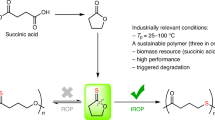
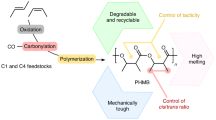
Abstract
Bio-based compounds with unique chemical functionality can be obtained through selective transformations of plant and other non-fossil, biogenic feedstocks for the development of new polymers to displace those produced from fossil carbon feedstocks. Although substantial efforts have been invested to produce bio-based polymers that are chemically identical to and directly replace those from petroleum, a long-pursued goal is to synthesize new, sustainable, bio-based polymers that either functionally replace or exhibit performance advantages relative to incumbent polymers. Owing to anthropogenic climate change and the environmental consequences of global plastics pollution, the need to realize a bio-based materials economy at scale is critical. To that end, in this Review we describe the concept of performance-advantaged, bio-based polymers (PBPs), highlighting examples wherein superior performance is facilitated by the inherent chemical functionality of bio-based feedstocks. We focus on PBPs with C–O and C–N inter-unit chemical bonds, as these are often readily accessible from bio-based feedstocks, which are heteroatom-rich relative to petroleum-derived feedstocks. Finally, we outline guiding principles and challenges to aid progress in the development of PBPs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Andrady, A. L. & Neal, M. A. Applications and societal benefits of plastics. Phil. Trans. R. Soc. B 364, 1977–1984 (2009).
Davis, S. J., Caldeira, K. & Matthews, H. D. Future CO2 emissions and climate change from existing energy infrastructure. Science 329, 1330–1333 (2010).
World Economic Forum, Ellen MacArthur Foundation & McKinsey. The new plastics economy — rethinking the future of plastics (World Economic Forum, 2016).
MacArthur, E. Beyond plastic waste. Science 358, 843–843 (2017).
Hermann, B. G., Blok, K. & Patel, M. K. Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ. Sci. Technol. 41, 7915–7921 (2007).
Dodds, D. R. & Gross, R. A. Chemicals from biomass. Science 318, 1250–1251 (2007).
Bozell, J. J. & Petersen, G. R. Technology development for the production of biobased products from biorefinery carbohydrates — the US Department of Energy’s “top 10” revisited. Green Chem. 12, 539–554 (2010).
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012).
Shen, L., Worrell, E. & Patel, M. K. Comparing life cycle energy and GHG emissions of bio-based PET, recycled PET, PLA, and man-made cellulosics. Biofuel Bioprod. Biorefin. 6, 625–639 (2012).
Weiss, M. et al. A review of the environmental impacts of biobased materials. J. Ind. Ecol. 16, S169–S181 (2012).
Chen, G.-Q. & Patel, M. K. Plastics derived from biological sources: present and future: a technical and environmental review. Chem. Rev. 112, 2082–2099 (2012).
Babu, R. P., O’Connor, K. & Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2, 8–23 (2013).
Sheldon, R. A. Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem. 16, 950–963 (2014).
Gandini, A. & Lacerda, T. M. From monomers to polymers from renewable resources: recent advances. Prog. Polym. Sci. 48, 1–39 (2015).
Isikgor, F. H. & Becer, C. R. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 6, 4497–4559 (2015).
Zhu, Y., Romain, C. & Williams, C. K. Sustainable polymers from renewable resources. Nature 540, 354–362 (2016).
Delidovich, I. et al. Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 116, 1540–1599 (2016).
Galbis, J. A., García-Martín, Md. G., de Paz, M. V. & Galbis, E. Synthetic polymers from sugar-based monomers. Chem. Rev. 116, 1600–1636 (2016).
Hillmyer, M. A. The promise of plastics from plants. Science 358, 868–870 (2017).
Shanks, B. H. & Keeling, P. L. Bioprivileged molecules: creating value from biomass. Green Chem. 19, 3177–3185 (2017).
Zhang, X., Fevre, M., Jones, G. O. & Waymouth, R. M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 118, 839–885 (2018).
Debuissy, T., Pollet, E. & Avérous, L. Biotic and abiotic synthesis of renewable aliphatic polyesters from short building blocks obtained from biotechnology. ChemSusChem 11, 3836–3870 (2018).
Hong, M. & Chen, E. Y.-X. Future directions for sustainable polymers. Trends Chem. 1, 148–151 (2019).
Nicholson, S. R., Rorrer, N. A., Carpenter, A. C. & Beckham, G. T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 5, 673–686 (2021).
Nikolau, B. J., Perera, M. A. D. N., Brachova, L. & Shanks, B. Platform biochemicals for a biorenewable chemical industry. Plant J. 54, 536–545 (2008).
Hermann, B. G. & Patel, M. Today’s and tomorrow’s bio-based bulk chemicals from white biotechnology. Appl. Biochem. Biotechnol. 136, 361–388 (2007).
Fitzgerald, N. D. Chemistry challenges to enable a sustainable bioeconomy. Nat. Rev. Chem. 1, 0080 (2017).
Fitzgerald, N. & Bailey, A. Moving Beyond Drop-in Replacements: Performance-advantaged Biobased Chemicals (US Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office, 2018).
Klemm, D., Heublein, B., Fink, H. P. & Bohn, A. Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 44, 3358–3393 (2005).
Upton, B. M. & Kasko, A. M. Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 116, 2275–2306 (2016).
Scheller, H. V. & Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 61, 263–289 (2010).
Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277 (2008).
Boerjan, W., Ralph, J. & Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 54, 519–546 (2003).
Chundawat, S. P., Beckham, G. T., Himmel, M. E. & Dale, B. E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2, 121–145 (2011).
Pollard, M., Beisson, F., Li, Y. & Ohlrogge, J. B. Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci. 13, 236–246 (2008).
Rinaudo, M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 31, 603–632 (2006).
Yan, N. & Chen, X. Sustainability: don’t waste seafood waste. Nature 524, 155–157 (2015).
Hülsey, M. J., Yang, H. & Yan, N. Sustainable routes for the synthesis of renewable heteroatom-containing chemicals. ACS Sus. Chem. Eng. 6, 5694–5707 (2018).
Ma, X. et al. Upcycling chitin-containing waste into organonitrogen chemicals via an integrated process. Proc. Natl Acad. Sci. USA 117, 7719–7728 (2020).
Lee, S. Y. et al. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2, 18–33 (2019).
Wheeldon, I., Christopher, P. & Blanch, H. Integration of heterogeneous and biochemical catalysis for production of fuels and chemicals from biomass. Curr. Opin. Biotechnol. 45, 127–135 (2017).
Zhou, X. et al. Computational framework for the identification of bioprivileged molecules. ACS Sus. Chem. Eng. 7, 2414–2428 (2019).
Huo, J. & Shanks, B. H. Bioprivileged molecules: integrating biological and chemical catalysis for biomass conversion. Annu. Rev. Chem. Biomol. Eng. 11, 63–85 (2020).
Nguyen, H. T. H., Qi, P., Rostagno, M., Feteha, A. & Miller, S. A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 6, 9298–9331 (2018).
Gandini, A. & Lacerda, T. M. Polymers from Plant Oils 2nd edn (Scrivener Publishing, 2019).
Mahajan, J. S., O’Dea, R. M., Norris, J. B., Korley, L. T. J. & Epps, T. H. Aromatics from lignocellulosic biomass: a platform for high-performance thermosets. ACS Sus. Chem. Eng. 8, 15072–15096 (2020).
Scott, A. Styrene leak in India kills at least 13. Chemical & Engineering News https://cen.acs.org/safety/industrialsafety/Styrene-leak-India-kills-least/98/web/2020/05 (2020).
Terasaki, M., Kazama, T., Shiraishi, F. & Makino, M. Identification and estrogenic characterization of impurities in commercial bisphenol A diglycidyl ether (BADGE). Chemosphere 65, 873–880 (2006).
Shi, M., Sekulovski, N., MacLean, J. A. & Hayashi, K. Effects of bisphenol A analogues on reproductive functions in mice. Reprod. Toxicol. 73, 280–291 (2017).
Ramskov Tetzlaff, C. N., Svingen, T., Vinggaard, A. M., Rosenmai, A. K. & Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 35, 543–552 (2020).
Koelewijn, S. F. et al. Sustainable bisphenols from renewable softwood lignin feedstock for polycarbonates and cyanate ester resins. Green Chem. 19, 2561–2570 (2017).
Rorrer, N. A., Vardon, D. R., Dorgan, J. R., Gjersing, E. J. & Beckham, G. T. Biomass-derived monomers for performance-differentiated fiber reinforced polymer composites. Green Chem. 19, 2812–2825 (2017).
Koelewijn, S. F. et al. Promising bulk production of a potentially benign bisphenol A replacement from a hardwood lignin platform. Green Chem. 20, 1050–1058 (2018).
Patel, A., Maiorana, A., Yue, L., Gross, R. A. & Manas-Zloczower, I. Curing kinetics of biobased epoxies for tailored applications. Macromolecules 49, 5315–5324 (2016).
Kurian, J. V. A new polymer platform for the future — Sorona® from corn derived 1,3-propanediol. Polym. Env. 13, 159–167 (2005).
Sarathchandran, C., Chan, C. H., Karim, S. R. B. A. & Thomas, S. in Physical Chemistry of Macromolecules Ch. 19 (eds Chan, C. H, Chia, C. H. & Thomas, S.) 573–617 (Apple Academic, 2014).
Bomgardner, M. Is clarity coming for biobased chemicals? C&EN Glob. Enterp. 98, 28–33 (2020).
Gowda, R. R. & Chen, E. Y.-X. in Encyclopedia of Polymer Science and Technology (Wiley, 2013).
Satoh, K. Controlled/living polymerization of renewable vinyl monomers into bio-based polymers. Polym. J. 47, 527–536 (2015).
Winnacker, M. & Rieger, B. Recent progress in sustainable polymers obtained from cyclic terpenes: synthesis, properties, and application potential. ChemSusChem 8, 2455–2471 (2015).
Kristufek, S. L., Wacker, K. T., Tsao, Y.-Y. T., Su, L. & Wooley, K. L. Monomer design strategies to create natural product-based polymer materials. Nat. Prod. Rep. 34, 433–459 (2017).
Winnacker, M. Pinenes: abundant and renewable building blocks for a variety of sustainable polymers. Angew. Chem. Int. Ed. 57, 14362–14371 (2018).
Gilsdorf, R. A., Nicki, M. A. & Chen, E. Y. X. High chemical recyclability of vinyl lactone acrylic bioplastics. Polym. Chem. 11, 4942–4950 (2020).
Hillmyer, M. A. & Tolman, W. B. Aliphatic polyester block polymers: renewable, degradable, and sustainable. Acc. Chem. Res. 47, 2390–2396 (2014).
Muñoz-Guerra, S., Lavilla, C., Japu, C. & Martínez de Ilarduya, A. Renewable terephthalate polyesters from carbohydrate-based bicyclic monomers. Green Chem. 16, 1716–1739 (2014).
Tullo, A. H. A biopolymer whose time has come. C&EN Glob. Enterp. 97, 20–21 (2019).
Martínez de Ilarduya, A. & Muñoz Guerra, S. Ring opening polymerization of macrocyclic oligoesters derived from renewable sources. Polym. Chem. 11, 4850–4860 (2020).
Gregory, G. L., López-Vidal, E. M. & Buchard, A. Polymers from sugars: cyclic monomer synthesis, ring-opening polymerisation, material properties and applications. ChemComm 53, 2198–2217 (2017).
Muhammadi, Shabina, Afzal, M. & Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 8, 56–77 (2015).
Anjum, A. et al. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int. J. Biol. Macromol. 89, 161–174 (2016).
Longo, J. M., Sanford, M. J. & Coates, G. W. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: structure–property relationships. Chem. Rev. 116, 15167–15197 (2016).
Sang, T., Wallis, C. J., Hill, G. & Britovsek, G. J. P. Polyethylene terephthalate degradation under natural and accelerated weathering conditions. Eur. Polym. J. 136, 109873 (2020).
Burgess, S. K., Karvan, O., Johnson, J. R., Kriegel, R. M. & Koros, W. J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 55, 4748–4756 (2014).
Eerhart, A. J. J. E., Faaij, A. P. C. & Patel, M. K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 5, 6407–6422 (2012).
Knoop, R. J. I., Vogelzang, W., van Haveren, J. & van Es, D. S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. A Polym. Chem. 51, 4191–4199 (2013).
Burgess, S. K. et al. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 47, 1383–1391 (2014).
Xu, C., Arancon, R. A. D., Labidi, J. & Luque, R. Lignin depolymerisation strategies: towards valuable chemicals and fuels. Chem. Soc. Rev. 43, 7485–7500 (2014).
Fache, M. et al. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 16, 1987–1998 (2014).
Mialon, L., Pemba, A. G. & Miller, S. A. Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid. Green Chem. 12, 1704–1706 (2010).
Mialon, L., Vanderhenst, R., Pemba, A. G. & Miller, S. A. Polyalkylenehydroxybenzoates (PAHBs): biorenewable aromatic/aliphatic polyesters from lignin. Macromol. Rapid Commun. 32, 1386–1392 (2011).
Nguyen, H. T. H., Reis, M. H., Qi, P. & Miller, S. A. Polyethylene ferulate (PEF) and congeners: polystyrene mimics derived from biorenewable aromatics. Green Chem. 17, 4512–4517 (2015).
Nguyen, H. T. H., Short, G. N., Qi, P. & Miller, S. A. Copolymerization of lactones and bioaromatics via concurrent ring-opening polymerization/polycondensation. Green Chem. 19, 1877–1888 (2017).
Schijndel, J. et al. Repeatable molecularly recyclable semi-aromatic polyesters derived from lignin. J. Polym. Sci. 58, 1655–1663 (2020).
Kaneko, T., Thi, T. H., Shi, D. J. & Akashi, M. Environmentally degradable, high-performance thermoplastics from phenolic phytomonomers. Nat. Mater. 5, 966–970 (2006).
Nsengiyumva, O. & Miller, S. A. Synthesis, characterization, and water-degradation of biorenewable polyesters derived from natural camphoric acid. Green Chem. 21, 973–978 (2019).
Beckham, G. T., Johnson, C. W., Karp, E. M., Salvachúa, D. & Vardon, D. R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 42, 40–53 (2016).
Johnson, C. W. et al. Enhancing muconic acid production from glucose and lignin-derived aromatic compounds via increased protocatechuate decarboxylase activity. Metab. Eng. Commun. 3, 111–119 (2016).
Settle, A. E. et al. Iodine-catalyzed isomerization of dimethyl muconate. ChemSusChem 11, 1768–1780 (2018).
Johnson, C. W. et al. Innovative chemicals and materials from bacterial aromatic catabolic pathways. Joule 3, 1523–1537 (2019).
Rorrer, N. A. et al. Renewable unsaturated polyesters from muconic acid. ACS Sus. Chem. Eng. 4, 6867–6876 (2016).
Rorrer, N. A. et al. Combining reclaimed PET with bio-based monomers enables plastics upcycling. Joule 3, 1006–1027 (2019).
Quinzler, D. & Mecking, S. Linear semicrystalline polyesters from fatty acids by complete feedstock molecule utilization. Angew. Chem. Int. Ed. 49, 4306–4308 (2010).
Stempfle, F., Quinzler, D., Heckler, I. & Mecking, S. Long-chain linear C19 and C23 monomers and polycondensates from unsaturated fatty acid esters. Macromolecules 44, 4159–4166 (2011).
Stempfle, F., Ritter, B. S., Mülhaupt, R. & Mecking, S. Long-chain aliphatic polyesters from plant oils for injection molding, film extrusion and electrospinning. Green Chem. 16, 2008–2014 (2014).
Roesle, P. et al. Synthetic polyester from algae oil. Angew. Chem. Int. Ed. 53, 6800–6804 (2014).
Stempfle, F., Ortmann, P. & Mecking, S. Long-chain aliphatic polymers to bridge the gap between semicrystalline polyolefins and traditional polycondensates. Chem. Rev. 116, 4597–4641 (2016).
Witt, T., Häußler, M., Kulpa, S. & Mecking, S. Chain multiplication of fatty acids to precise telechelic polyethylene. Angew. Chem. Int. Ed. 56, 7589–7594 (2017).
Genovese, L. et al. Biodegradable long chain aliphatic polyesters containing ether-linkages: synthesis, solid-state, and barrier properties. Ind. Eng. Chem. Res. 53, 10965–10973 (2014).
Jiang, G. et al. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 17, 1157 (2016).
Wang, S. et al. Biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) plastic under anaerobic sludge and aerobic seawater conditions: gas evolution and microbial diversity. Environ. Sci. Technol. 52, 5700–5709 (2018).
Winnacker, M. Polyhydroxyalkanoates: recent advances in their synthesis and applications. Eur. J. Lipid Sci. Technol. 121, 1900101 (2019).
Sangroniz, A. et al. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Comm. 10, 3559 (2019).
Myung, J., Flanagan, J. C. A., Waymouth, R. M. & Criddle, C. S. Methane or methanol-oxidation dependent synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by obligate type II methanotrophs. Process Biochem. 51, 561–567 (2016).
Flanagan, J. C. A., Myung, J., Criddle, C. S. & Waymouth, R. M. Poly(hydroxyalkanoate)s from waste biomass: a combined chemical–biological approach. ChemistrySelect 1, 2327–2331 (2016).
Myung, J., Flanagan, J. C. A., Waymouth, R. M. & Criddle, C. S. Expanding the range of polyhydroxyalkanoates synthesized by methanotrophic bacteria through the utilization of ω-hydroxyalkanoate co-substrates. AMB Express 7, 118 (2017).
Tang, X. & Chen, E. Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 9, 2345 (2018).
Tang, X., Westlie, A. H., Watson, E. M. & Chen, E. Y.-X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 366, 754–758 (2019).
Tang, X. et al. Biodegradable polyhydroxyalkanoates by stereoselective copolymerization of racemic diolides: stereocontrol and polyolefin-like properties. Angew. Chem. Int. Ed. 59, 7881–7890 (2020).
Westlie, A. H. & Chen, E. Y.-X. Catalyzed chemical synthesis of unnatural aromatic polyhydroxyalkanoate and aromatic–aliphatic PHAs with record-high glass-transition and decomposition temperatures. Macromolecules 53, 9906–9915 (2020).
Haider, T. P., Völker, C., Kramm, J., Landfester, K. & Wurm, F. R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 58, 50–62 (2019).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Lin, B., Hedrick, J. L., Park, N. H. & Waymouth, R. M. Programmable high-throughput platform for the rapid and scalable synthesis of polyester and polycarbonate libraries. J. Am. Chem. Soc. 141, 8921–8927 (2019).
Siracusa, V. Microbial degradation of synthetic biopolymers waste. Polymers 11, 1066 (2019).
Dusselier, M., Van Wouwe, P., Dewaele, A., Jacobs, P. A. & Sels, B. F. Shape-selective zeolite catalysis for bioplastics production. Science 349, 78–80 (2015).
de Roo, G., Kellerhals, M. B., Ren, Q., Witholt, B. & Kessler, B. Production of chiral R-3-hydroxyalkanoic acids and R-3-hydroxyalkanoic acid methylesters via hydrolytic degradation of polyhydroxyalkanoate synthesized by pseudomonads. Biotechnol. Bioeng. 77, 717–722 (2002).
van der Meulen, I. et al. Catalytic ring-opening polymerization of renewable macrolactones to high molecular weight polyethylene-like polymers. Macromolecules 44, 4301–4305 (2011).
Witt, T. & Mecking, S. Large-ring lactones from plant oils. Green Chem. 15, 2361–2364 (2013).
Hodge, P. Entropically driven ring-opening polymerization of strainless organic macrocycles. Chem. Rev. 114, 2278–2312 (2014).
Wilson, J. A., Hopkins, S. A., Wright, P. M. & Dove, A. P. Synthesis of ω-pentadecalactone copolymers with independently tunable thermal and degradation behavior. Macromolecules 48, 950–958 (2015).
Myers, D. et al. Ring opening polymerization of macrolactones: high conversions and activities using an yttrium catalyst. Polym. Chem. 8, 5780–5785 (2017).
Witt, T., Häußler, M. & Mecking, S. No strain, no gain? Enzymatic ring-opening polymerization of strainless aliphatic macrolactones. Macromol. Rapid Commun. 38, 1600638 (2017).
Li, C. et al. Lipase-catalyzed ring-opening copolymerization of ω-pentadecalactone and δ-valerolactone by reactive extrusion. Green Chem. 22, 662–668 (2020).
Vendamme, R., Schüwer, N. & Eevers, W. Recent synthetic approaches and emerging bio-inspired strategies for the development of sustainable pressure-sensitive adhesives derived from renewable building blocks. J. Appl. Polym. Sci. 131, 40669 (2014).
Heinrich, L. A. Future opportunities for bio-based adhesives — advantages beyond renewability. Green Chem. 21, 1866–1888 (2019).
Brutman, J. P., De Hoe, G. X., Schneiderman, D. K., Le, T. N. & Hillmyer, M. A. Renewable, degradable, and chemically recyclable cross-linked elastomers. Ind. Eng. Chem. Res. 55, 11097–11106 (2016).
De Hoe, G. X. et al. Sustainable polyester elastomers from lactones: synthesis, properties, and enzymatic hydrolyzability. J. Am. Chem. Soc. 140, 963–973 (2018).
Schneiderman, D. K. et al. Chemically recyclable biobased polyurethanes. ACS Macro Lett. 5, 515–518 (2016).
Shin, J. et al. Pressure-sensitive adhesives from renewable triblock copolymers. Macromolecules 44, 87–94 (2011).
Vendamme, R. et al. Interplay between viscoelastic and chemical tunings in fatty-acid-based polyester adhesives: engineering biomass toward functionalized step-growth polymers and soft networks. Biomacromolecules 13, 1933–1944 (2012).
Shin, J., Lee, Y., Tolman, W. B. & Hillmyer, M. A. Thermoplastic elastomers derived from menthide and tulipalin A. Biomacromolecules 13, 3833–3840 (2012).
Sulley, G. S. et al. Switchable catalysis improves the properties of CO2-derived polymers: poly(cyclohexene carbonate-b-ε-decalactone-b-cyclohexene carbonate) adhesives, elastomers, and toughened plastics. J. Am. Chem. Soc. 142, 4367–4378 (2020).
Winkler, M., Romain, C., Meier, M. A. R. & Williams, C. K. Renewable polycarbonates and polyesters from 1,4-cyclohexadiene. Green Chem. 17, 300–306 (2015).
Xiong, M., Schneiderman, D. K., Bates, F. S., Hillmyer, M. A. & Zhang, K. Scalable production of mechanically tunable block polymers from sugar. Proc. Natl Acad. Sci. USA 111, 8357–8362 (2014).
Watts, A., Kurokawa, N. & Hillmyer, M. A. Strong, resilient, and sustainable aliphatic polyester thermoplastic elastomers. Biomacromolecules 18, 1845–1854 (2017).
Robert, C., de Montigny, F. & Thomas, C. M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Commun. 2, 586 (2011).
Peña Carrodeguas, L., Martín, C. & Kleij, A. W. Semiaromatic polyesters derived from renewable terpene oxides with high glass transitions. Macromolecules 50, 5337–5345 (2017).
Van Zee, N. J. & Coates, G. W. Alternating copolymerization of propylene oxide with biorenewable terpene-based cyclic anhydrides: a sustainable route to aliphatic polyesters with high glass transition temperatures. Angew. Chem. Int. Ed. 54, 2665–2668 (2015).
Sanford, M. J., Peña Carrodeguas, L., Van Zee, N. J., Kleij, A. W. & Coates, G. W. Alternating copolymerization of propylene oxide and cyclohexene oxide with tricyclic anhydrides: access to partially renewable aliphatic polyesters with high glass transition temperatures. Macromolecules 49, 6394–6400 (2016).
Snyder, R. L. et al. Mechanically robust and reprocessable imine exchange networks from modular polyester pre-polymers. Polym. Chem. 11, 5346–5355 (2020).
Sommerfeld, S. D., Zhang, Z., Costache, M. C., Vega, S. L. & Kohn, J. Enzymatic surface erosion of high tensile strength polycarbonates based on natural phenols. Biomacromolecules 15, 830–836 (2014).
Xu, J., Feng, E. & Song, J. Renaissance of aliphatic polycarbonates: new techniques and biomedical applications. J. Appl. Polym. Sci. 131, 39822 (2014).
Byrne, C. M., Allen, S. D., Lobkovsky, E. B. & Coates, G. W. Alternating copolymerization of limonene oxide and carbon dioxide. J. Am. Chem. Soc. 126, 11404–11405 (2004).
Auriemma, F. et al. Stereocomplexed poly(limonene carbonate): a unique example of the cocrystallization of amorphous enantiomeric polymers. Angew. Chem. Int. Ed. 54, 1215–1218 (2015).
Kristufek, T. S. et al. Rapidly-cured isosorbide-based cross-linked polycarbonate elastomers. Polym. Chem. 7, 2639–2644 (2016).
Stößer, T. et al. Bio-derived polymers for coating applications: comparing poly(limonene carbonate) and poly(cyclohexadiene carbonate). Polym. Chem. 8, 6099–6105 (2017).
Hauenstein, O., Reiter, M., Agarwal, S., Rieger, B. & Greiner, A. Bio-based polycarbonate from limonene oxide and CO2 with high molecular weight, excellent thermal resistance, hardness and transparency. Green Chem. 18, 760–770 (2016).
von der Assen, N. & Bardow, A. Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: insights from an industrial case study. Green Chem. 16, 3272–3280 (2014).
Langanke, J. et al. Carbon dioxide (CO2) as sustainable feedstock for polyurethane production. Green Chem. 16, 1865–1870 (2014).
Allen, S. D. et al. Polycarbonate polyol compositions and methods. US Patent 8,247,520 B2 (2012).
Zhang, Z. et al. A non-phosgene process for bioderived polycarbonate with high molecular weight and advanced property profile synthesized using amino acid ionic liquids as catalysts. Green Chem. 22, 2534–2542 (2020).
Park, S.-A. et al. Sustainable and recyclable super engineering thermoplastic from biorenewable monomer. Nat. Commun. 10, 2601 (2019).
Li, C., Sablong, R. J., van Benthem, R. A. T. M. & Koning, C. E. Unique base-initiated depolymerization of limonene-derived polycarbonates. ACS Macro Lett. 6, 684–688 (2017).
Neumann, S., Leitner, L.-C., Schmalz, H., Agarwal, S. & Greiner, A. Unlocking the processability and recyclability of biobased poly(limonene carbonate). ACS Sus. Chem. Eng. 8, 6442–6448 (2020).
Ma, S. & Webster, D. C. Naturally occurring acids as cross-linkers to yield VOC-free, high-performance, fully bio-based, degradable thermosets. Macromolecules 48, 7127–7137 (2015).
Hevus, I., Ricapito, N. G., Tymoshenko, S., Raja, S. N. & Webster, D. C. Biobased carboxylic acids as components of sustainable and high-performance coating systems. ACS Sus. Chem. Eng. 8, 5750–5762 (2020).
Zhang, S. et al. Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives. Green Chem. 20, 2995–3000 (2018).
Toldy, A., Szolnoki, B. & Marosi, G. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications. Polym. Degrad. Stab. 96, 371–376 (2011).
Maiorana, A., Spinella, S. & Gross, R. A. Bio-based alternative to the diglycidyl ether of bisphenol A with controlled materials properties. Biomacromolecules 16, 1021–1031 (2015).
Zago, E. et al. Synthesis of bio-based epoxy monomers from natural allyl- and vinyl phenols and the estimation of their affinity to the estrogen receptor α by molecular docking. New J. Chem. 40, 7701–7710 (2016).
Winne, J. M., Leibler, L. & Du Prez, F. E. Dynamic covalent chemistry in polymer networks: a mechanistic perspective. Polym. Chem. 10, 6091–6108 (2019).
Scheutz, G. M., Lessard, J. J., Sims, M. B. & Sumerlin, B. S. Adaptable crosslinks in polymeric materials: resolving the intersection of thermoplastics and thermosets. J. Am. Chem. Soc. 141, 16181–16196 (2019).
Liu, X. & Zhang, J. High-performance biobased epoxy derived from rosin. Polym. Int. 59, 607–609 (2010).
Pan, X., Sengupta, P. & Webster, D. C. High biobased content epoxy–anhydride thermosets from epoxidized sucrose esters of fatty acids. Biomacromolecules 12, 2416–2428 (2011).
Chrysanthos, M., Galy, J. & Pascault, J.-P. Preparation and properties of bio-based epoxy networks derived from isosorbide diglycidyl ether. Polymer 52, 3611–3620 (2011).
Hong, J., Radojč, D., Ionescu, M., Petrovič, Z. S. & Eastwood, E. Advanced materials from corn: isosorbide-based epoxy resins. Polym. Chem. 5, 5360–5368 (2014).
Hu, F., La Scala, J. J., Sadler, J. M. & Palmese, G. R. Synthesis and characterization of thermosetting furan-based epoxy systems. Macromolecules 47, 3332–3342 (2014).
Liu, W., Zhou, R., Goh, H. L. S., Huang, S. & Lu, X. From waste to functional additive: toughening epoxy resin with lignin. ACS Appl. Mater. Interfaces 6, 5810–5817 (2014).
Qin, J., Liu, H., Zhang, P., Wolcott, M. & Zhang, J. Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym. Int. 63, 760–765 (2014).
Gandini, A., Lacerda, T. M., Carvalho, A. J. F. & Trovatti, E. Progress of polymers from renewable resources: furans, vegetable oils, and polysaccharides. Chem. Rev. 116, 1637–1669 (2016).
Zhang, C., Garrison, T. F., Madbouly, S. A. & Kessler, M. R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 71, 91–143 (2017).
Li, R. et al. Use of hempseed-oil-derived polyacid and rosin-derived anhydride acid as cocuring agents for epoxy materials. ACS Sus. Chem. Eng. 6, 4016–4025 (2018).
Zhao, S., Huang, X., Whelton, A. J. & Abu-Omar, M. M. Renewable epoxy thermosets from fully lignin-derived triphenols. ACS Sus. Chem. Eng. 6, 7600–7608 (2018).
Ocando, C., Ecochard, Y., Decostanzi, M., Caillol, S. & Avérous, L. Dynamic network based on eugenol-derived epoxy as promising sustainable thermoset materials. Eur. Polym. J. 135, 109860 (2020).
Hollande, L. et al. Preparation of renewable epoxy–amine resins with tunable thermo-mechanical properties, wettability and degradation abilities from lignocellulose- and plant oils-derived components. Front. Chem. 7, 159 (2019).
Gandini, A., Carvalho, A. J. F., Trovatti, E., Kramer, R. K. & Lacerda, T. M. Macromolecular materials based on the application of the Diels–Alder reaction to natural polymers and plant oils. Eur. J. Lipid Sci. Technol. 120, 1700091 (2018).
Hernandez, E. D., Bassett, A. W., Sadler, J. M., La Scala, J. J. & Stanzione, J. F. Synthesis and characterization of bio-based epoxy resins derived from vanillyl alcohol. ACS Sus. Chem. Eng. 4, 4328–4339 (2016).
Liu, T. et al. A self-healable high glass transition temperature bioepoxy material based on vitrimer chemistry. Macromolecules 51, 5577–5585 (2018).
Zhao, S. & Abu-Omar, M. M. Recyclable and malleable epoxy thermoset bearing aromatic imine bonds. Macromolecules 51, 9816–9824 (2018).
Yu, Q. et al. Vanillin-based degradable epoxy vitrimers: reprocessability and mechanical properties study. Eur. Polym. J. 117, 55–63 (2019).
Ma, S. et al. Readily recyclable, high-performance thermosetting materials based on a lignin-derived spiro diacetal trigger. J. Mater. Chem. A 7, 1233–1243 (2019).
Wang, S. et al. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem. 21, 1484–1497 (2019).
Marchildon, K. Polyamides — still strong after seventy years. Macromol. React. Eng. 5, 22–54 (2011).
Barnes, C. E. Nylon 4 — development and commercialization. Lenzing. Ber. 62, 62–66 (1987).
Kim, H. T. et al. Development of metabolically engineered corynebacterium glutamicum for enhanced production of cadaverine and its use for the synthesis of bio-polyamide 510. ACS Sus. Chem. Eng. 8, 129–138 (2020).
Lane, J. Terryl, a next-generation fiber: innovative, cost-competitive, biobased polyamide for textiles. Biofuels Digest http://www.biofuelsdigest.com/bdigest/2014/11/10/terryl-a-next-generation-fiber-innovative-cost-competitive-biobased-polyamide-for-textiles/ (2014).
Caswell, P. J. Terryl. Presented at the Biotechnology Innovation Organization (BIO) World Congress (2014).
Yi, Z., Bingbing, Q. & Chi, L. Blended fiber and preparation method thereof and fabric comprising the blended fiber. CN Patent 105,040,156 A (2014).
Winnacker, M. & Rieger, B. Biobased polyamides: recent advances in basic and applied research. Macromol. Rapid Commun. 37, 1391–1413 (2016).
Froidevaux, V., Negrell, C., Caillol, S., Pascault, J.-P. & Boutevin, B. Biobased amines: from synthesis to polymers; present and future. Chem. Rev. 116, 14181–14224 (2016).
Pingen, D. et al. Diamines for polymer materials via direct amination of lipid- and lignocellulose-based alcohols with NH3. ChemCatChem 10, 3027–3033 (2018).
Citoler, J., Derrington, S. R., Galman, J. L., Bevinakatti, H. & Turner, N. J. A biocatalytic cascade for the conversion of fatty acids to fatty amines. Green Chem. 21, 4932–4935 (2019).
Firdaus, M. & Meier, M. A. R. Renewable polyamides and polyurethanes derived from limonene. Green Chem. 15, 370–380 (2013).
Türünç, O., Firdaus, M., Klein, G. & Meier, M. A. R. Fatty acid derived renewable polyamides via thiol–ene additions. Green Chem. 14, 2577–2583 (2012).
Jiang, Y., Maniar, D., Woortman, A. J. J., Alberda van Ekenstein, G. O. R. & Loos, K. Enzymatic polymerization of furan-2,5-dicarboxylic acid-based furanic–aliphatic polyamides as sustainable alternatives to polyphthalamides. Biomacromolecules 16, 3674–3685 (2015).
Mitiakoudis, A. & Gandini, A. Synthesis and characterization of furanic polyamides. Macromolecules 24, 830–835 (1991).
Song, L. et al. Ultra-strong long-chain polyamide elastomers with programmable supramolecular interactions and oriented crystalline microstructures. Nat. Commun. 10, 1315 (2019).
Stockmann, P. N. et al. Biobased chiral semi-crystalline or amorphous high-performance polyamides and their scalable stereoselective synthesis. Nat. Commun. 11, 509 (2020).
Stockmann, P. N. et al. New bio-polyamides from terpenes: α-pinene and (+)-3-carene as valuable resources for lactam production. Macromol. Rapid Commun. 40, 1800903 (2019).
Winnacker, M., Neumeier, M., Zhang, X., Papadakis, C. M. & Rieger, B. Sustainable chiral polyamides with high melting temperature via enhanced anionic polymerization of a menthone-derived lactam. Macromol. Rapid Commun. 37, 851–857 (2016).
Winnacker, M., Sag, J., Tischner, A. & Rieger, B. Sustainable, stereoregular, and optically active polyamides via cationic polymerization of ε-lactams derived from the terpene β-pinene. Macromol. Rapid Commun. 38, 1600787 (2017).
Winnacker, M. & Sag, J. Sustainable terpene-based polyamides via anionic polymerization of a pinene-derived lactam. ChemComm 54, 841–844 (2018).
Maisonneuve, L., Lamarzelle, O., Rix, E., Grau, E. & Cramail, H. Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s. Chem. Rev. 115, 12407–12439 (2015).
Luo, X., Xiao, Y., Wu, Q. & Zeng, J. Development of high-performance biodegradable rigid polyurethane foams using all bioresource-based polyols: lignin and soy oil-derived polyols. Int. J. Biol. Macromol. 115, 786–791 (2018).
Guo, A., Javni, I. & Petrovic, Z. Rigid polyurethane foams based on soybean oil. J. Appl. Polym. Sci. 77, 467–473 (2000).
Zlatanić, A., Lava, C., Zhang, W. & Petrović, Z. S. Effect of structure on properties of polyols and polyurethanes based on different vegetable oils. J. Polym. Sci. B Polym. Phys. 42, 809–819 (2004).
Babb, D. A. in Synthetic Biodegradable Polymers (eds Rieger, B. et al.) 315–360 (Springer, 2012).
Peyrton, J., Chambaretaud, C., Sarbu, A. & Avérous, L. Biobased polyurethane foams based on new polyol architectures from microalgae oil. ACS Sus. Chem. Eng. 8, 12187–12196 (2020).
Lysenko, Z. et al. Vegetable oil based polyols and polyurethanes made therefrom. WO Patent 2,004,096,882 A1 (2004).
Gurusamy-Thangavelu, S. A. et al. Polyurethanes based on renewable polyols from bioderived lactones. Polym. Chem. 3, 2941–2948 (2012).
Gunawan, N. R. et al. Rapid biodegradation of renewable polyurethane foams with identification of associated microorganisms and decomposition products. Bioresour. Technol. 11, 100513 (2020).
Cornille, A. et al. Promising mechanical and adhesive properties of isocyanate-free poly(hydroxyurethane). Eur. Polym. J. 84, 404–420 (2016).
Zhang, K. et al. Non-isocyanate poly(amide-hydroxyurethane)s from sustainable resources. Green Chem. 18, 4667–4681 (2016).
Carré, C., Ecochard, Y., Caillol, S. & Avérous, L. From the synthesis of biobased cyclic carbonate to polyhydroxyurethanes: a promising route towards renewable non-isocyanate polyurethanes. ChemSusChem 12, 3410–3430 (2019).
Kühnel, I., Saake, B. & Lehnen, R. A new environmentally friendly approach to lignin-based cyclic carbonates. Macromol. Chem. Phys. 219, 1700613 (2018).
Chen, X., Li, L., Jin, K. & Torkelson, J. M. Reprocessable polyhydroxyurethane networks exhibiting full property recovery and concurrent associative and dissociative dynamic chemistry via transcarbamoylation and reversible cyclic carbonate aminolysis. Polym. Chem. 8, 6349–6355 (2017).
Schimpf, V., Ritter, B. S., Weis, P., Parison, K. & Mülhaupt, R. High purity limonene dicarbonate as versatile building block for sustainable non-isocyanate polyhydroxyurethane thermosets and thermoplastics. Macromolecules 50, 944–955 (2017).
Tamami, B., Sohn, S. & Wilkes, G. L. Incorporation of carbon dioxide into soybean oil and subsequent preparation and studies of nonisocyanate polyurethane networks. J. Appl. Polym. Sci. 92, 883–891 (2004).
Liaw, D.-J. et al. Advanced polyimide materials: syntheses, physical properties and applications. Prog. Polym. Sci. 37, 907–974 (2012).
Lau, K. S. Y. in Handbook of Thermoset Plastics 3rd edn Ch. 10 (eds Dodiuk, H. & Goodman, S. H.) 297–424 (William Andrew Publishing, 2014).
McNamara, J., Harvey, J. D., Graham, M. J., Scherger, C. Optically transparent polyimides. WO Patent 2019/156,717 A2 (2019).
Serber, Z., et al. Microbial strain improvement by a HTP genomic engineering platform. WO Patent 2017/100,377 A1 (2018).
Zymergen. Zymergen Reimagines Electronics with Breakthrough Bio-fabricated Materials (Zymergen, 2020).
Lane, J. Super clear, super thin, super durable: Zymergen bends it like Beckham, electronics-wise. Biofuels Digest http://www.biofuelsdigest.com/bdigest/2020/04/06/super-clear-super-thin-super-durable-zymergen-bends-it-like-beckham-electronics-wise/ (2020).
Santhosh Kumar, K. S. & Reghunadhan Nair, C. P. in Handbook of Thermoset Plastics 3rd edn Ch. 3 (eds Dodiuk, H. & Goodman, S. H.) 45–73 (William Andrew Publishing, 2014).
Dumas, L., Bonnaud, L., Olivier, M., Poorteman, M. & Dubois, P. Chavicol benzoxazine: ultrahigh Tg biobased thermoset with tunable extended network. Eur. Polym. J. 81, 337–346 (2016).
Puchot, L. et al. Breaking the symmetry of dibenzoxazines: a paradigm to tailor the design of bio-based thermosets. Green Chem. 18, 3346–3353 (2016).
Teng, N. et al. Making benzoxazine greener and stronger: renewable resource, microwave irradiation, green solvent, and excellent thermal properties. ACS Sus. Chem. Eng. 7, 8715–8723 (2019).
Zhang, K., Liu, Y., Han, M. & Froimowicz, P. Smart and sustainable design of latent catalyst-containing benzoxazine-bio-resins and application studies. Green Chem. 22, 1209–1219 (2020).
Whiteley, J. M., Taynton, P., Zhang, W. & Lee, S.-H. Ultra-thin solid-state Li-ion electrolyte membrane facilitated by a self-healing polymer matrix. Adv. Mater. 27, 6922–6927 (2015).
Taynton, P. et al. Re-healable polyimine thermosets: polymer composition and moisture sensitivity. Polym. Chem. 7, 7052–7056 (2016).
Taynton, P. et al. Repairable woven carbon fiber composites with full recyclability enabled by malleable polyimine networks. Adv. Mater. 28, 2904–2909 (2016).
Dhers, S., Vantomme, G. & Avérous, L. A fully bio-based polyimine vitrimer derived from fructose. Green Chem. 21, 1596–1601 (2019).
Hajj, R., Duval, A., Dhers, S. & Avérous, L. Network design to control polyimine vitrimer properties: physical versus chemical approach. Macromolecules 53, 3796–3805 (2020).
Geng, H. et al. Vanillin-based polyschiff vitrimers: reprocessability and chemical recyclability. ACS Sus. Chem. Eng. 6, 15463–15470 (2018).
Wang, S. et al. Robust, fire-safe, monomer-recovery, highly malleable thermosets from renewable bioresources. Macromolecules 51, 8001–8012 (2018).
Kim, C., Chandrasekaran, A., Huan, T. D., Das, D. & Ramprasad, R. Polymer genome: a data-powered polymer informatics platform for property predictions. J. Phys. Chem. C. 122, 17575–17585 (2018).
Hackett, M., Zang, L., Viciu, L. & Masuda, T. Lactic Acid, Its Salts, and Esters (IHS Markit, 2018).
Montazeri, M., Zaimes, G. G., Khanna, V. & Eckelman, M. J. Meta-analysis of life cycle energy and greenhouse gas emissions for priority biobased chemicals. ACS Sus. Chem. Eng. 4, 6443–6454 (2016).
Acknowledgements
This work was authored in part by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the US Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding provided by the US DOE, Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office. The views expressed in the article do not necessarily represent the views of the DOE or the US Government. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for US Government purposes. E.Y.-X.C acknowledges support from the US National Science Foundation (NSF-1955482).
Author information
Authors and Affiliations
Contributions
R.M.C., N.A.R., C.B.H. and G.T.B. wrote the manuscript and designed the figures. G.T.B. and E.Y.-X.C. prepared the initial draft outline. All authors edited the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Materials thanks the other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Polymers: A Property Database: https://poly.chemnetbase.com/faces/polymers/PolymerSearch.xhtml
Supplementary information
Rights and permissions
About this article
Cite this article
Cywar, R.M., Rorrer, N.A., Hoyt, C.B. et al. Bio-based polymers with performance-advantaged properties. Nat Rev Mater 7, 83–103 (2022). https://doi.org/10.1038/s41578-021-00363-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00363-3
This article is cited by
-
The multifaceted challenges of bioplastics
Nature Reviews Bioengineering (2024)
-
Designing a circular carbon and plastics economy for a sustainable future
Nature (2024)
-
Polyamides go circular
Nature Sustainability (2024)
-
Optimizing bioplastics translation
Nature Reviews Bioengineering (2024)
-
Facile fabrication of Fe/Fe3C@starch-derived hierarchical porous carbon for microwave absorption
Journal of Materials Science: Materials in Electronics (2024)