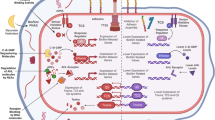
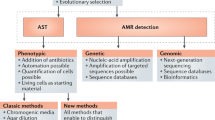
Abstract
An optimal antimicrobial dose provides enough drug to achieve a clinical response while minimizing toxicity and development of drug resistance. There can be considerable variability in pharmacokinetics, for example, owing to comorbidities or other medications, which affects antimicrobial pharmacodynamics and, thus, treatment success. Although current approaches to antimicrobial dose optimization address fixed variability, better methods to monitor and rapidly adjust antimicrobial dosing are required to understand and react to residual variability that occurs within and between individuals. We review current challenges to the wider implementation of antimicrobial dose optimization and highlight novel solutions, including biosensor-based, real-time therapeutic drug monitoring and computer-controlled, closed-loop control systems. Precision antimicrobial dosing promises to improve patient outcome and is important for antimicrobial stewardship and the prevention of antimicrobial resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hernando-Amado, S., Coque, T. M., Baquero, F. & Martínez, J. L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 4, 1432–1442 (2019).
Baur, D. et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect. Dis. 17, 990–1001 (2017).
Davey, P. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2, CD003543 (2017).
Karanika, S., Paudel, S., Grigoras, C., Kalbasi, A. & Mylonakis, E. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob. Agents Chemother. 60, 4840–4852 (2016).
Drusano, G. L., Hope, W., Macgowan, A. & Louie, A. Suppression of emergence of resistance in pathogenic bacteria: keeping our powder dry, part 2. 60, 1194–1201 (2016).
Drusano, G. L., Louie, A., Macgowan, A. & Hope, W. Suppression of emergence of resistance in pathogenic bacteria: keeping our powder dry, part 1. 60, 1183–1193 (2016). Together with Drusano et al. (2016), this article reviews state-of-the-art approaches to antimicrobial dosing and suppression of resistance.
Destache, C. J., Meyer, S. K., Bittner, M. J. & Hermann, K. G. Impact of a clinical pharmacokinetic service on patients treated with aminoglycosides: a cost–benefit analysis. Ther. Drug. Monit. 12, 419–426 (1990).
Pippenger, C. E. The cost-effectiveness of therapeutic drug monitoring. Ther. Drug Monit. 12, 418 (1990).
Destache, C. J., Meyer, S. K. & Rowley, K. M. Does accepting pharmacokinetic recommendations impact hospitalization? A cost–benefit analysis. Ther. Drug Monit. 12, 427–433 (1990).
Ambrose, P. G. et al. Pharmacokinetics–pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin. Infect. Dis. 44, 79–86 (2007).
Bauer, K. A., Perez, K. K., Forrest, G. N. & Goff, D. A. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin. Inect Dis. 59, S134–S135 (2014).
Messacar, K., Parker, S. K., Todd, J. K. & Dominguez, S. R. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J. Clin. Microbiol. 55, 715–723 (2017).
Drancourt, M., Michel-Lepage, A., Boyer, S. & Raoult, D. The point-of-care laboratory in clinical microbiology. Clin. Microbiol. Rev. 29, 429–447 (2016).
Onufrak, N. J., Forrest, A., Gonzalez, D. & Author, C. T. Pharmacokinetic and pharmacodynamic principles of anti-infective dosing. Clin. Ther. 38, 1930–1947 (2016).
Zander, J. et al. Piperacillin concentration in relation to therapeutic range in critically ill patients — a prospective observational study. Crit. Care 20, 79 (2016).
Udy, A. et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care 19, 28 (2015).
Udy, A. A., Roberts, J. A. & Lipman, J. Antibiotic Pharmacokinetic/Pharmacodynamic Considerations in the Critically Ill (Springer, 2018).
Meng, L., Mui, E., Holubar, M. K. & Deresinski, S. C. Comprehensive guidance for antibiotic dosing in obese adults. Pharmacotherapy 37, 1415–1431 (2017).
Benson, J. M. Antimicrobial pharmacokinetics and pharmacodynamics in older adults. Infect. Dis. Clin. North. Am. 31, 609–617 (2017).
Asín-Prieto, E., Rodríguez-Gascón, A. & Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 21, 319–329 (2015).
Craig, W. A. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North. Am. 17, 479–501 (2003).
Neely, M. N. et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob. Agents Chemother. 58, 309–316 (2014).
Thomas, J. K. et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely Ill patients during therapy. Antimicrob. Agents Chemother. 42, 521–527 (1998).
Hyatt, J. M. & Schentag, J. J. Pharmacodynamic modeling of risk factors for ciprofloxacin resistance in Pseudomonas aeruginosa. Infect. Control. Hosp. Epidemiol. 21, S9–S11 (2000).
Álvarez, R., López Cortés, L. E., Molina, J., Cisneros, J. M. & Pachón, J. Optimizing the clinical use of vancomycin. Antimicrob. Agents Chemother. 60, 2601–2609 (2016).
Roberts, J. A. et al. Therapeutic drug monitoring of β-lactams in critically ill patients: proof of concept. Int. J. Antimicrob. Agents 36, 332–339 (2010).
Roberts, J. A. et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 58, 1072–1083 (2014).
Holmes, A. H. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387, 176–187 (2016).
Buerger, C., Plock, N., Dehghanyar, P., Joukhadar, C. & Kloft, C. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob. Agents Chemother. 50, 2455–2463 (2006).
Mouton, J. W. et al. MIC-based dose adjustment: facts and fables. J. Antimicrob. Chemother. 73, 564–568 (2018).
Huurneman, L. J. et al. Pharmacodynamics of voriconazole in children: further steps along the path to true individualized therapy. Antimicrob. Agents Chemother. 60, 2336–2342 (2016).
Ramos-Martín, V. et al. Population pharmacokinetics and pharmacodynamics of teicoplanin in neonates: making better use of C-reactive protein to deliver individualized therapy. J. Antimicrob. Chemother. 71, 3168–3178 (2016).
Rawson, T. M. et al. Exploring the Use of C-reactive protein to estimate the pharmacodynamics of vancomycin. Therap. Drug Monit. 40, 315–321 (2018). Together with Huurneman et al. (2016) and Ramos-Martín et al. (2016), this article explores use of in vivo markers of antimicrobial pharmacodynamics.
Liu, P., Mü Ller, M. & Derendorf, H. Rational dosing of antibiotics: the use of plasma concentrations versus tissue concentrations. Int. J. Antimicrob. Agents. 19, 285–290 (2002).
European Committee on Antimicrobial Susceptibility Testing. Clinical breakpoints - breakpoints and guidance. EUCAST https://eucast.org/clinical_breakpoints/ (2020).
The Clinical & Laboratory Standards Institute (CLSI). CLSI Breakpoints. CLSI https://clsi.org/standards/products/free-resources/access-our-free-resources/ (2020).
Rawson, T. M. et al. Delivering precision antimicrobial therapy through closed-loop control systems. J. Antimicrob. Chemother. 73, 835–843 (2018).
Andersson, D. I. & Hughes, D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat. Rev. Microbiol. 8, 260–271 (2010). This article reviews the concept of antimicrobial resistance development and how we may be able to address this problem.
Andersson, D. I. & Hughes, D. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist. Updat. 15, 162–172 (2012).
Phua, J. et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit. Care 17, R202 (2013).
Zarb, P. & Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): value of a point-prevalence survey of antimicrobial use across Europe. Drugs 71, 745–755 (2011).
Cassell, A. et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br. J. Gen. Pract. 68, e245–e251 (2018).
Aubert, C. E. et al. Patterns of multimorbidity in internal medicine patients in Swiss university hospitals: a multicentre cohort study. Swiss Med. Wkly. 149, w20094 (2019).
Menditto, E. et al. Patterns of multimorbidity and polypharmacy in young and adult population: systematic associations among chronic diseases and drugs using factor analysis. PLoS ONE 14, e0210701 (2019).
Sester, M. et al. Challenges and perspectives for improved management of HIV/Mycobacterium tuberculosis co-infection. Eur. Respir. J. 36, 1242–1247 (2010).
Everts, R. J. et al. Probenecid and food effects on flucloxacillin pharmacokinetics and pharmacodynamics in healthy volunteers. J. Infect. 80, 42–53 (2020).
Grayson, M. L. et al. Once-daily intravenous cefazolin plus oral probenecid is equivalent to once-daily intravenous ceftriaxone plus oral placebo for the treatment of moderate-to-severe cellulitis in adults. Clin. Infect. Dis. 34, 1440–1448 (2002).
Robbins, N., Koch, S. E., Tranter, M. & Rubinstein, J. The history and future of probenecid. Cardiovasc. Toxicol. 12, 1–9 (2012).
Yew, W. W. Clinically significant interactions with drugs used in the treatment of tuberculosis. Drug Saf. 25, 111–113 (2002).
Chen, J. & Raymond, K. Roles of rifampicin in drug–drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann. Clin. Microbiol. Antimicrob. 5, 3 (2006).
Ma, Z., Guo, F., Qi, J., Xiang, W. & Zhang, J. Meta-analysis shows that obesity may be a significant risk factor for prosthetic joint infections. Int. Orthop. 40, 659–667 (2016).
Barras, M., Hospital, P. A. & Legg, A. Drug dosing in obese adults. Aust. Prescr. 40, 189–193 (2017).
Charani, E., Gharbi, M., Frost, G., Drumright, L. & Holmes, A. Antimicrobial therapy in obesity: a multicentre cross-sectional study. BMJ. Open 70, 2906–2912 (2015).
Lazzerini, M. & Tickell, D. Antibiotics in severely malnourished children: systematic review of efficacy, safety and pharmacokinetics. Bull. World Heal. Organ. 89, 593–606 (2011).
Grace, E. Altered vancomycin pharmacokinetics in obese and morbidly obese patients: what we have learned over the past 30 years. J. Antimicrob. Chemother. 67, 1305–1310 (2012).
Corcione, S. et al. Pharmacokinetics of high dosage of linezolid in two morbidly obese patients. J. Antimicrob. Chemother. 70, 8–9 (2015).
Huttner, A., Harbarth, S., Hope, W. W., Lipman, J. & Roberts, J. A. Therapeutic drug monitoring of the β-lactam antibiotics: what is the evidence and which patients should we be using it for? J. Antimicrob. Chemother. 70, 3178–3183 (2015).
Theuretzbacher, U. Pharmacokinetic and pharmacodynamic issues for antimicrobial therapy in patients with cancer. Clin. Infect. Dis. 54, 1785–1792 (2012).
Nielsen, E. I. & Friberg, L. E. Pharmacokinetic–pharmacodynamic modeling of antibacterial drugs. Pharmacol. Rev. 65, 1053–1090 (2013).
Roberts, J. A., Norris, R., Paterson, D. L. & Martin, J. H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 73, 27–36 (2012).
Pea, F. et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob. Agents Chemother. 54, 4605–4610 (2010).
Drlica, K. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 52, 11–17 (2003).
Tam, V. H. et al. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49, 4920–4927 (2005). This article presents evidence of suppression of antimicrobial resistance through optimized antimicrobial dosing.
Abdul-Aziz, M. H. et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 46, 1127–1153 (2020).
Sumi, C. D., Heffernan, A. J., Lipman, J., Roberts, J. A. & Sime, F. B. What antibiotic exposures are required to suppress the emergence of resistance for Gram-negative bacteria? A systematic review. Clin. Pharmacokinet. 58, 1407–1443 (2019).
Shahi, F., Redeker, K. & Chong, J. Rethinking antimicrobial stewardship paradigms in the context of the gut microbiome. JAC Antimicrobial Resist. 1, dlz015 (2019).
Penders, J., Stobberingh, E. E., Savelkoul, P. H. M. & Wolffs, P. F. G. The human microbiome as a reservoir of antimicrobial resistance. Front. Microbiol. 4, 87 (2013).
Cantón, R. & Morosini, M.-I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 35, 977–991 (2011).
Bhalodi, A. A., van Engelen, T. S. R., Virk, H. S. & Wiersinga, W. J. Impact of antimicrobial therapy on the gut microbiome. J. Antimicrob. Chemother. 74, i6–i15 (2019).
Pea, F. et al. TDM coupled with Bayesian forecasting should be considered an invaluable tool for optimizing vancomycin daily exposure in unstable critically ill patients. Int. J. Antimicrob. Agents 20, 326–332 (2002).
Pea, F. et al. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob. Agents Chemother. 53, 1863–1867 (2009).
Williams, P., Beall, G., Cotta, M. O. & Roberts, J. A. Antimicrobial dosing in critical care: a pragmatic adult dosing nomogram. Int. J. Antimicrob. Agents 55, 105837 (2020).
Bartal, C. et al. Pharmacokinetic dosing of aminoglycosides: a controlled trial. Am. J. Med. 114, 194–198 (2003).
Minichmayr, I. K. et al. Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J. Antimicrob. Chemother. 73, 1330–1339 (2018).
Charani, E. et al. Lack of weight recording in patients being administered narrow therapeutic index antibiotics: a prospective cross-sectional study. BMJ Open 5, e006092 (2015).
Rybak, M. J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42 (Suppl. 1), S35–S39 (2006).
Neely, M. N. et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob. Agents Chemother. 62, 2042–2059 (2018). This article presents a prospective trial of Bayesian forecasting to support antimicrobial dose optimization.
Avent, M. L. & Rogers, B. A. Optimising antimicrobial therapy through the use of Bayesian dosing programs. Int. J. Clin. Pharm. 41, 1121–1130 (2019).
Roberts, J. A. et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 14, 498–509 (2014).
Ates, H. C. et al. On-site therapeutic drug monitoring. Trends Biotechnol. 38, 1262–1277 (2020).
O’Hare, D. in Body Sensor Networks (ed. Yang, G.) 55–115 (Springer, 2014).
Turner, A. P. F. Biosensors: sense and sensibility. Chem. Soc. Rev. 42, 3184 (2013).
Grieshaber, D., Mackenzie, R., Vörös, J. & Reimhult, E. Electrochemical biosensors — sensor principles and architectures. Sensors 8, 1400–1458 (2008).
Monzó, J., Insua, I., Fernandez-Trillo, F. & Rodriguez, P. Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst 140, 7116–7128 (2015).
Bakker, E. & Qin, Y. Electrochemical sensors. Anal. Chem. 78, 3965–3984 (2006).
Moreno-Bondi, M. C. & Benito-Peña, E. in Optical Chemical Sensors 323–352 (Kluwer Academic, 2006).
Mascini, M., Palchetti, I. & Tombelli, S. Nucleic acid and peptide aptamers: fundamentals and bioanalytical aspects. Angew. Chem. Int. Ed. 51, 1316–1332 (2012).
Mungroo, N. A. & Neethirajan, S. Biosensors for the detection of antibiotics in poultry industry — a review. Biosensors 4, 472–493 (2014).
Reder-Christ, K. & Bendas, G. Biosensor applications in the field of antibiotic research — a review of recent developments. Sensors 11, 9450–9466 (2011).
Hayat, A. & Marty, J. L. Aptamer based electrochemical sensors for emerging environmental pollutants. Front. Chem. 2, 41 (2014).
Gorchkov, D. V., Soldatkin, A. P., Maupas, H., Martelet, C. & Jaffrezic-Renault, N. Correlation between the electrical charge properties of polymeric membranes and the characteristics of ion field effect transistors or penicillinase based enzymatic field effect transistors. Anal. Chim. Acta 331, 217–223 (1996).
Ferguson, B. S. et al. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci. Transl. Med. 5, 213ra165–213ra165 (2013).
Kittichan, K. Aptamer biosensors. Imperial College London https://spiral.imperial.ac.uk/handle/10044/1/39048 (2016).
Guo, Y., Wang, X. & Sun, X. Aptamer biosensor for antibiotic residues detection in food analysis. Sens. Tranducers 156, 368–373 (2013).
Rawson, T. M. et al. Public acceptability of computer-controlled antibiotic management: an exploration of automated dosing and opportunities for implementation. J. Infect. 78, 75–86 (2018).
Trouillon, R., Cheung, C., Patel, B. A. & O’Hare, D. Comparative study of poly(styrene-sulfonate)/poly(l-lysine) and fibronectin as biofouling-preventing layers in dissolved oxygen electrochemical measurements. Analyst 134, 784–793 (2009).
Trouillon, R., Cheung, C., Patel, B. A. & O’Hare, D. Electrochemical study of the intracellular transduction of vascular endothelial growth factor induced nitric oxide synthase activity using a multi-channel biocompatible microelectrode array. Biochim. Biophys. Acta 1800, 929–936 (2010).
Wisniewski, N., Moussy, F. & Reichert, W. M. Characterization of implantable biosensor membrane biofouling. Fresenius’ J. Anal. Chem. 366, 611–621 (2000).
Gray, M. et al. Implantable biosensors and their contribution to the future of precision medicine. Veterinary J. 239, 21–29 (2018).
Frost, M. & Meyerhoff, M. E. In vivo chemical sensors: tackling biocompatibility. Anal. Chem. 78, 7370–7377 (2006).
Soto, R. J., Hall, J. R., Brown, M. D., Taylor, J. B. & Schoenfisch, M. H. In vivo chemical sensors: role of biocompatibility on performance and utility. Anal. Chem. 89, 276–299 (2017).
Smith, N. A. et al. Fluorescent Ca2+ indicators directly inhibit the Na,K-ATPase and disrupt cellular functions. Sci. Signal. 11, eaal2039 (2018).
Vigneshvar, S., Sudhakumari, C. C., Senthilkumaran, B. & Prakash, H. Recent advances in biosensor technology for potential applications — an overview. Front. Bioeng. Biotechnol. 4, 11 (2016).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Helton, K. L., Ratner, B. D., Wisniewski, N. A. & Wisniewski, N. Biomechanics of the sensor–tissue interface — effects of motion, pressure, and design on sensor performance and the foreign body response — part II: examples and application. J. Diabetes Sci. Technol. 5, 647–656 (2011).
Helton, K. L., Ratner, B. D. & Wisniewski, N. A. Biomechanics of the sensor–tissue interface — effects of motion, pressure, and design on sensor performance and the foreign body response — part I: theoretical framework. J. Diabetes Sci. Technol. 5, 632–646 (2011).
Liang, S. et al. Measuring luteinising hormone pulsatility with a robotic aptamer-enabled electrochemical reader. Nat. Commun. 10, 852 (2019).
Rowe, A. A., Miller, E. A. & Plaxco, K. W. Reagentless measurement of aminoglycoside antibiotics in blood serum via an electrochemical, ribonucleic acid aptamer-based biosensor. Anal. Chem. 82, 7090–7095 (2010).
Gowers, S. et al. Development of a minimally-invasive microneedle-based sensor for continuous monitoring of β-lactam antibiotic concentrations in vivo. ACS Sens. 4, 1072–1080 (2019).
Rawson, T. M. et al. Towards a minimally invasive device for β-lactam monitoring in humans. Electrochem. Commun. 82, 1–5 (2017).
Rawson, T. M. et al. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: a first-in-human evaluation in healthy volunteers. Lancet Digit. Heal. 1, 335–343 (2019). This article presents a first-in-human trial of microneedle biosensors for real-time monitoring of antimicrobial concentrations.
Wolf, M. B. & Deland, E. C. A mathematical model of blood-interstitial acid–base balance: application to dilution acidosis and acid–base status. J. Appl. Physiol. 110, 988–1002 (2011).
Niedzwiecki, M. M. et al. Human suction blister fluid composition determined using high-resolution metabolomics. Anal. Chem. 90, 3786–3792 (2018).
Google Patents. Aptamer-coated microneedle-based diagnostic skin patch. Google https://patents.google.com/patent/WO2017007271A1/en (2019).
Li, H., Dauphin-Ducharme, P., Ortega, G. & Plaxco, K. W. Calibration-free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J. Am. Chem. Soc. 139, 11207–11213 (2017).
Brown, S. A. et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N. Engl. J. Med. 381, 1707–1717 (2019).
Madhavan, J. S., Puri, G. D. & Mathew, P. J. Closed-loop isoflurane administration with bispectral index in open heart surgery: randomized controlled trial with manual control. Acta Anaesthesiol. Taiwan. 49, 130–135 (2011).
Salam, M. T., Mirzaei, M., Ly, M. S., Nguyen, D. K. & Sawan, M. An implantable closedloop asynchronous drug delivery system for the treatment of refractory epilepsy. IEEE Trans. Neural Syst. Rehabil. Eng. 20, 432–442 (2012).
Li, J., Liang, J. Y., Laken, S. J., Langer, R. & Traverso, G. Feature review clinical opportunities for continuous biosensing and closed-loop therapies. Trends Chem. 2, 319–340 (2020).
Scholten, K. & Meng, E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 544, 319–334 (2018).
Herrero, P. et al. Closed-loop control for precision antimicrobial delivery: an in silico proof-of-concept. IEEE Trans. Biomed. Eng. 65, 2231–2236 (2018). This article is an in silico demonstration of closed-loop control of antimicrobial delivery.
Johnson, M. A. in PID Control 1–46 (Springer-Verlag, 2005).
Sutton, R. S. & Barto, A. G. Reinforcement Learning: An Introduction 2nd ed. (MIT Press, 2015).
Arroyo-Currás, N. et al. High-precision control of plasma drug levels using feedback-controlled dosing. ACS Pharmacol. Transl. Sci. 1, 110–118 (2018). This article is an in vivo demonstration of closed-loop control of antimicrobial delivery.
Padmanabhan, R., Meskin, N. & Haddad, W. M. Reinforcement learning-based control of drug dosing for cancer chemotherapy treatment. Math. Biosci. 293, 11–20 (2017).
Tejedor, M., Woldaregay, A. Z. & Godtliebsen, F. Reinforcement learning application in diabetes blood glucose control: a systematic review. Artif. Intell. Med. 104, 101836 (2020).
Cescon, M., Deshpande, S., Nimri, R., Doyle, F. J. III & Dassau, E. Using iterative learning for insulin dosage optimization in multiple-daily-injections therapy for people with type 1 diabetes. IEEE Trans. Biomed. Eng. 68, 482–491 (2021).
Reddy, M. et al. Clinical safety and feasibility of the advanced bolus calculator for type 1 diabetes based on case-based reasoning: a 6-week nonrandomized single-arm pilot study. Diabetes Technol. Ther. 18, 487–493 (2016).
Bulik, C. C. et al. PK–PD compass: bringing infectious diseases pharmacometrics to the patient’s bedside. J. Pharmacokinet. Pharmacodyn. 44, 161–177 (2017).
Ming, D. et al. Connectivity of rapid-testing diagnostics and surveillance of infectious diseases. Bull. World Health Organ. 97, 242–244 (2019).
Komorowski, M., Celi, L. A., Badawi, O., Gordon, A. C. & Faisal, A. A. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med. 24, 1716–1720 (2018).
Rawson, T. M. et al. A real-world evaluation of a case-based reasoning algorithm to support antimicrobial prescribing decisions in acute care. Clin. Infect. Dis. 4, ciaa383 (2020).
Rawson, T. M. et al. Supervised machine learning for the prediction of infection on admission to hospital: a prospective observational cohort study. J. Antimicrob. Chemother. 74, 1108–1115 (2019).
Ribba, B., Dudal, S., Lavé, T. & Peck, R. W. Model-informed artificial intelligence: reinforcement learning for precision dosing. Clin. Pharmacol. Ther. 107, 853–857 (2020).
Rawson, T. M. et al. Mapping the decision pathways of acute infection management in secondary care among UK medical physicians: a qualitative study. BMC Med. 14, 208 (2016).
Acknowledgements
The authors acknowledge the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with Public Health England and the NIHR Imperial Patient Safety Translational Research Centre. The Department of Health and Social Care-funded Centre for Antimicrobial Optimization (CAMO), Imperial College London provides state-of-the-art research facilities and consolidates multidisciplinary academic excellence, clinical expertise, Imperial’s NIHR/Wellcome-funded Clinical Research Facility (CRF) and partnerships with the National Health Service (NHS) to support and deliver innovative research on antimicrobial optimization and precision prescribing. The authors also thank the Department of Health and Social Care-funded Centre of Excellence in Infectious Diseases Research (CEIDR), Liverpool University, which focuses on infection therapeutics and the NIHR HPRU. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the UK Department of Health.
Author information
Authors and Affiliations
Contributions
T.M.R. and A.H.H. drafted the initial manuscript with support from other authors on their areas of expertise (R.C.W., D.O’H., P.H., A.K., M.L., M.E., P.G., A.C., W.W.H.). All authors contributed significantly to subsequent iterations and finalization of the manuscript for submission to the journal. T.M.R., R.C.W., P.H., A.C. and A.H.H. produced the figures, finalizing them for submission to the journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antimicrobial resistance
-
The development of resistance to the effects of an antimicrobial drug that once was effective against a bacterium, fungus or other microorganism.
- Antimicrobial stewardship
-
An approach to optimize antimicrobial use to ensure optimal therapeutic outcomes while minimizing the harmful consequences of therapy on the individual (toxicity) and the wider population (antimicrobial resistance).
- Pharmacokinetics
-
The movement of a drug into, through and out of the body (what the body does to a drug). Pharmacokinetics can be described by, for example, concentration over time.
- Pharmacodynamics
-
The relationship between drug concentration and an observed effect (what the drug does to the body).
- Biofilm
-
A collection of microorganisms in which the cells stick to each other and/or a surface. The adherent cells are protected by an extracellular matrix of polymeric substances.
- Hetero-resistance
-
Resistance to an antimicrobial occurring in a subset of an otherwise susceptible microbial population.
- Therapeutic index
-
A relative measure of drug safety. Defined as a ratio between the amount of drug that causes toxicity and the amount of drug that leads to a therapeutic effect. A narrow therapeutic index (low ratio) therefore has a small window between therapeutic success and toxicity.
- Volume of distribution
-
A theoretical volume that would be required to contain the total amount of an administered drug at the concentration that is observed in the plasma.
- Minimum inhibitory concentration
-
(MIC). The minimum antimicrobial concentration required to prevent the visible growth of bacteria in vitro. Measurement is performed under standardized conditions.
- C-reactive protein
-
(CRP). An acute-phase response protein produced by the liver in response to inflammation, including that caused by infection.
- Galactomannan
-
A polysaccharide component of the cell wall of certain fungi, such as Aspergillus spp.
- Breakpoints
-
Chosen concentrations (milligrams per litre) of antimicrobial that define whether an organism is sensitive or resistant based on the minimum inhibitory concentration. Defined based on the highest drug concentration that can likely be achieved in a patient.
- Area under the concentration–time curve
-
(AUC). The total integrated area under a drug concentration–time curve.
- EC50
-
The concentration of drug that gives a half-maximal response.
- Bayesian posterior estimate
-
An estimate that enables the inference of a value (for example, drug concentration at a certain time), derived using a prior assumption and a likelihood function, forming a statistical model for observed data.
- Nomogram
-
A diagram that represents the relationship between three or more variables, designed so that one variable can be estimated, for example, by drawing a straight line intersecting the other variables at measured values.
- Aptamer
-
An oligonucleotide or peptide molecule that is selected to bind to a specific target molecule.
- Square wave voltammetry
-
An electrochemical technique that is a type of linear potential sweep voltammetry, using combined square wave and staircase potentials applied to a stationary electrode.
- Chronoamperometry
-
An electrochemical technique that involves stepping the potential of an electrode. The step in potential results in a current that can be monitored as a function of time.
- Artificial intelligence
-
The theory and development of computer systems that can perform tasks that normally require human intelligence.
Rights and permissions
About this article
Cite this article
Rawson, T.M., Wilson, R.C., O’Hare, D. et al. Optimizing antimicrobial use: challenges, advances and opportunities. Nat Rev Microbiol 19, 747–758 (2021). https://doi.org/10.1038/s41579-021-00578-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-021-00578-9
This article is cited by
-
Drug stewardship in chronic kidney disease to achieve effective and safe medication use
Nature Reviews Nephrology (2024)
-
What’s new in therapeutic drug monitoring of antimicrobials?
Intensive Care Medicine (2023)
-
An efficient cat hunting optimization-biased ReLU neural network for healthcare monitoring system
Wireless Networks (2023)
-
Awareness regarding antimicrobial resistance and confidence to prescribe antibiotics in dentistry: a cross-continental student survey
Antimicrobial Resistance & Infection Control (2022)
-
Anti-tuberculosis treatment strategies and drug development: challenges and priorities
Nature Reviews Microbiology (2022)