eur. J. Clin. Nutr. 54, 306–313 (2000).
Louis, P., Hold, G. L. & Flint, H. J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672 (2014).
von Schwartzenberg, R. J. et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature 595, 272–277 (2021).
van Best, N. et al. Bile acids drive the newborn’s gut microbiota maturation. Nat. Commun. 11, 3692 (2020).
Ridlon, J. M., Kang, D. J., Hylemon, P. B. & Bajaj, J. S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 30, 332–338 (2014).
Sannasiddappa, T. H., Lund, P. A. & Clarke, S. R. In vitro antibacterial activity of unconjugated and conjugated bile salts on Staphylococcus aureus. Front. Microbiol. 8, 1581 (2017).
Tian, Y. et al. The microbiome modulating activity of bile acids. Gut Microbes 11, 979–996 (2020).
Begley, M., Gahan, C. G. M. & Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 (2005).
Miller, S. I. Antibiotic resistance and regulation of the Gram-negative bacterial outer membrane barrier by host innate immune molecules. mBio 7, e01541-16 (2016).
Thanassi, D. G., Cheng, L. W. & Nikaido, H. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179, 2512–2518 (1997).
Liu, T., Zhang, L., Joo, D. & Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2, 17023 (2017).
Inagaki, T. et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl Acad. Sci. USA 103, 3920–3925 (2006).
Li, Y., Tang, R., Leung, P. S. C., Gershwin, M. E. & Ma, X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 16, 885–896 (2017).
Cabrera-Rubio, R., Patterson, A. M., Cotter, P. D. & Beraza, N. Cholestasis induced by bile duct ligation promotes changes in the intestinal microbiome in mice. Sci. Rep. 9, 12324 (2019).
Rea, M. C. et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl Acad. Sci. USA 107, 9352–9357 (2010).
Ng, K. M. et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99 (2013).
Theriot, C. M., Bowman, A. A. & Young, V. B. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere 1, e00045-15 (2016).
Robinson, J. I. et al. Metabolomic networks connect host-microbiome processes to human Clostridioides difficile infections. J. Clin. Invest. 129, 3792–3806 (2019).
Sorg, J. A. & Sonenshein, A. L. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190, 2505–2512 (2008).
Thanissery, R., Winston, J. A. & Theriot, C. M. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe 45, 86–100 (2017).
Buffie, C. G. et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208 (2015).
Kang, J. D. et al. Bile acid 7α-dehydroxylating gut bacteria secrete antibiotics that inhibit Clostridium difficile: role of secondary bile acids. Cell Chem. Biol. 26, 27–34.e4 (2019).
Tam, J. et al. Intestinal bile acids directly modulate the structure and function of C. difficile TcdB toxin. Proc. Natl Acad. Sci. USA 117, 6792–6800 (2020).
Weingarden, A. R. et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G310–G319 (2014).
Louie, T. J. et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364, 422–431 (2011).
Qian, X. et al. Ridinilazole, a narrow spectrum antibiotic for treatment of Clostridioides difficile infection, enhances preservation of microbiota-dependent bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 319, G227–G237 (2020).
Franzosa, E. A. et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 4, 293–305 (2019).
Manichanh, C. et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55, 205–211 (2006).
Gevers, D. et al. The treatment-naïve microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392 (2014).
Duboc, H. et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 62, 531–539 (2013).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Heinken, A. et al. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 7, 75 (2019).
Vavassori, P., Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 183, 6251–6261 (2009).
Mencarelli, A. et al. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur. J. Pharmacol. 668, 317–324 (2011).
Gadaleta, R. M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).
Shah, Y. M., Ma, X., Morimura, K., Kim, I. & Gonzalez, F. J. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-κB target gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1114–G1122 (2007).
Wilson, A., Almousa, A., Teft, W. A. & Kim, R. B. Attenuation of bile acid-mediated FXR and PXR activation in patients with Crohn’s disease. Sci. Rep. 10, 1866 (2020).
Alberti, K. G. M. M. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009).
Xiao, J. et al. Prevalence of metabolic syndrome and its risk factors among rural adults in Nantong, China. Sci. Rep. 6, 38089 (2016).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Kootte, R. S. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619.e6 (2017).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7 (2012).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
He, Y. et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 6, 172 (2018).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Falony, G. et al. Population-level analysis of gut microbiome variation. Science 352, 560–564 (2016).
Walters, W. A., Xu, Z. & Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 588, 4223–4233 (2014).
Bisanz, J. E., Upadhyay, V., Turnbaugh, J. A., Ly, K. & Turnbaugh, P. J. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 26, 265–272.e4 (2019).
Vijay-Kumar, M. et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2010).
Jiao, N. et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67, 1881–1891 (2018).
Katsuma, S., Hirasawa, A. & Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 329, 386–390 (2005).
Thomas, C., Pellicciari, R., Pruzanski, M., Auwerx, J. & Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693 (2008).
Pathak, P. et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588 (2018).
Watanabe, M. et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113, 1408–1418 (2004).
Joyce, S. A. et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl Acad. Sci. USA 111, 7421–7426 (2014).
Ryan, K. K. et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188 (2014).
Ðanić, M. et al. Pharmacological applications of bile acids and their derivatives in the treatment of metabolic syndrome. Front. Pharmacol. 9, 1382 (2018).
Zhang, H., Dong, M. & Liu, X. Obeticholic acid ameliorates obesity and hepatic steatosis by activating brown fat. Exp. Ther. Med. 22, 991 (2021).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e1 (2013).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Parséus, A. et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 66, 429–437 (2017).
Zhang, Y. et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl Acad. Sci. USA 103, 1006–1011 (2006).
Watanabe, M. et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem. 286, 26913–26920 (2011).
Ma, Y., Huang, Y., Yan, L., Gao, M. & Liu, D. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm. Res. 30, 1447–1457 (2013).
Jia, W., Xie, G. & Jia, W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15, 111–128 (2018).
Cook, J. W., Kennaway, E. L. & Kennaway, N. M. Production of tumours in mice by deoxycholic acid. Nature 145, 627–627 (1940).
McGarr, S. E., Ridlon, J. M. & Hylemon, P. B. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J. Clin. Gastroenterol. 39, 98–109 (2005).
Xie, G. et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int. J. Cancer 139, 1764–1775 (2016).
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516 (2012).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Bernstein, H., Bernstein, C., Payne, C. M., Dvorakova, K. & Garewal, H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 589, 47–65 (2005).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Qiao, D., Gaitonde, S. V., Qi, W. & Martinez, J. D. Deoxycholic acid suppresses p53 by stimulating proteasome-mediated p53 protein degradation. Carcinogenesis 22, 957–964 (2001).
Payne, C. M., Bernstein, C., Dvorak, K. & Bernstein, H. Hydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesis. Clin. Exp. Gastroenterol. 1, 19–47 (2008).
Washo-Stultz, D. et al. Role of mitochondrial complexes I and II, reactive oxygen species and arachidonic acid metabolism in deoxycholate-induced apoptosis. Cancer Lett. 177, 129–144 (2002).
Yu, J. et al. Farnesoid X receptor antagonizes Wnt/β-catenin signaling in colorectal tumorigenesis. Cell Death Dis. 11, 640 (2020).
Lax, S. et al. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int. J. Cancer 130, 2232–2239 (2012).
Maran, R. R. M. et al. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J. Pharmacol. Exp. Ther. 328, 469–477 (2009).
Yang, F. et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 67, 863–867 (2007).
Wolfe, A. et al. Increased activation of the Wnt/β-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J. Pharmacol. Exp. Ther. 338, 12–21 (2011).
Gadaleta, R. M. et al. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κB signaling in the intestine. Biochim. Biophys. Acta Mol. Basis Dis. 1812, 851–858 (2011).
Ridlon, J. M., Wolf, P. G. & Gaskins, H. R. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 7, 201–215 (2016).
Laidlaw, S., Grosvenor, M. & Kopple, J. The taurine content of common foodstuffs. J. Parenter. Enter. Nutr. 14, 183–188 (1990).
Reissig, C. J., Strain, E. C. & Griffiths, R. R. Caffeinated energy drinks — a growing problem. Drug. Alcohol. Depend. 99, 1–10 (2009).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Seekatz, A. M. et al. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe 53, 64–73 (2018).
Mullish, B. H. et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut 68, 1791–1800 (2019).
Paramsothy, S. et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 156, 1440–1454.e2 (2019).
Zhao, L. et al. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J. Clin. Invest. 130, 438–450 (2020).
Bárcena, C. et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242 (2019).
Kazemian, N. et al. The trans-kingdom battle between donor and recipient gut microbiome influences fecal microbiota transplantation outcome. Sci. Rep. 10, 18349 (2020).
Bibiloni, R. et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 100, 1539–1546 (2005).
Degirolamo, C., Rainaldi, S., Bovenga, F., Murzilli, S. & Moschetta, A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 7, 12–18 (2014).
Mencarelli, A. et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS ONE 6, e22978 (2011).
Appleyard, C. B. et al. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G1004–G1013 (2011).
Jones, M. L., Martoni, C. J. & Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur. J. Clin. Nutr. 66, 1234–1241 (2012).
Alberts, D. S. et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J. Natl Cancer Inst. 97, 846–853 (2005).
Oyama, K., Shiota, G., Ito, H., Murawaki, Y. & Kawasaki, H. Reduction of hepatocarcinogenesis by ursodeoxycholic acid in rats. Carcinogenesis 23, 885–892 (2002).
Serfaty, L. et al. Ursodeoxycholic acid therapy and the risk of colorectal adenoma in patients with primary biliary cirrhosis: an observational study. Hepatology 38, 203–209 (2003).
Zhang, H., Xu, H., Zhang, C., Tang, Q. & Bi, F. Ursodeoxycholic acid suppresses the malignant progression of colorectal cancer through TGR5-YAP axis. Cell Death Discov. 7, 207 (2021).
Pearson, T. et al. Effects of ursodeoxycholic acid on the gut microbiome and colorectal adenoma development. Cancer Med. 8, 617–628 (2019).
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Adhikari, A. A. et al. A gut-restricted lithocholic acid analog as an inhibitor of gut bacterial bile salt hydrolases. ACS Chem. Biol. 16, 1401–1412 (2021).
Paik, D. et al. Human gut bacteria produce ΤΗ17-modulating bile acid metabolites. Nature 603, 907–912 (2022).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
van der Lelie, D. et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat. Commun. 12, 3105 (2021).
Wang, K. et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235.e5 (2019).
Nevens, F. et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 375, 631–643 (2016).
Huijghebaert, S. M. & Eyssen, H. J. Specificity of bile salt sulfatase activity from Clostridium sp. strains S1. Appl. Environ. Microbiol. 44, 1030–1034 (1982).
Huijghebaert, S., Parmentier, G. & Eyssen, H. Specificity of bile salt sulfatase activity in man, mouse and rat intestinal microflora. J. Steroid Biochem. 20, 907–912 (1984).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Thibaut, M. M. & Bindels, L. B. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol. Med. 28, 223–236 (2022).
Molinaro, A., Wahlström, A. & Marschall, H.-U. Role of bile acids in metabolic control. Trends Endocrinol. Metab. 29, 31–41 (2018).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Parks, D. J. et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 284, 1365–1368 (1999).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005).
Holt, J. A. et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 17, 1581–1591 (2003).
Goodwin, B. et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6, 517–526 (2000).
Lu, T. T. et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 6, 507–515 (2000).
Sinal, C. J. et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 (2000).
Sanyal, S. et al. Involvement of corepressor complex subunit GPS2 in transcriptional pathways governing human bile acid biosynthesis. Proc. Natl Acad. Sci. USA 104, 15665–15670 (2007).
Pircher, P. C. et al. Farnesoid X receptor regulates bile acid-amino acid conjugation. J. Biol. Chem. 278, 27703–27711 (2003).
Stedman, C. A. M. et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc. Natl Acad. Sci. USA 102, 2063–2068 (2005).
Saini, S. P. S. et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol. Pharmacol. 65, 292–300 (2004).
Makishima, M. et al. Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002).
Sonoda, J. et al. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc. Natl Acad. Sci. USA 99, 13801–13806 (2002).
Staudinger, J. L. et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA 98, 3369–3374 (2001).
McCarthy, T. C., Li, X. & Sinal, C. J. Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J. Biol. Chem. 280, 23232–23242 (2005).
Schmidt, D. R. et al. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem. 285, 14486–14494 (2010).
Bhalla, S., Ozalp, C., Fang, S., Xiang, L. & Kemper, J. K. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1α: functional implications in hepatic cholesterol and glucose metabolism. J. Biol. Chem. 279, 45139–45147 (2004).
Miao, J., Fang, S., Bae, Y. & Kemper, J. K. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J. Biol. Chem. 281, 14537–14546 (2006).
Maruyama, T. et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298, 714–719 (2002).
Kawamata, Y. et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 (2003).
Wang, Y.-D., Chen, W.-D., Yu, D., Forman, B. M. & Huang, W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatol. Baltim. Md. 54, 1421–1432 (2011).
Alemi, F. et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144, 145–154 (2013).
Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009).
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Meixiong, J., Vasavda, C., Snyder, S. H. & Dong, X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc. Natl Acad. Sci. USA 116, 10525–10530 (2019).
Yu, H. et al. MRGPRX4 is a bile acid receptor for human cholestatic itch. eLife 8, e48431 (2019).
Cao, C. et al. Structure, function and pharmacology of human itch GPCRs. Nature 600, 170–175 (2021).
Author information
Authors and Affiliations
Contributions
S.L.C. researched data for the article. All authors contributed to discussion of the content, and reviewed and edited the manuscript before submission. S.L.C., C.D.O., A.D.P. and J.G.S. wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Laure Bindels, Morgane Thibaut, Lixin Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Amphipathic
-
Containing both hydrophilic (polar) and hydrophobic (nonpolar) regions.
- Micelles
-
A collection or aggregate of amphipathic molecules that self-assemble in aqueous solution so that the hydrophobic portions are shielded from water.
- Cholestasis
-
A liver disease characterized by blockage of or reduced bile flow.
- Brown fat
-
Adipose tissue containing high numbers of mitochondria, involved in metabolizing energy sources to produce heat.
- Pouchitis
-
Inflammation of the J-pouch in surgery for ulcerative colitis.
- J-pouch
-
A J-shaped surgical reformation of the ileum, connected directly to the anus after the removal of diseased colon in patients with ulcerative colitis.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Collins, S.L., Stine, J.G., Bisanz, J.E. et al. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol 21, 236–247 (2023). https://doi.org/10.1038/s41579-022-00805-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-022-00805-x
This article is cited by
-
Critical role of the gut microbiota in immune responses and cancer immunotherapy
Journal of Hematology & Oncology (2024)
-
Two intestinal microbiota-derived metabolites, deoxycholic acid and butyrate, synergize to enhance host defense peptide synthesis and alleviate necrotic enteritis
Journal of Animal Science and Biotechnology (2024)
-
Orally biomimetic metal-phenolic nanozyme with quadruple safeguards for intestinal homeostasis to ameliorate ulcerative colitis
Journal of Nanobiotechnology (2024)
-
Effect of bile reflux on gastric juice microbiota in patients with different histology phenotypes
Gut Pathogens (2024)
-
Enhancing milk quality and modulating rectal microbiota of dairy goats in starch-rich diet: the role of bile acid supplementation
Journal of Animal Science and Biotechnology (2024)