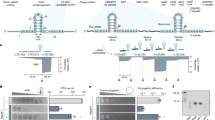
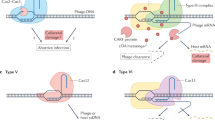
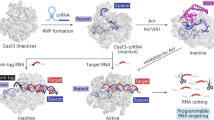
Abstract
CRISPR–Cas is a widespread adaptive immune system in bacteria and archaea that protects against viral infection by targeting specific invading nucleic acid sequences. Whereas some CRISPR–Cas systems sense and cleave viral DNA, type III and type VI CRISPR–Cas systems sense RNA that results from viral transcription and perhaps invasion by RNA viruses. The sequence-specific detection of viral RNA evokes a cell-wide response that typically involves global damage to halt the infection. How can one make sense of an immune strategy that encompasses broad, collateral effects rather than specific, targeted destruction? In this Review, we summarize the current understanding of RNA-targeting CRISPR–Cas systems. We detail the composition and properties of type III and type VI systems, outline the cellular defence processes that are instigated upon viral RNA sensing and describe the biological rationale behind the broad RNA-activated immune responses as an effective strategy to combat viral infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koonin, E. V., Wolf, Y. I. & Katsnelson, M. I. Inevitability of the emergence and persistence of genetic parasites caused by evolutionary instability of parasite-free states. Biol. Direct 12, 1–12 (2017).
Frost, L. S., Leplae, R., Summers, A. O. & Toussaint, A. Mobile genetic elements: The agents of open source evolution. Nat. Rev. Microbiol. 3, 722–732 (2005).
Van Valen, L. A new evolutionary law. Evol. Theory 1, 1–30 (1973).
Koonin, E. V. Viruses and mobile elements as drivers of evolutionary transitions. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150442 (2016).
Bernheim, A. & Sorek, R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 18, 113–119 (2020).
Tesson, F. et al. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat. Commun. 13, 2561 (2022).
Rocha, E. P. C. & Bikard, D. Microbial defenses against mobile genetic elements and viruses: who defends whom from what? PLoS Biol. 20, e3001514 (2022).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Deveau, H. et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400 (2008).
Barrangou, R. & Horvath, P. A decade of discovery: CRISPR functions and applications. Nat. Microbiol. 2, 17092 (2017).
Datsenko, K. A. et al. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat. Commun. 3, 1–7 (2012).
Swarts, D. C., Mosterd, C., van Passel, M. W. J. & Brouns, S. J. J. CRISPR interference directs strand specific spacer acquisition. PLoS ONE 7, e35888 (2012).
Nussenzweig, P. M., McGinn, J. & Marraffini, L. A. Cas9 cleavage of viral genomes primes the acquisition of new immunological memories. Cell Host Microbe 26, 515–526.e6 (2019).
Carte, J., Wang, R., Li, H., Terns, R. M. & Terns, M. P. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 22, 3489–3496 (2008).
East-Seletsky, A. et al. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 538, 270–273 (2016).
Brouns, S. J. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Hille, F. et al. The biology of CRISPR-Cas: backward and forward. Cell 172, 1239–1259 (2018).
Bernheim, A., Bikard, D., Touchon, M. & Rocha, E. P. C. Atypical organizations and epistatic interactions of CRISPRs and cas clusters in genomes and their mobile genetic elements. Nucleic Acids Res. 48, 748–760 (2020).
Jia, N. & Patel, D. J. Structure-based functional mechanisms and biotechnology applications of anti-CRISPR proteins. Nat. Rev. Mol. Cell Biol. 22, 563–579 (2021).
Wilkinson, M. et al. Structural basis for the inhibition of RecBCD by Gam and its synergistic antibacterial effect with quinolones. Elife 5, 1–12 (2016).
Shah, M. et al. A phage-encoded anti-activator inhibits quorum sensing in Pseudomonas aeruginosa. Mol. Cell 81, 571–583.e6 (2021).
Bandyopadhyay, P. K., Studier, F. W., Hamilton, D. L. & Yuan, R. Inhibition of the type I restriction-modification enzymes EcoB and EcoK by the gene 0.3 protein of bacteriophage T7. J. Mol. Biol. 182, 567–578 (1985).
Deveau, H., Garneau, J. E. & Moineau, S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu. Rev. Microbiol. 64, 475–493 (2010).
Hutinet, G., Lee, Y., de Crécy-lagard, V. & Weigele, P. R. Hypermodified DNA in viruses of E. coli and Salmonella. EcoSalPlus 9, eESP00282019 (2021).
Bryson, A. L. et al. Covalent modification of bacteriophage T4 DNA inhibits CRISPRCas9. mBio https://doi.org/10.1128/mBio.00648-15 (2015).
Vlot, M. et al. Bacteriophage DNA glucosylation impairs target DNA binding by type I and II but not by type V CRISPR–Cas effector complexes. Nucleic Acids Res. 46, 873–885 (2018).
Mendoza, S. D. et al. A bacteriophage nucleus-like compartment shields DNA from CRISPR nucleases. Nature 577, 244–248 (2020).
Malone, L. M. et al. A jumbo phage that forms a nucleus-like structure evades CRISPR–Cas DNA targeting but is vulnerable to type III RNA-based immunity. Nat. Microbiol. 5, 48–55 (2020).
Jiang, W., Samai, P. & Marraffini, L. A. Degradation of phage transcripts by CRISPR-associated RNases enables type III CRISPR-Cas immunity. Cell 164, 710–721 (2016).
Lopatina, A., Tal, N. & Sorek, R. Abortive infection: bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 7, 371–384 (2020).
Neri, U. et al. A five-fold expansion of the global RNA virome reveals multiple new clades of RNA bacteriophages. bioRxiv https://doi.org/10.1101/2022.02.15.480533 (2022).
Wolf, Y. I. et al. Doubling of the known set of RNA viruses by metagenomic analysis of an aquatic virome. Nat. Microbiol. 5, 1262–1270 (2020).
Artamonova, D. et al. Spacer acquisition by type III CRISPR–Cas system during bacteriophage infection of Thermus thermophilus. Nucleic Acids Res. 48, 9787–9803 (2020).
Maniv, I., Jiang, W., Bikard, D. & Marraffini, L. A. Impact of different target sequences on type III CRISPR-Cas immunity. J. Bacteriol. 198, 941–950 (2016).
Manica, A., Zebec, Z., Steinkellner, J. & Schleper, C. Unexpectedly broad target recognition of the CRISPR-mediated virus defence system in the archaeon Sulfolobus solfataricus. Nucleic Acids Res. 41, 10509–10517 (2013).
Johnson, K., Learn, B. A., Estrella, M. A. & Bailey, S. Target sequence requirements of a type III-B CRISPR-Cas immune system. J. Biol. Chem. 294, 10290–10299 (2019).
Pyenson, N. C., Gayvert, K., Varble, A., Elemento, O. & Marraffini, L. A. Broad targeting specificity during bacterial type III CRISPR-Cas immunity constrains viral escape. Cell Host Microbe 22, 343–353.e3 (2017).
Gleditzsch, D. et al. PAM identification by CRISPR-Cas effector complexes: diversified mechanisms and structures. RNA Biol. 16, 504–517 (2019).
Meeske, A. J. & Marraffini, L. A. RNA guide complementarity prevents self-targeting in type VI CRISPR systems. Mol. Cell 71, 791–801.e3 (2018).
Marraffini, L. A. & Sontheimer, E. J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463, 568–571 (2010).
Elmore, J. R. et al. Bipartite recognition of target RNAs activates DNA cleavage by the type III-B CRISPR–Cas system. Genes Dev. 30, 447–459 (2016).
Vink, J. N. A., Baijens, J. H. L. & Brouns, S. J. J. PAM-repeat associations and spacer selection preferences in single and co-occurring CRISPR-Cas systems. Genome Biol. 22, 1–25 (2021).
Majumdar, S. et al. Three CRISPR-Cas immune effector complexes coexist in Pyrococcus furiosus. RNA 21, 1147–1158 (2015).
Silas, S. et al. Type III CRISPR-Cas systems can provide redundancy to counteract viral escape from type I systems. Elife 6, e27601 (2017).
Hoikkala, V. et al. Cooperation between different CRISPR-Cas types enables adaptation in an RNA-targeting system. mBio 12, e03338-20 (2021).
Chaikeeratisak, V. et al. Assembly of a nucleus-like structure during viral replication in bacteria. Science 355, 194–197 (2017).
Harrison, E. & Brockhurst, M. A. Ecological and evolutionary benefits of temperate phage: what does or doesn’t kill you makes you stronger. Bioessays 39, 1700112 (2017).
Goldberg, G. W., Jiang, W., Bikard, D. & Marraffini, L. A. Conditional tolerance of temperate phages via transcription-dependent CRISPR-Cas targeting. Nature 514, 633–637 (2014).
Goldberg, G. W. et al. Incomplete prophage tolerance by type III-A CRISPR-Cas systems reduces the fitness of lysogenic hosts. Nat. Commun. 9, 61 (2018).
Koonin, E. V. & Makarova, K. S. Discovery of oligonucleotide signaling mediated by CRISPR-associated polymerases solves two puzzles but leaves an enigma. ACS Chem. Biol. 13, 309–312 (2018).
Coleman, G. A. et al. A rooted phylogeny resolves early bacterial evolution. Science. 372, (2021).
Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020). Presents the latest evolutionary classification of CRISPR–Cas systems and cas genes.
Molina, R., Sofos, N. & Montoya, G. Structural basis of CRISPR-Cas type III prokaryotic defence systems. Curr. Opin. Struct. Biol. 65, 119–129 (2020). Summarizes the structural insights into the mechanisms governing type III CRISPR–Cas immunity.
Tamulaitis, G. Venclovas, Č. & Siksnys, V. Type III CRISPR-Cas immunity: major differences brushed aside. Trends Microbiol. 25, 49–61 (2017).
Rouillon, C. et al. Structure of the CRISPR interference complex CSM reveals key similarities with cascade. Mol. Cell 52, 124–134 (2013).
Zhang, J. et al. Structure and Mechanism of the CMR complex for CRISPR-mediated antiviral immunity. Mol. Cell 45, 303–313 (2012).
Hale, C. R. et al. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139, 945–956 (2009). Describes the type III CRISPR–Cas effector for the first time.
Makarova, K. S. et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Grüschow, S., Adamson, C. S. & White, M. F. Specificity and sensitivity of an RNA targeting type III CRISPR complex coupled with a NucC endonuclease effector. Nucleic Acids Res. 49, 13122–13134 (2021).
Sokolowski, R. D., Graham, S. & White, M. F. Cas6 specificity and CRISPR RNA loading in a complex CRISPR-Cas system. Nucleic Acids Res. 42, 6532–6541 (2014).
Walker, F. C., Chou-Zheng, L., Dunkle, J. A. & Hatoum-Aslan, A. Molecular determinants for CRISPR RNA maturation in the Cas10-Csm complex and roles for non-Cas nucleases. Nucleic Acids Res. 45, 2112–2123 (2017).
Chou-Zheng, L. & Hatoum-Aslan, A. A type III-A CRISPR-Cas system employs degradosome nucleases to ensure robust immunity. Elife 8, 1–25 (2019).
van Beljouw, S. P. B. et al. The gRAMP CRISPR-Cas effector is an RNA endonuclease complexed with a caspase-like peptidase. Science 3, eabk2718 (2021).
Özcan, A. et al. Programmable RNA targeting with the single-protein CRISPR effector Cas7-11. Nature 597, 720–725 (2021).
Tamulaitis, G. et al. Programmable RNA shredding by the type III-A CRISPR-Cas system of Streptococcus thermophilus. Mol. Cell 56, 506–517 (2014).
Kazlauskiene, M., Tamulaitis, G., Kostiuk, G., Venclovas, Č. & Siksnys, V. Spatiotemporal control of type III-A CRISPR-Cas immunity: coupling DNA degradation with the target RNA recognition. Mol. Cell 62, 295–306 (2016).
Steens, J. A. et al. SCOPE enables type III CRISPR-Cas diagnostics using flexible targeting and stringent CARF ribonuclease activation. Nat. Commun. 12, 1–12 (2021).
Estrella, M. A., Kuo, F. T. & Bailey, S. RNA-activated DNA cleavage by the type III-B CRISPR–Cas effector complex. Genes Dev. 30, 460–470 (2016).
Wang, L. et al. Dynamics of Cas10 govern discrimination between self and non-self in type III CRISPR-Cas immunity. Mol. Cell 73, 278–290.e4 (2019).
Staals, R. H. J. et al. RNA targeting by the type III-A CRISPR-Cas Csm complex of Thermus thermophilus. Mol. Cell 56, 518–530 (2014).
Jia, N. et al. Type III-A CRISPR-Cas Csm complexes: assembly, periodic RNA cleavage, DNase activity regulation, and autoimmunity. Mol. Cell 73, 264–277.e5 (2019).
Staals, R. H. J. et al. Structure and activity of the RNA-targeting type III-B CRISPR-Cas complex of Thermus thermophilus. Mol. Cell 52, 135–145 (2013).
Hale, C. R., Cocozaki, A., Li, H., Terns, R. M. & Terns, M. P. Target RNA capture and cleavage by the Cmr type III-B CRISPR–Cas effector complex. Genes Dev. 28, 2432–2443 (2014).
Hale, C. R. et al. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Mol. Cell 45, 292–302 (2012).
Rouillon, C., Athukoralage, J. S., Graham, S., Grüschow, S. & White, M. F. Control of cyclic oligoadenylate synthesis in a type III CRISPR system. Elife 7, 1–22 (2018).
Samai, P. et al. Co-transcriptional DNA and RNA cleavage during type III CRISPR-Cas immunity. Cell 161, 1164–1174 (2015).
Park, K.-H. et al. RNA activation-independent DNA targeting of the type III CRISPR-Cas system by a Csm complex. EMBO Rep. 18, 826–840 (2017).
Liu, T. Y., Liu, J. J., Aditham, A. J., Nogales, E. & Doudna, J. A. Target preference of type III-A CRISPR-Cas complexes at the transcription bubble. Nat. Commun. 10, 3001 (2019).
Mo, C. Y. et al. Type III-A CRISPR immunity promotes mutagenesis of staphylococci. Nature 592, 611–615 (2021).
Rostøl, J. T. & Marraffini, L. A. Non-specific degradation of transcripts promotes plasmid clearance during type III-A CRISPR–Cas immunity. Nat. Microbiol. 4, 656–662 (2019).
Han, W. et al. A Type III-B Cmr effector complex catalyzes the synthesis of cyclic oligoadenylate second messengers by cooperative substrate binding. Nucleic Acids Res. 46, 10319–10330 (2018).
Cocozaki, A. I. et al. Structure of the Cmr2 subunit of the CRISPR-Cas RNA silencing complex. Structure 20, 545–553 (2012).
Nasef, M. et al. Regulation of cyclic oligoadenylate synthesis by the Staphylococcus epidermidis Cas10-Csm complex. RNA 25, 948–962 (2019).
Kazlauskiene, M., Kostiuk, G., Venclovas, Č., Tamulaitis, G. & Siksnys, V. A cyclic oligonucleotide signaling pathway in type III CRISPR-Cas systems. Science 357, 605–609 (2017).
Niewoehner, O. et al. Type III CRISPR-Cas systems produce cyclic oligoadenylate second messengers. Nature 548, 543–548 (2017). Describes the discovery of cOA-based signalling upon RNA activation in type III CRISPR–Cas systems.
Shah, S. A. et al. Comprehensive search for accessory proteins encoded with archaeal and bacterial type III CRISPR-cas gene cassettes reveals 39 new cas gene families. RNA Biol. 16, 530–542 (2019). Identifies a vast range of protein families associated with type III CRISPR–Cas systems.
Makarova, K. S. et al. Evolutionary and functional classification of the CARF domain superfamily, key sensors in prokaryotic antivirus defense. Nucleic Acids Res. 48, 8828–8847 (2020). Identifies a vast range of protein families associated with type III CRISPR–Cas systems.
Makarova, K. S., Anantharaman, V., Grishin, N. V., Koonin, E. V. & Aravind, L. CARF and WYL domains: Ligand-binding regulators of prokaryotic defense systems. Front. Genet. 5, 102 (2014).
Garcia-Doval, C. et al. Activation and self-inactivation mechanisms of the cyclic oligoadenylate-dependent CRISPR ribonuclease Csm6. Nat. Commun. 11, 1596 (2020).
Niewoehner, O. & Jinek, M. Structural basis for the endoribonuclease activity of the type III-A CRISPR-associated protein Csm6. RNA 22, 318–329 (2016).
Han, W., Pan, S., López-Méndez, B., Montoya, G. & She, Q. Allosteric regulation of Csx1, a type IIIB-associated CARF domain ribonuclease by RNAs carrying a tetraadenylate tail. Nucleic Acids Res. 45, 10740–10750 (2017).
Zhao, Y. et al. Structural insights into the CRISPR-Cas-associated ribonuclease activity of Staphylococcus epidermidis Csm3 and Csm6. Sci. Bull. 63, 691–699 (2018).
McMahon, S. A. et al. Structure and mechanism of a type III CRISPR defence DNA nuclease activated by cyclic oligoadenylate. Nat. Commun. 11, 500 (2020).
Zhu, W. et al. The CRISPR ancillary effector Can2 is a dual-specificity nuclease potentiating type III CRISPR defence. Nucleic Acids Res. 49, 2777–2789 (2021).
Rostøl, J. T. et al. The Card1 nuclease provides defence during type-III CRISPR immunity. Nature 590, 624–629 (2021).
Athukoralage, J. S., Graham, S., Grüschow, S., Rouillon, C. & White, M. F. A type III CRISPR ancillary ribonuclease degrades its cyclic oligoadenylate activator. J. Mol. Biol. 431, 2894–2899 (2019).
Hinton, D. M. Transcriptional control in the prereplicative phase of T4 development. Virol. J. 7, 1–16 (2010).
Chevallereau, A. et al. Next-generation “-omics” approaches reveal a massive alteration of host RNA metabolism during bacteriophage infection of Pseudomonas aeruginosa. PLoS Genet. 12, 1–20 (2016).
Blasdel, B. G., Chevallereau, A., Monot, M., Lavigne, R. & Debarbieux, L. Comparative transcriptomics analyses reveal the conservation of an ancestral infectious strategy in two bacteriophage genera. ISME J. 11, 1988–1996 (2017).
Nemudraia, A. et al. Sequence-specific capture and concentration of viral RNA by type III CRISPR system enhances diagnostic. Res. Sq. https://doi.org/10.21203/rs.3.rs-1466718/v1 (2022).
Smalakyte, D. et al. Type III-A CRISPR-associated protein Csm6 degrades cyclic hexa-adenylate activator using both CARF and HEPN domains. Nucleic Acids Res. 48, 9204–9217 (2020).
Grüschow, S., Adamson, C. S. & White, M. F. Specificity and sensitivity of an RNA targeting type III CRISPR complex coupled with a NucC endonuclease effector. 49 13122–13134 (2021).
Lau, R. K. et al. Structure and mechanism of a cyclic trinucleotide-activated bacterial endonuclease mediating bacteriophage immunity. Mol. Cell 77, 723–733.e6 (2020).
Mayo-Muñoz, D. et al. Type III CRISPR–Cas provides resistance against nucleus-forming jumbo phages via abortive infection. bioRxiv https://doi.org/10.1101/2022.06.20.496707 (2022).
Burroughs, A. M., Zhang, D., Schäffer, D. E., Iyer, L. M. & Aravind, L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling. Nucleic Acids Res. 43, 10633–10654 (2015).
Lowey, B. et al. CBASS immunity uses CARF-related effectors to sense 3′–5′- and 2′–5′-linked cyclic oligonucleotide signals and protect bacteria from phage infection. Cell 182, 38–49.e17 (2020).
Rouillon, C. et al. SAVED by a toxin: structure and function of the CRISPR Lon protease. bioRxiv https://doi.org/10.1101/2021.12.06.471393 (2021).
Athukoralage, J. S. et al. The dynamic interplay of host and viral enzymes in type III CRISPR-mediated cyclic nucleotide signalling. Elife 9, 1–16 (2020).
Athukoralage, J. S., Rouillon, C., Graham, S., Grüschow, S. & White, M. F. Ring nucleases deactivate type III CRISPR ribonucleases by degrading cyclic oligoadenylate. Nature 562, 277–280 (2018).
Brown, S., Gauvin, C. C., Charbonneau, A. A., Burman, N. & Martin Lawrence, C. Csx3 is a cyclic oligonucleotide phosphodiesterase associated with type III CRISPR-Cas that degrades the second messenger cA4. J. Biol. Chem. 295, 14963–14972 (2020).
Foster, K., Grüschow, S., Bailey, S., White, M. F. & Terns, M. P. Regulation of the RNA and DNA nuclease activities required for Pyrococcus furiosus type III-B CRISPR-Cas immunity. Nucleic Acids Res. 48, 4418–4434 (2020).
Jia, N., Jones, R., Yang, G., Ouerfelli, O. & Patel, D. J. CRISPR-Cas III-A Csm6 CARF domain is a ring nuclease triggering stepwise cA4 cleavage with ApA>p formation terminating RNase activity. Mol. Cell 75, 944–956.e6 (2019).
Samolygo, A., Athukoralage, J. S., Graham, S. & White, M. F. Fuse to defuse: a self-limiting ribonuclease-ring nuclease fusion for type III CRISPR defence. Nucleic Acids Res. 48, 6149–6156 (2020).
Athukoralage, J. S. & White, M. F. Cyclic oligoadenylate signaling and regulation by ring nucleases during type III CRISPR defense. RNA 27, 855–867 (2021).
Zhao, R. et al. A membrane-associated DHH-DHHA1 nuclease degrades type III CRISPR second messenger. Cell Rep. 32, 108133 (2020).
Athukoralage, J. S. et al. An anti-CRISPR viral ring nuclease subverts type III CRISPR immunity. Nature 577, 572–575 (2020).
Charbonneau, A. A., Eckert, D. M., Gauvin, C. C., Lintner, N. G. & Lawrence, C. M. Cyclic tetra-adenylate (cA4) recognition by Csa3; implications for an integrated Class 1 CRISPR-Cas immune response in Saccharolobus solfataricus. Biomolecules 11, 1852 (2021).
Ye, Q. et al. CRISPR-associated factor Csa3b regulates CRISPR adaptation and Cmr-mediated RNA interference in Sulfolobus islandicus. Front. Microbiol. 11, 1–12 (2020).
Xia, P., Dutta, A., Gupta, K., Batish, M. & Parashar, V. Structural basis of cyclic oligoadenylate binding to the transcription factor Csa3 outlines cross talk between type III and type I CRISPR systems. J. Biol. Chem. 298, 101591 (2022).
Zhang, S. et al. Mycobacterium tuberculosis CRISPR/Cas system Csm1 holds clues to the evolutionary relationship between DNA polymerase and cyclase activity. Int. J. Biol. Macromol. 170, 140–149 (2021).
Liu, L. et al. Two distant catalytic sites are responsible for C2c2 RNase activities. Cell 168, 121–134.e12 (2017).
Liu, L. et al. The molecular architecture for RNA-guided RNA cleavage by Cas13a. Cell 170, 714–726.e10 (2017).
Abudayyeh, O. O. et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573 (2016). Characterizes the effector protein Cas13 (then known as C2c2) for the first time.
Xu, C. et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat. Methods 18, 499–506 (2021).
Yan, W. X. et al. Cas13d is a compact RNA-targeting type VI CRISPR effector positively modulated by a WYL-domain-containing accessory protein. Mol. Cell 70, 327–339.e5 (2018).
Smargon, A. A. et al. Cas13b is a type VI-B CRISPR-associated RNA-guided RNase differentially regulated by accessory proteins Csx27 and Csx28. Mol. Cell 65, 618–630.e7 (2017).
Gootenberg, J. S. et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442 (2017).
Tambe, A., East-Seletsky, A., Knott, G. J., Doudna, J. A. & O’Connell, M. R. RNA Binding and HEPN-nuclease activation are decoupled in CRISPR-Cas13a. Cell Rep. 24, 1025–1036 (2018).
Meeske, A. J., Nakandakari-Higa, S. & Marraffini, L. A. Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature 570, 241–245 (2019). Demonstrates that the ancillary nuclease activity of Cas13 halts host growth to abort viral infection.
Adler, B. A. et al. RNA-targeting CRISPR-Cas13 provides broad-spectrum phage immunity. bioRxiv https://doi.org/10.1101/2022.03.25.485874 (2022).
Meeske, A. J. et al. A phage-encoded anti-CRISPR enables complete evasion of type VI-A CRISPR-Cas immunity. Science 369, 54–59 (2020).
VanderWal, A. R., Park, J.-U., Polevoda, B., Kellogg, E. H. & O’Connell, M. R. CRISPR-Csx28 forms a Cas13b-activated membrane pore required for robust CRISPR-Cas adaptive immunity. bioRxiv https://doi.org/10.1101/2021.11.02.466367 (2021).
Guan, J., Bosch, A. O., Mendoza, S. D., Karambelkar, S. & Berry, J. RNA targeting with CRISPR-Cas13a facilitates bacteriophage genome engineering. bioRxiv https://doi.org/10.1101/2022.02.14.480438 (2022).
Bandaru, S. et al. Structure-based design of gRNA for Cas13. Sci. Rep. 10, 1–12 (2020).
Wang, B. et al. Structural basis for self-cleavage prevention by tag:anti-tag pairing complementarity in type VI Cas13 CRISPR systems. Mol. Cell 81, 1100–1115.e5 (2021).
O’Connell, M. R. Molecular mechanisms of RNA targeting by Cas13-containing type VI CRISPR–Cas systems. J. Mol. Biol. 431, 66–87 (2019). Evaluates the structural insights into the architecture and mechanisms of type VI CRISPR–Cas effectors.
East-Seletsky, A., O’Connell, M. R., Burstein, D., Knott, G. J. & Doudna, J. A. RNA targeting by functionally orthogonal type VI-A CRISPR-Cas enzymes. Mol. Cell 66, 373–383.e3 (2017).
Gootenberg, J. S. et al. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a and Csm6. Science 360, 439–444 (2018).
Williams, M. C. et al. Phage genome cleavage enables resuscitation from Cas13 induced bacterial dormancy. bioRxiv https://doi.org/10.1101/2022.07.05.498905 (2022).
Jain, I. et al. tRNA anticodon cleavage by target-activated CRISPR-Cas13a effector. bioRxiv https://doi.org/10.1101/2021.11.10.468108 (2021).
Su, Z., Wilson, B., Kumar, P. & Dutta, A. Noncanonical roles of tRNAs: tRNA fragments and beyond. Annu. Rev. Genet. 54, 47–69 (2020).
Makarova, K. S., Gao, L., Zhang, F. & Koonin, E. V. Unexpected connections between type VI-B CRISPR-Cas systems, bacterial natural competence, ubiquitin signaling network and DNA modification through a distinct family of membrane proteins. FEMS Microbiol. Lett. 366, 1–6 (2019).
Shivram, H., Cress, B. F., Knott, G. J. & Doudna, J. A. Controlling and enhancing CRISPR systems. Nat. Chem. Biol. 17, 10–19 (2021).
Zhang, H., Dong, C., Li, L., Wasney, G. A. & Min, J. Structural insights into the modulatory role of the accessory protein WYL1 in the type VI-D CRISPR-Cas system. Nucleic Acids Res. 47, 5420–5428 (2019).
Rittershaus, E. S. C., Baek, S. H. & Sassetti, C. M. The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe 13, 643–651 (2013).
Dworkin, J. & Shah, I. M. Exit from dormancy in microbial organisms. Nat. Rev. Microbiol. 8, 890–896 (2010).
Abudayyeh, O. O. et al. RNA targeting with CRISPR-Cas13. Nature 550, 280–284 (2017).
Koonin, E. V. & Zhang, F. Coupling immunity and programmed cell suicide in prokaryotes: life-or-death choices. BioEssays 39, 1–9 (2017). Provides the conceptual basis on how viral immunity in bacteria and archaea is related to programmed cell death.
Makarova, K. S., Anantharaman, V., Aravind, L. & Koonin, E. V. Live virus-free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes. Biol. Direct 7, 1–10 (2012).
Mendoza, S. D. & Bondy-Denomy, J. Cas13 helps bacteria play dead when the enemy strikes. Cell Host Microbe 26, 1–2 (2019).
Weigel, C. & Seitz, H. Bacteriophage replication modules. FEMS Microbiol. Rev. 30, 321–381 (2006).
Muñoz-Espín, D., Serrano-Heras, G. & Salas, M. Role of Host Factors in Bacteriophage φ29 DNA Replication. in Advances in Virus Research vol. 82 351–383 (Academic Press Inc., 2012).
Chevallereau, A., Pons, B. J., van Houte, S. & Westra, E. R. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol. 20, 49–62 (2022).
Vink, J. N. A. et al. Direct visualization of native CRISPR target search in live bacteria reveals cascade DNA surveillance mechanism. Mol. Cell 77, 39–50.e10 (2020).
Dimitriu, T. et al. Bacteriostatic antibiotics promote CRISPR-Cas adaptive immunity by enabling increased spacer acquisition. Cell Host Microbe 30, 31–40.e5 (2022).
de Jonge, P. A., Nobrega, F. L., Brouns, S. J. J. & Dutilh, B. E. Molecular and evolutionary determinants of bacteriophage host range. Trends Microbiol. 27, 51–63 (2019).
Ndhlovu, A., Durand, P. M. & Ramsey, G. Programmed cell death as a black queen in microbial communities. Mol. Ecol. 30, 1110–1119 (2021).
Peeters, S. H. & de Jonge, M. I. For the greater good: programmed cell death in bacterial communities. Microbiol. Res. 207, 161–169 (2018).
Weigele, P. & Raleigh, E. A. Biosynthesis and function of modified bases in bacteria and their viruses. Chem. Rev. 116, 12655–12687 (2016).
Leenay, R. T. & Beisel, C. L. Deciphering, communicating, and engineering the CRISPR PAM. J. Mol. Biol. 429, 177–191 (2017).
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 15, 169–182 (2017).
Acknowledgements
S.J.J.B. is supported by the Netherlands Organization for Scientific Research (VICI grant no. VI.C.182.027) and has received funding from the European Research Council Consolidator Grant programme under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101003229). The authors thank members of the Brouns laboratory for helpful discussions.
Author information
Authors and Affiliations
Contributions
S.P.B.v.B., J.S., A.R.-M. and S.J.J.B. researched data for the article and contributed substantially to discussion of the content. S.P.B.v.B. wrote the manuscript. S.P.B.v.B. and A.R.-M. designed figures. S.P.B.v.B. and S.J.J.B. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Michael Terns, Malcolm White and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Mobile genetic elements
-
(MGEs). Simple genetic organisms (such as phages, plasmids, conjugative elements, transposons, introns and phage-inducible chromosomal islands) capable of horizontal transfer within or between genomes.
- Dormancy
-
A non-replicating cellular state defined by low or inactive metabolism that promotes survival during stressful conditions.
- Protospacer adjacent motif
-
(PAM). A small DNA sequence which serves as a recognition motif for various CRISPR–Cas nucleases, positioned next to a target sequence in an invading DNA-based mobile genetic element but absent from the host genome, allowing the discrimination between self and non-self.
- Protospacer flanking site
-
(PFS). A small RNA sequence positioned next to a target sequence in RNA which needs to be non-complementary to the repeat-derived portion of the CRISPR RNA for the type III and type VI CRISPR–Cas effectors to become active, thereby preventing activation by a self-transcript.
- CRISPR adaptation
-
The process by which a sequence of an invading mobile genetic element is extracted and stored in the CRISPR array.
- Palm domain
-
A domain in the Cas10 subunit of various type III CRISPR–Cas effectors with structural similarity to nucleotidyl cyclases and nucleotide polymerases, catalysing the synthesis of cyclic oligoadenylate molecules from ATP.
- Cyclic oligonucleotide-based antiphage signalling system
-
A family of defence systems in bacteria and archaea related to animal cGAS–STING immunity in which a cyclase generates cyclic oligonucleotides to activate a cell death effector protein in response to viral infection.
- Restriction–modification systems
-
Genetic compositions encoding nucleases and methylases in which specific methylation patterns generated by the methylases prevent or facilitate cleavage by the nucleases, presenting a means for discriminating self from non-self.
- Membrane depolarization
-
The process by which the basal electrochemical potential across cellular membranes changes from a negative state to a positive state.
- Toxin–antitoxin systems
-
Genetic compositions encoding at least a toxin and a counteracting antitoxin whereby the toxin component becomes active when the antitoxin is absent.
- Kin selection
-
A theory of natural selection describing behaviour that favours the fitness of closely related kin over the fitness of the individual enacting the behaviour.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Beljouw, S.P.B., Sanders, J., Rodríguez-Molina, A. et al. RNA-targeting CRISPR–Cas systems. Nat Rev Microbiol 21, 21–34 (2023). https://doi.org/10.1038/s41579-022-00793-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-022-00793-y
This article is cited by
-
Structures, mechanisms and applications of RNA-centric CRISPR–Cas13
Nature Chemical Biology (2024)
-
Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment
Signal Transduction and Targeted Therapy (2024)
-
Insights into Superinfection Immunity Regulation of Xanthomonas axonopodis Filamentous Bacteriophage cf
Current Microbiology (2024)
-
Crop bioengineering via gene editing: reshaping the future of agriculture
Plant Cell Reports (2024)
-
Craspase: A novel CRISPR/Cas dual gene editor
Functional & Integrative Genomics (2023)