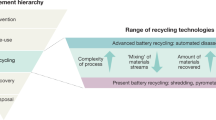
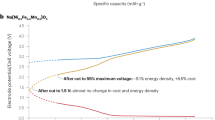
Abstract
Sodium-ion batteries (SIBs) are promising electrical power sources complementary to lithium-ion batteries (LIBs) and could be crucial in future electric vehicles and energy storage systems. Spent LIBs and SIBs both face many of the same environmental and economic challenges in their recycling, but SIB recycling has a much higher economic barrier. Although LIB recycling can be profitable by recovering high-valued metals of lithium and cobalt, the lower material valuation of spent SIBs diminishes profitability and may hinder industrial recycling. Pre-emptive strategies to facilitate recycling spent SIBs should be made during the early commercialization stage to ensure that SIBs are designed for ease of recycling, low operation costs and optimum efficiency. This Perspective article summarizes the material components of SIBs, discusses strategies for their recycling and outlines the associated challenges and future outlook of SIB recycling. The insights presented should aid scientists and engineers in creating a circular economy for the SIB industry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Federal Consortium for Advanced Batteries. National blueprint for lithium batteries 2021–2030 (FCAB, 2021).
Slowik, P., Lutsey N. & Hsu, C. How technology, recycling, and policy can mitigate supply risks to long-term transition to zero-emission vehicles (ICCT, 2020).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Lin, M. C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324–328 (2015).
Arroyo-de Dompablo, M. E., Ponrouch, A., Johansson, P. & Palacín, M. R. Achievements, challenges, and prospects of calcium batteries. Chem. Rev. 120, 6331–6357 (2019).
Xu, C., Li, B., Du, H. & Kang, F. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew. Chem. Int. Ed. 124, 957–959 (2012).
Tarascon, J. M. Na-ion versus Li-ion batteries: complementarity rather than competitiveness. Joule 4, 1616–1620 (2020).
Vaalma, C., Buchholz, D., Weil, M. & Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 3, 18013 (2018).
Contemporary Amperex Technology. CATL releases the first generation of sodium-ion batteries. CATL https://www.catl.com/news/994.html (2021).
EVTank. White paper on the development of China’s sodium-ion battery industry. EVTank http://www.evtank.cn/DownloadDetail.aspx?ID=443 (2022).
TOB New Energy. In 2023, the production capacity of sodium-ion batteries will increase by 10x. TOB https://www.tobmachine.com/in-2023-the-production-capacity-of-sodium-ion-batteries-will-increase-by-10x_n662 (2023).
Castillo, S., Ansart, F., Laberty-Robert, C. & Portal, J. Advances in the recovering of spent lithium battery compounds. J. Power Sources 112, 247–254 (2002).
Contestabile, M., Panero, S. & Scrosati, B. A laboratory-scale lithium battery recycling process. J. Power Sources 83, 75–78 (1999).
Lain, M. J. Recycling of lithium ion cells and batteries. J. Power Sources 97–98, 736–738 (2001).
Contestabile, M., Panero, S. & Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 92, 65–69 (2001).
Azhari, L., Bong, S., Ma, X. & Wang, Y. Recycling for all solid-state lithium-ion batteries. Matter 3, 1845–1861 (2020).
Li, W. et al. Thermally depolymerizable polyether electrolytes for convenient and low-cost recycling of LiTFSI. Angew. Chem. Int. Ed. 134, e202209 (2022).
Yin, Y. et al. Fire-extinguishing, recyclable liquefied gas electrolytes for temperature-resilient lithium-metal batteries. Nat. Energy 7, 548–559 (2022).
Bauer, A. et al. The scale‐up and commercialization of nonaqueous Na‐ion battery technologies. Adv. Energy Mater. 8, 1702869 (2018).
Chayambuka, K., Mulder, G., Danilov, D. L. & Notten, P. H. From Li-ion batteries toward Na-ion chemistries: challenges and opportunities. Adv. Energy Mater. 10, 2001310 (2020).
Wang, P. F., You, Y., Yin, Y. X. & Guo, Y. G. Layered oxide cathodes for sodium‐ion batteries: phase transition, air stability, and performance. Adv. Energy Mater. 8, 1701912 (2018).
Barpanda, P., Lander, L., Nishimura, S. I. & Yamada, A. Polyanionic insertion materials for sodium‐ion batteries. Adv. Energy Mater. 8, 1703055 (2018).
Peng, J. et al. Prussian blue analogues for sodium-ion batteries: past, present, and future. Adv. Mater. 34, 2108384 (2022).
Hirsh, H. S. et al. Sodium‐ion batteries paving the way for grid energy storage. Adv. Energy Mater. 10, 2001274 (2020).
Irisarri, E., Ponrouch, A. & Palacin, M. R. Hard carbon negative electrode materials for sodium-ion batteries. J. Electrochem. Soc. 162, A2476 (2015).
Bommier, C. & Ji, X. Electrolytes, SEI formation, and binders: a review of nonelectrode factors for sodium‐ion battery anodes. Small 14, 1703576 (2018).
Sommerville, R., Shaw-Stewart, J., Goodship, V., Rowson, N. & Kendrick, E. A review of physical processes used in the safe recycling of lithium ion batteries. Sustain. Mater. Technol. 25, e00197 (2020).
Makuza, B., Tian, Q., Guo, X., Chattopadhyay, K. & Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: a comprehensive review. J. Power Sources 491, 229622 (2021).
Lu, Y., Peng, K. & Zhang, L. Sustainable recycling of electrode materials in spent Li-ion batteries through direct regeneration processes. ACS Est. Eng. 2, 586–605 (2022).
Zhao, Y. et al. Precise separation of spent lithium-ion cells in water without discharging for recycling. Energy Storage Mater. 45, 1092–1099 (2022).
Zhao, Y. et al. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Met. 40, 1431–1436 (2021).
Zhao, Y. et al. Regeneration and reutilization of cathode materials from spent lithium-ion batteries. Chem. Eng. J. 383, 123089 (2020).
Yao, Y. et al. Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review. ACS Sustain. Chem. Eng. 6, 13611–13627 (2018).
Shen, Y. Recycling cathode materials of spent lithium-ion batteries for advanced catalysts production. J. Power Sources 528, 231220 (2022).
Du, H. et al. Easily recyclable lithium-ion batteries: recycling-oriented cathode design using highly soluble LiFeMnPO4 with a water-soluble binder. Battery Energy 2, 20230011 (2023).
Ma, L. A., Naylor, A. J., Nyholm, L. & Younesi, R. Strategies for mitigating dissolution of solid electrolyte interphases in sodium‐ion batteries. Angew. Chem. Int. Ed. 60, 4855–4863 (2021).
Gupta, V. et al. Scalable direct recycling of cathode black mass from spent lithium-ion batteries. Adv. Energy Mater. 13, 2203093 (2023).
Ojanen, S., Lundström, M., Santasalo-Aarnio, A. & Serna-Guerrero, R. Challenging the concept of electrochemical discharge using salt solutions for lithium-ion battery recycling. Waste Manag. 76, 242–249 (2018).
Bai, Y., Muralidharan, N., Li, J., Essehli, R. & Belharouak, I. Sustainable direct recycling of lithium‐ion batteries via solvent recovery of electrode materials. ChemSusChem 13, 5664–5670 (2020).
He, Y. et al. A critical review of current technologies for the liberation of electrode materials from foils in the recycling process of spent lithium-ion batteries. Sci. Total Environ. 766, 142382 (2021).
Du, H. et al. Recovery of lithium salt from spent lithium‐ion battery by less polar solvent wash and water extraction. Carbon Neutralization https://doi.org/10.1002/cnl2.73 (2023).
Park, J., Park, S., Beak, M., Jeong, S. & Kwon, K. Impacts of residual electrolyte components of spent lithium-ion batteries on the physical/electrochemical properties of resynthesized cathode active materials. J. Clean. Prod. 379, 134570 (2022).
Peng, C. et al. Role of impurity copper in Li-ion battery recycling to LiCoO2 cathode materials. J. Power Sources 450, 227630 (2020).
Lu, J. et al. Surplus energy utilization of spent lithium‐ion batteries for high‐profit organolithiums. Carbon Energy 5, 6058 (2022).
Xu, P. et al. Efficient direct recycling of lithium-ion battery cathodes by targeted healing. Joule 4, 2609–2626 (2020).
European Commission. Ecodesign for sustainable products (European Commission, 2022).
Han, F., Gao, T., Zhu, Y., Gaskell, K. J. & Wang, C. A battery made from a single material. Adv. Mater. 27, 3473–3483 (2015).
Ma, X. et al. Recycled cathode materials enabled superior performance for lithium-ion batteries. Joule 5, 2955–2970 (2021).
Sohn, J. S., Yang, D. H., Shin, S. M. & Kang, J. G. Recovery of cobalt in sulfuric acid leaching solution using oxalic acid. Geosyst. Eng. 9, 81–86 (2006).
Dai, Q. et al. Everbatt: a closed-loop battery recycling cost and environmental impacts model (ANL, 2019).
Acknowledgements
This research was financially supported by the Key-Area Research and Development Program of Guangdong Province (No. 2020B090919003), National Natural Science Foundation of China (Nos 52261160384 and 52072208), Fundamental Research Project of Shenzhen (No. JCYJ20220818101004009), Local Innovative and Research Teams Project of Guandong Pearl River Talents Program (No. 2017BT01N111), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515110531) and China Postdoctoral Science Foundation (No. 2022M721800).
Author information
Authors and Affiliations
Contributions
Y.Z. and B.L. planned and supervised this project. Y.Z., J.W., T.L., N.T. and Y.K. collected and discussed recycling related data. Y.Z., J.L., H.D., C.L. and Y.K. discussed practical sodium-ion battery technology and their possible recycling strategies. Y.Z., Y.K., J.W., N.T., F.K. and B.L. summarized battery recycling strategies, challenges and future perspectives. Y.Z. wrote and edited the manuscript with N.T., J.W. and B.L. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Renjie Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
2015 Paris Climate Agreement: https://unfccc.int/sites/default/files/english_paris_agreement.pdf
2017 Climate Act: https://cdn.climatepolicyradar.org/navigator/SWE/2017/the-swedish-climate-policy-framework_4d29ca793f2bf5c7782ed55f5f62c434.pdf
2060 Carbon Neutralization Plan: https://en.ndrc.gov.cn/policies/202110/t20211024_1300725.html
Contemporary Amperex Technology: https://www.catl.com
Faradion: https://faradion.co.uk
HiNa: https://www.hinabattery.com
icbattery: http://www.icbattery.com
Natron Energy: https://natron.energy
Tiamat: http://www.tiamat-energy.com
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Kang, Y., Wozny, J. et al. Recycling of sodium-ion batteries. Nat Rev Mater 8, 623–634 (2023). https://doi.org/10.1038/s41578-023-00574-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-023-00574-w
This article is cited by
-
Exploration of the two-dimensional transition metal phosphide MoP2 as anode for Na/K ion batteries
npj 2D Materials and Applications (2024)
-
Insights into Nano- and Micro-Structured Scaffolds for Advanced Electrochemical Energy Storage
Nano-Micro Letters (2024)