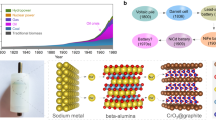
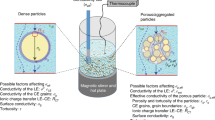
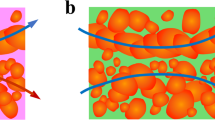
Abstract
Solid-state batteries (SSBs) have recently been revived to increase the energy density and eliminate safety concerns associated with conventional Li-ion batteries with flammable liquid electrolytes. To achieve large-scale, low-cost production of SSBs as soon as possible, it would be advantageous to modify the mature manufacturing platform, involving slurry casting and roll-to-roll technologies, used for conventional Li-ion batteries for application to SSBs. However, the manufacturing of SSBs depends on the development of suitable solid electrolytes. Inorganic–polymer composite electrolytes combine the advantages of inorganic and polymer solid electrolytes, making them particularly suitable for the mass production of SSBs. In this Review, we discuss the properties of solid electrolytes comprising inorganic–polymer composites and outline the design of composite electrolytes for realizing high-performance devices. We also assess the challenges of integrating the composite electrolytes into batteries, which will enable the mass production of SSBs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Goodenough, J. B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 7, 14–18 (2014).
Castelvecchi, D. & Stoye, E. World-changing batteries win Nobel. Nature 574, 308 (2019).
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Etacheri, V., Marom, R., Elazari, R., Salitra, G. & Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243–3262 (2011).
Goodenough, J. B. & Park, K.-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Liu, K., Liu, Y. Y., Lin, D. C., Pei, A. & Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 4, eaas9820 (2018).
Li, W. D., Erickson, E. M. & Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 5, 26–34 (2020).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Mauger, A., Julien, C. M., Paolella, A., Armand, M. & Zaghib, K. Building better batteries in the solid state: a review. Materials 12, 3892 (2019).
Randau, S. et al. Benchmarking the performance of all-solid-state lithium batteries. Nat. Eenrgy 5, 259–270 (2020).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densitie. Nat. Rev. Mater. 1, 16013 (2016).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Manthiram, A., Yu, X. W. & Wang, S. F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Kim, C.-R., Tajitsu, N. & Nussey, S. Toyota set to sell long-range, fast charging electric cars in 2022: paper. Reuters https://www.reuters.com/article/idUSKBN1AA035 (2017).
Lienert, P. QuantumScape’s solid-state battery could power electric planes - director. Reuters https://www.reuters.com/article/idUSKBN28I2Y3 (2020).
Schnella, J. et al. All-solid-state lithium-ion and lithium metal batteries–paving the way to large-scale production. J. Power Sources 382, 160–175 (2018).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Lin, D. C., Liu, Y. Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Cheng, X.-B., Zhang, R., Zhao, C.-Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Xiao, J. et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020).
Varzi, A., Raccichini, R., Passerini, S. & Scrosati, B. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries. J. Mater. Chem. 4, 17251–17259 (2016).
Dorfler, S. et al. Challenges and key parameters of lithium-sulfur batteries on pouch cell level. Joule 4, 539–554 (2020).
Yang, X., Luo, J. & Sun, X. Towards high-performance solid-state Li–S batteries: from fundamental understanding to engineering design. Chem. Soc. Rev. 49, 2140–2195 (2020).
Freunberger, S. A. et al. The lithium–oxygen battery with ether-based electrolytes. Angew. Chem. Int. Ed. 50, 8609–8613 (2011).
Hu, Y. S. Batteries: getting solid. Nat. Energy 1, 16042 (2016).
Günther, T. et al. The manufacturing of electrodes: key process for the future success of lithium-ion batteries. Adv. Mater. Res. 1140, 304–311 (2016).
Mindemark, J., Lacey, M. J., Bowden, T. & Brandell, D. Beyond PEO — alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 81, 114–143 (2018).
Lopez, J., Mackanic, D. G., Cui, Y. & Bao, Z. N. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 4, 312–330 (2019).
Zhao, Q., Stalin, S., Zhao, C.-Z. & Archer, L. A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5, 229–252 (2020).
Famprikis, T., Canepa, P., Dawson, J. A., Islam, M. S. & Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18, 1278–1291 (2019).
Ren, Y. et al. Oxide electrolytes for lithium batteries. J. Am. Ceram. Soc. 98, 3603–3623 (2015).
Wang, C. et al. Garnet-type solid-state electrolytes: materials, interfaces, and batteries. Chem. Rev. 120, 4257–4300 (2020).
Chen, R., Li, Q., Yu, X., Chen, L. & Li, H. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem. Rev. 120, 6820–6877 (2020).
Li, X. et al. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 13, 1429–1461 (2020).
Tan, D. H. S., Banerjee, A., Chen, Z. & Meng, Y. S. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 15, 170–180 (2020).
Zou, Z. et al. Mobile ions in composite solids. Chem. Rev. 120, 4169–4221 (2020).
Fenton, D. E., Parker, J. M. & Wright, P. V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 14, 589 (1973).
Xue, Z. G., He, D. & Xie, X. L. Poly(ethylene oxide)-based electrolytes for lithium ion batteries. J. Mater. Chem. A 3, 19218–19253 (2015).
Stephan, A. M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 42, 21–42 (2006).
Jacoby, M. Batteries get flexible. Chem. Eng. News 91, 13–18 (2013).
Xia, Y. Y., Fujieda, T., Tatsumi, K., Prosini, P. P. & Sakai, T. Thermal and electrochemical stability of cathode materials in solid polymer electrolyte. J. Power Sources 92, 234–243 (2001).
Li, Q. et al. Cycling performances and interfacial properties of a Li/PEO-Li(CF3SO2)2N-ceramic filler/LiNi0.8Co0.2O2 cell. J. Power Sources 97–98, 795–797 (2001).
Liang, J. et al. Stabilizing and understanding the interface between nickel-rich cathode and PEO-based electrolyte by lithium niobium oxide coating for high-performance all-solid-state batteries. Nano Energy 78, 105107 (2020).
Kimura, K., Yajima, M. & Tominaga, Y. A highly-concentrated poly(ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature. Electrochem. Commun. 66, 46–48 (2016).
Brissot, C., Rosso, M., Chazalviel, J.-N. & Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 81, 925–929 (1999).
Harry, K. J., Hallinan, D. T., Parkinson, D. Y., MacDowell, A. A. & Balsara, N. P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2014).
Yang, X. et al. High-areal-capacity all-solid-state lithium batteries enabled by rational design of fast ion transport channels in vertically-aligned composite polymer electrodes. Nano Energy 61, 567–575 (2019).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Zhu, Y. Z., He, X. F. & Mo, Y. F. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015).
Ohtomo, T. & Hayashi, A. Suppression of H2S gas generation from the 75Li2S·25P2S5 glass electrolyte by additives. J. Mater. Sci. 48, 4137–4142 (2013).
Wenzel, S. et al. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode. Chem. Mater. 28, 2400–2407 (2016).
Li, X. et al. Unravelling the chemistry and microstructure evolution of a cathodic interface in sulfide-based all-solid-state Li-ion batteries. ACS Energy Lett. 4, 2480–2488 (2019).
Haruyama, J., Sodeyama, K., Han, L., Takada, K. & Tateyama, Y. Space–charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 26, 4248–4255 (2014).
Luntz, A. C., Voss, J. & Reuter, K. Interfacial challenges in solid-state Li ion batteries. J. Phys. Chem. Lett. 6, 4599–4604 (2015).
Deng, S. et al. Dual-functional interfaces for highly stable Ni-rich layered cathodes in sulfide all-solid-state batteries. Energy Storage Mater. 27, 117–123 (2020).
Lee, Y.-G. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 5, 299–308 (2020).
Stramare, S., Thangadurai, V. & Weppner, W. Lithium lanthanum titanates: a review. Chem. Mater. 15, 3974–3990 (2003).
Jian, Z., Hu, Y. S., Ji, X. & Chen, W. NASICON-structured materials for energy storage. Adv. Mater. 29, 1601925 (2017).
Thangadurai, V., Sumaletha, N. & Dana, P. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem. Soc. Rev. 43, 4714–4727 (2014).
Zhao, N. et al. Solid garnet batteries. Joule 3, 1190–1199 (2019).
Bates, J. B., Dudney, N. J., Neudecker, B., Ueda, A. & Evans, C. D. Thin-film lithium and lithium-ion batteries. Solid State Ion. 135, 33–45 (2000).
Sakabe, Y. Multilayer ceramic capacitors. Curr. Opin. Solid State Mater. Sci. 2, 584–587 (1997).
Dirican, M., Yan, C., Zhu, P. & Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 136, 27–46 (2019).
Li, S. et al. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 7, 1903088 (2020).
Liang, J. et al. Recent progress on solid-state hybrid electrolytes for solid-state lithium batteries. Energy Storage Mater. 21, 308–334 (2019).
Liu, Y. et al. Composition modulation and structure design of inorganic-in-polymer composite solid electrolytes for advanced lithium batteries. Small 16, 1902813 (2019).
Cui, G. Reasonable design of high-energy-density solid-state lithium-metal batteries. Matter 2, 805–815 (2020).
Gadjourova, Z., Andreev, Y. G., Tunstall, D. P. & Bruce, P. G. Ionic conductivity in crystalline polymer electrolytes. Nature 412, 520–523 (2001).
Xue, S. et al. Diffusion of lithium ions in amorphous and crystalline poly(ethylene oxide)3:LiCF3SO3 polymer electrolytes. Electrochim. Acta 235, 122–128 (2017).
Zhou, Q., Ma, J., Dong, S., Li, X. & Cui, G. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv. Mater. 31, 1902029 (2019).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Lin, D. et al. A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 30, 1802661 (2018).
Lin, D. C. et al. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide). Nano Lett. 16, 459–465 (2016).
Chung, S. C. et al. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J. Power Sources 97–98, 644–648 (2001).
Liu, W., Lin, D. C., Sun, J., Zhou, G. M. & Cui, Y. Improved lithium ionic conductivity in composite polymer electrolytes with oxide-ion conducting nanowires. ACS Nano 10, 11407–11413 (2016).
Huo, H. Y. et al. Anion-immobilized polymer electrolyte achieved by cationic metal-organic framework filler for dendrite-free solid-state batteries. Energy Storage Mater. 18, 59–67 (2019).
Wang, Z. N. et al. Covalently linked metal–organic framework (MOF)-polymer all-solid-state electrolyte membranes for room temperature high performance lithium batteries. J. Mater. Chem. A 6, 17227–17234 (2018).
Chen, L., Li, W. X., Fan, L.-Z., Nan, C.-W. & Zhang, Q. Intercalated electrolyte with high transference number for dendrite-free solid-state lithium batteries. Adv. Funct. Mater. 29, 1901047 (2019).
Zhu, Q. Y., Wang, X. M. & Miller, J. D. Advanced nanoclay-based nanocomposite solid polymer electrolyte for lithium iron phosphate batteries. ACS Appl. Mater. Interfaces 11, 8954–8960 (2019).
Yao, P. C. et al. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Lett. 18, 6113–6120 (2018).
Wang, Z. X., Huang, X. J. & Chen, L. Q. Understanding of effects of nano-Al2O3 particles on ionic conductivity of composite polymer electrolytes. Electrochem. Solid State Lett. 6, E40–E44 (2003).
Nan, C. W., Fan, L. Z., Lin, Y. H. & Cai, Q. Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys. Rev. Lett. 91, 266104 (2003).
Zhang, X. K. et al. Vertically aligned and continuous nanoscale ceramic–polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity. Nano Lett. 18, 3829–3838 (2018).
Wang, W. M., Yi, E., Fici, A. J., Laine, R. M. & Kieffer, J. Lithium ion conducting poly(ethylene oxide)-based solid electrolytes containing active or passive ceramic nanoparticles. J. Phys. Chem. C 121, 2563–2573 (2017).
Chen, L. et al. PEO/garnet composite electrolytes for solid-state lithium batteries: from “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 46, 176–184 (2018).
Zhang, X. et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 139, 13779–13785 (2017).
Zhang, X. et al. Effects of Li6.75La3Zr1.75Ta0.25O12 on chemical and electrochemical properties of polyacrylonitrile-based solid electrolytes. Solid State Ion. 327, 32–38 (2018).
Zhang, J. X. et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 28, 447–454 (2016).
Zhang, Y. et al. Free-standing sulfide/polymer composite solid electrolyte membranes with high conductance for all-solid-state lithium batteries. Energy Storage Mater. 25, 145–153 (2020).
Pan, K. et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries. Adv. Mater. 32, 2000399 (2020).
Zheng, J., Tang, M. X. & Hu, Y.-Y. Lithium ion pathway within Li7La3Zr2O12-polyethylene oxide composite electrolytes. Angew. Chem. Int. Ed. 128, 12726–12730 (2016).
Zheng, J., Dang, H., Feng, X. Y., Chien, P.-H. & Hu, Y.-Y. Li-ion transport in a representative ceramic–polymer–plasticizer composite electrolyte: Li7La3Zr2O12–polyethylene oxide–tetraethylene glycol dimethyl ether. J. Mater. Chem. A 5, 18457–18463 (2017).
Yang, T., Zheng, J., Cheng, Q., Hu, Y. Y. & Chan, C. K. Composite polymer electrolytes with Li7La3Zr2O12 garnet-type nanowires as ceramic fillers: mechanism of conductivity enhancement and role of doping and morphology. ACS Appl. Mater. Interfaces 9, 21773–21780 (2017).
Zheng, J. & Hu, Y.-Y. New insights into the compositional dependence of Li-ion transport in polymer–ceramic composite electrolytes. ACS Appl. Mater. Interfaces 10, 4113–4120 (2018).
Bae, J. et al. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte. Angew. Chem. Int. Ed. 57, 2096–2100 (2018).
Li, Z., Xie, H. X., Zhang, X. Y. & Guo, X. In situ thermally polymerized solid composite electrolytes with a broad electrochemical window for all-solid-state lithium metal batteries. J. Mater. Chem. A 8, 3892–3900 (2020).
Li, Y., Zhang, W., Dou, Q. Q., Wong, K. W. & Ng, K. M. Li7La3Zr2O12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. J. Mater. Chem. A 7, 3391–3398 (2019).
Fan, R. et al. Versatile strategy for realizing flexible room-temperature all-solid-state battery through a synergistic combination of salt affluent PEO and Li6.75La3Zr1.75Ta0.25O12 nanofibers. ACS Appl. Mater. Interfaces 12, 7222–7231 (2020).
Zhu, P. et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. J. Mater. Chem. A 6, 4279–4285 (2018).
Li, B. et al. Li0.35La0.55TiO3 nanofibers enhanced poly(vinylidene fluoride)-based composite polymer electrolytes for all-solid-state batteries. ACS Appl. Mater. Interfaces 11, 42206–42213 (2019).
Liu, W. et al. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett. 15, 2740–2745 (2015).
Pignanelli, F., Romero, M., Faccio, R., Fernández-Werner, L. & Mombrú, A. W. Enhancement of lithium-ion transport in poly(acrylonitrile) with hydrogen titanate nanotube fillers as solid polymer electrolytes for lithium-ion battery applications. J. Phys. Chem. C 122, 1492–1499 (2018).
Cheng, L. et al. The origin of high electrolyte–electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys. Chem. Chem. Phys. 16, 18294–18300 (2014).
Kumar, S. K., Ganesan, V. & Riggleman, R. A. Perspective: Outstanding theoretical questions in polymer-nanoparticle hybrids. J. Chem. Phys. 147, 020901 (2017).
Nan, C. W. Physics of inhomogeneous inorganic materials. Prog. Mater. Sci. 37, 1–116 (1993).
Wang, X. Z. et al. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries. ACS Appl. Mater. Interfaces 10, 24791–24798 (2018).
Fu, K. K. et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc. Natl Acad. Sci. USA 113, 7094–7099 (2016).
Yang, H. et al. Chemical interaction and enhanced interfacial ion transport in a ceramic nanofiber–polymer composite electrolyte for all-solid-state lithium metal batteries. J. Mater. Chem. A 8, 7261–7272 (2020).
Xie, H. et al. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose. Adv. Energy Mater. 8, 1703474 (2018).
Gong, Y. H. et al. Lithium-ion conductive ceramic textile: a new architecture for flexible solid-state lithium metal batteries. Mater. Today 21, 594–601 (2018).
Li, Z., Sha, W. X. & Guo, X. Three-dimensional garnet framework-reinforced solid composite electrolytes with high lithium-ion conductivity and excellent stability. ACS Appl. Mater. Interfaces 11, 26920–26927 (2019).
Bae, J. et al. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Mater. 15, 46–52 (2018).
Wang, X. et al. Rechargeable solid-state lithium metal batteries with vertically aligned ceramic nanoparticle/polymer composite electrolyte. Nano Energy 60, 205–212 (2019).
Zhai, H. W. et al. A flexible solid composite electrolyte with vertically aligned and connected ion-conducting nanoparticles for lithium batteries. Nano Lett. 17, 3182–3187 (2017).
Zekoll, S. et al. Hybrid electrolytes with 3D bicontinuous ordered ceramic and polymer microchannels for all-solid-state batteries. Energy Environ. Sci. 11, 185–201 (2018).
Wang, S. et al. High-conductivity free-standing Li6PS5Cl/poly(vinylidene difluoride) composite solid electrolyte membranes for lithium-ion batteries. J. Materiomics 6, 70–76 (2020).
Lu, Y. et al. Stable cycling of lithium metal batteries using high transference number electrolytes. Adv. Energy Mater. 5, 1402073 (2015).
Keller, M., Barzi, A. & Passerini, S. Hybrid electrolytes for lithium metal batteries. J. Power Sources 392, 206–225 (2018).
Tominaga, Y. & Yamazaki, K. Fast Li-ion conduction in poly(ethylene carbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem. Commun. 50, 4448–4450 (2014).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396–A404 (2005).
Ni, J. E., Case, E. D., Sakamoto, J. S., Rangasamy, E. & Wolfenstine, J. B. Room temperature elastic moduli and Vickers hardness of hot-pressed LLZO cubic garnet. J. Mater. Sci. 47, 7978–7985 (2012).
Weston, J. E. & Steele, B. C. H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion. 7, 75–79 (1982).
Zhao, Y. et al. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries. Solid State Ion. 295, 65–71 (2016).
Li, D., Chen, L., Wang, T. S. & Fan, L. Z. 3D fiber-network-reinforced bicontinuous composite solid electrolyte for dendrite-free lithium metal batteries. ACS Appl. Mater. Interfaces 10, 7069–7078 (2018).
Zhou, B. et al. A flexible, self-healing and highly stretchable polymer electrolyte via quadruple hydrogen bonding for lithium-ion batteries. J. Mater. Chem. A 6, 11725–11733 (2018).
Munaoka, T. et al. Ionically conductive self-healing binder for low cost Si microparticles anodes in Li-ion batteries. Adv. Energy Mater. 8, 1703138 (2018).
Guo, Y. et al. A self-healable and easily recyclable supramolecular hydrogel electrolyte for flexible supercapacitors. J. Mater. Chem. A 4, 8769–8776 (2016).
Huo, H. Y. et al. Rational design of hierarchical “ceramic-in-polymer” and “polymer-in-ceramic” electrolytes for dendrite-free solid-state batteries. Adv. Energy Mater. 9, 1804004 (2019).
Wang, C. H. et al. Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 9, 13694–13702 (2017).
Ren, Y., Shen, Y., Lin, Y. & Nan, C. W. Direct observation of lithium dendrites inside garnet-type lithium-ion solid electrolyte. Electrochem. Commun. 57, 27–30 (2015).
Cheng, E. J., Sharafi, A. & Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 223, 85–91 (2017).
Fu, C. et al. Universal chemomechanical design rules for solid-ion conductors to prevent dendrite formation in lithium metal batteries. Nat. Mater. 19, 758–766 (2020).
Perea, A., Dontigny, M. & Zaghib, K. Safety of solid-state Li metal battery: solid polymer versus liquid electrolyte. J. Power Sources 359, 182–185 (2017).
Keller, M. et al. Electrochemical performance of a solvent-free hybrid ceramic polymer electrolyte based on Li7La3Zr2O12 in P(EO)15LiTFSI. J. Power Sources 353, 287–297 (2017).
Xu, X. Y. et al. Li7P3S11/poly(ethylene oxide) hybrid solid electrolytes with excellent interfacial compatibility for all-solid-state batteries. J. Power Sources 400, 212–217 (2018).
Chen, B. et al. A new composite solid electrolyte PEO/Li10GeP2S12/SN for all-solid-state lithium battery. Electrochim. Acta 210, 905–914 (2016).
Liang, J. Y. et al. Engineering Janus interfaces of ceramic electrolyte via distinct functional polymers for stable high-voltage Li-metal batteries. J. Am. Chem. Soc. 141, 9165–9169 (2019).
Liu, K., Zhang, R. H., Sun, J., Wu, M. & Zhao, T. S. Polyoxyethylene (PEO)|PEO-perovskite|PEO composite electrolyte for all-solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 11, 46930–46937 (2019).
Goodenough, J. B. & Kim, Y. Challenages for rechargable Li batteries. Chem. Mater. 22, 587–603 (2010).
Zhu, Y. Z., He, X. F. & Mo, Y. F. First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 4, 3253–3266 (2016).
Xu, C. et al. Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study. J. Mater. Chem. A 2, 7256–7264 (2014).
Jung, Y.-C., Lee, S.-M., Choi, J.-H., Jang, S. S. & Kim, D.-W. All solid-state lithium batteries assembled with hybrid solid electrolytes. J. Electrochem. Soc. 162, A704–A710 (2015).
Choi, J.-H., Lee, C.-H., Yu, J.-H., Doh, C.-H. & Lee, S.-M. Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J. Power Sources 274, 458–463 (2015).
Chen, S. J., Zhao, Y. R., Yang, J., Yao, L. L. & Xu, X. X. Hybrid solid electrolytes with excellent electrochemical properties and their applications in all-solid-state cells. Ionics 23, 2603–2611 (2017).
Zhao, Y. R. et al. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries. J. Power Sources 301, 47–53 (2016).
Yue, L. P. et al. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 5, 139–164 (2016).
Hu, P. et al. Progress in nitrile-based polymer electrolytes for high performance lithium batteries. J. Mater. Chem. A 4, 10070–10083 (2016).
Li, W., Song, B. & Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017).
Gauthier, M. et al. Electrode–electrolyte interface in Li-ion batteries: current understanding and new insights. J. Phys. Chem. Lett. 6, 4653–4672 (2015).
Seki, S. et al. Degradation mechanism analysis of all-solid-state lithium polymer secondary batteries by using the impedance measurement. J. Power Sources 146, 741–744 (2005).
Wetjen, M. et al. Thermal and electrochemical properties of PEO-LiTFSI-Pyr14TFSI-based composite cathodes, incorporating 4 V-class cathode active materials. J. Power Sources 246, 846–857 (2014).
Zhang, Q. et al. Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries. Adv. Mater. 31, 1901131 (2019).
Oh, D. Y. et al. Slurry-fabricable Li+-conductive polymeric binders for practical all-solid-state lithium-ion batteries enabled by solvate ionic liquids. Adv. Energy Mater. 9, 1802927 (2019).
Cao, D. et al. Stable thiophosphate-based all-solid-state lithium batteries through conformally interfacial nanocoating. Nano Lett. 20, 1483–1490 (2020).
Wang, L.-P. et al. Ameliorating the interfacial problems of cathode and solid-state electrolytes by interface modification of functional polymers. Adv. Energy Mater. 8, 1801528 (2018).
Xiao, Y. H. et al. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 5, 105–126 (2020).
Culver, S. P., Koerver, R., Zeier, W. G. & Janek, J. On the functionality of coatings for cathode active materials in thiophosphate-based all-solid-state batteries. Adv. Energy Mater. 9, 1900626 (2019).
Wu, M. Y. et al. Toward an ideal polymer binder design for high-capacity battery anodes. J. Am. Chem. Soc. 135, 12048–12056 (2013).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016).
Yang, C. P., Yin, Y. X., Zhang, S. F., Li, N. W. & Guo, Y. G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 6, 8058 (2015).
Zhang, C. et al. Vertically aligned lithiophilic CuO nanosheets on a Cu collector to stabilize lithium deposition for lithium metal batteries. Adv. Energy Mater. 8, 1703404 (2018).
Ye, H. et al. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J. Am. Chem. Soc. 139, 5916–5922 (2017).
Chi, S. S., Liu, Y. C., Song, W. L., Fan, L. Z. & Zhang, Q. Prestoring lithium into stable 3D nickel foam host as dendrite-free lithium metal anode. Adv. Funct. Mater. 27, 1700348 (2017).
Huang, S. B. et al. Chemical energy release driven lithiophilic layer on 1 m2 commercial brass mesh toward highly stable lithium metal batteries. Nano Lett. 19, 1832–1837 (2019).
Huang, S. B. et al. Early lithium plating behavior in confined nanospace of 3D lithiophilic carbon matrix for stable solid-state lithium metal batteries. Small 15, e1904216 (2019).
Liang, Z. et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl Acad. Sci. USA 113, 2862–2867 (2016).
Niu, C. et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601 (2019).
Zhang, R. et al. Coralloid carbon fiber-based composite lithium anode for robust lithium metal batteries. Joule 2, 764–777 (2018).
Liu, Y. et al. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 7, 10992 (2016).
Fan, L. et al. Stable lithium electrodeposition at ultra-high current densities enabled by 3D PMF/Li composite anode. Adv. Energy Mater. 8, 1703360 (2018).
Shi, X. et al. In situ forming LiF nano-decorated electrolyte/electrode interfaces for stable all-solid-state batteries. Mater. Today Nano 10, 100079 (2020).
Jiang, T. et al. Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solid-state lithium batteries. Adv. Energy Mater. 10, 1903376 (2020).
Yan, C. et al. Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 30, 1707629 (2018).
Yan, C. et al. An armored mixed conductor interphase on a dendrite-free lithium-metal anode. Adv. Mater. 30, 1804461 (2018).
Zhang, X. et al. Self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly(vinylidene difluoride)-based solid electrolytes. Adv. Mater. 31, 1806082 (2019).
Zhao, C. Z. et al. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl Acad. Sci. USA 114, 11069–11074 (2017).
Chi, S. S. et al. Solid polymer electrolyte soft interface layer with 3D lithium anode for all-solid-state lithium batteries. Energy Storage Mater. 17, 309–316 (2019).
Li, Y. T. et al. Hybrid polymer/garnet electrolyte with a small interfacial resistance for lithium-ion batteries. Angew. Chem. Int. Ed. 56, 753–756 (2017).
Zhou, W. D. et al. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 138, 9385–9388 (2016).
Zhou, W. D. et al. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv. Mater. 31, 1805574 (2018).
Ju, J. W. et al. Integrated interface strategy toward room temperature solid-state lithium batteries. ACS Appl. Mater. Interfaces 10, 13588–13597 (2018).
Chai, J. C. et al. In situ generation of poly(vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv. Sci. 4, 1600377 (2017).
Chai, J. C. et al. Dendrite-free lithium deposition via flexible-rigid coupling composite network for LiNi0.5Mn1.5O2/Li metal batteries. Small 14, 1802244 (2018).
Duan, H. et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries. Adv. Mater. 31, 1807789 (2019).
Duan, H. et al. Dendrite-free Li-metal battery enabled by a thin asymmetric solid electrolyte with engineered layers. J. Am. Chem. Soc. 140, 82–85 (2017).
Gai, J. L. et al. Flexible organic–inorganic composite solid electrolyte with asymmetric structure for room temperature solid-state Li-ion batteries. ACS Sustain. Chem. Eng. 7, 15896–15903 (2019).
Chen, X. Z., He, W. J., Ding, L. X., Wang, S. Q. & Wang, H. H. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 12, 938–944 (2019).
Whiteley, J. M., Taynton, P., Zhang, W. & Lee, S.-H. Ultra-thin solid-state Li-ion electrolyte membrane facilitated by a self-healing polymer matrix. Adv. Mater. 27, 6922–6927 (2015).
Villaluenga, I. et al. Compliant glass–polymer hybrid single ion-conducting electrolytes for lithium batteries. Proc. Natl Acad. Sci. USA 113, 52–57 (2016).
Fan, L., Wei, S. Y., Li, S. Y. & Lu, Y. Y. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater. 8, 1702657 (2018).
Acknowledgements
C.-W.N. and L.-Z.F. acknowledge support from the Basic Science Center Program of the National Natural Science Foundation of China (NSFC) under grant nos. 51788104 and 51532002. The authors are grateful to L. Chen, Y. Liang, X. Liu, G. Wang, F. Liu and J. Yi for help with the drawing of graphics.
Author information
Authors and Affiliations
Contributions
All authors contributed to the discussion of content, and writing and editing of the article prior to submission. C.-W.N. conceived the outline and L.-Z.F. researched the data.
Corresponding author
Ethics declarations
Competing interests
H.H. is employed at Qingtao Energy Development Inc., which develops and commercializes solid-state batteries. C.-W.N. is one of the co-founders of Qingtao. L.-Z.F. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, LZ., He, H. & Nan, CW. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat Rev Mater 6, 1003–1019 (2021). https://doi.org/10.1038/s41578-021-00320-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00320-0
This article is cited by
-
Sulfide-based composite solid electrolyte films for all-solid-state batteries
Communications Materials (2024)
-
External-pressure–electrochemistry coupling in solid-state lithium metal batteries
Nature Reviews Materials (2024)
-
Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries
Nature Communications (2024)
-
Precisely succinonitrile-functionalized PEO electrolytes toward room-temperature all-solid-state lithium batteries
Science China Materials (2024)
-
Solid-state lithium-ion batteries for grid energy storage: opportunities and challenges
Science China Chemistry (2024)