Abstract
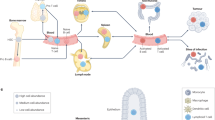
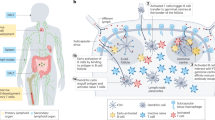
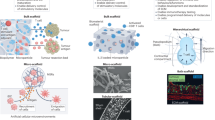
Immunoengineering applies quantitative and materials-based approaches for the investigation of the immune system and for the development of therapeutic solutions for various diseases, such as infection, cancer, inflammatory diseases and age-related malfunctions. The design of immunomodulatory and cell therapies requires the precise understanding of immune cell formation and activation in primary, secondary and ectopic tertiary immune organs. However, the study of the immune system has long been limited to in vivo approaches, which often do not allow multidimensional control of intracellular and extracellular processes, and to 2D in vitro models, which lack physiological relevance. 3D models built with synthetic and natural materials enable the structural and functional recreation of immune tissues. These models are being explored for the investigation of immune function and dysfunction at the cell, tissue and organ levels. In this Review, we discuss 2D and 3D approaches for the engineering of primary, secondary and tertiary immune structures at multiple scales. We highlight important insights gained using these models and examine multiscale engineering strategies for the design and development of immunotherapies. Finally, dynamic 4D materials are investigated for their potential to provide stimuli-dependent and context-dependent scaffolds for the generation of immune organ models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Joshi, N. S. et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015).
Li, W. et al. Bronchus-associated lymphoid tissue-resident Foxp3+ T lymphocytes prevent antibody-mediated lung rejection. J. Clin. Invest. 129, 556–568 (2019).
Garcia-Hernandez, M. L. et al. A unique cellular and molecular microenvironment is present in tertiary lymphoid organs of patients with spontaneous prostate cancer regression. Front. Immunol. 8, 563 (2017).
Eddens, T. et al. Pneumocystis-driven inducible bronchus-associated lymphoid tissue formation requires Th2 and Th17 immunity. Cell Rep. 18, 3078–3090 (2017).
Neelapu, S. S. et al. Chimeric antigen receptor T cell therapy — assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018). In this Review, the authors discuss cellular immunotherapy and provide recommendations for monitoring, grading and managing acute toxicities that can occur in patients.
Weiner, G. J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 15, 361–370 (2015).
Chan, A. C. & Carter, P. J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 10, 301–316 (2010).
Burak, M. F. et al. Development of a therapeutic monoclonal antibody that targets secreted fatty acid-binding protein aP2 to treat type 2 diabetes. Sci. Transl Med. 7, 319ra205 (2015).
Harrison, C. Autoimmune disease: targeting IL-7 reverses type 1 diabetes. Nat. Rev. Drug Discov. 11, 599 (2012).
Penaranda, C. et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc. Natl Acad. Sci. USA 109, 12668–12673 (2012).
Li, W. et al. A neutralizing anti-Nogo66 receptor monoclonal antibody reverses inhibition of neurite outgrowth by central nervous system myelin. J. Biol. Chem. 279, 43780–43788 (2004).
Mothe, A. J. et al. RGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injury. Sci. Rep. 7, 10529 (2017).
Tran, H. T. et al. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell Rep. 7, 2054–2065 (2014).
Stephenson, R. & Singh, A. Drug discovery and therapeutic delivery for the treatment of B and T cell tumors. Adv. Drug Deliv. Rev. 114, 285–300 (2017).
Tokatlian, T. et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363, 649–654 (2019).
Wilson, D. S. et al. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat. Mater. 18, 175–185 (2019).
Mosquera, M. J. et al. Immunomodulatory nanogels overcome restricted immunity in a murine model of gut microbiome-mediated metabolic syndrome. Sci. Adv. 5, eaav9788 (2019).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Gordon, J. & Manley, N. R. Mechanisms of thymus organogenesis and morphogenesis. Development 138, 3865–3878 (2011).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014). This paper describes the current understanding and controversies regarding the cellular composition and localization of haematopoietic stem cell niches within the bone marrow.
Nagasawa, T. Microenvironmental niches in the bone marrow required for B cell development. Nat. Rev. Immunol. 6, 107–116 (2006).
Wilson, A. & Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 6, 93–106 (2006).
Chabannon, C. et al. Hematopoietic stem cell transplantation in its 60s: a platform for cellular therapies. Sci. Transl Med. 10, eaap9630 (2018).
Cornelissen, J. J. et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat. Rev. Clin. Oncol. 9, 579–590 (2012).
Atkins, H. L. et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 388, 576–585 (2016).
Kawai, T. et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am. J. Transplant. 14, 1599–1611 (2014).
Ogonek, J. et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 7, 507 (2016).
Bello, A. B., Park, H. & Lee, S. H. Current approaches in biomaterial-based hematopoietic stem cell niches. Acta Biomater. 72, 1–15 (2018).
Chinn, I. K., Blackburn, C. C., Manley, N. R. & Sempowski, G. D. Changes in primary lymphoid organs with aging. Semin. Immunol. 24, 309–320 (2012).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 (2016).
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Braccini, A. et al. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells 23, 1066–1072 (2005).
Ferreira, M. S. et al. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 33, 6987–6997 (2012).
Leisten, I. et al. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 33, 1736–1747 (2012).
Miyoshi, H., Murao, M., Ohshima, N. & Tun, T. Three-dimensional culture of mouse bone marrow cells within a porous polymer scaffold: effects of oxygen concentration and stromal layer on expansion of haematopoietic progenitor cells. J. Tissue Eng. Regen. Med. 5, 112–118 (2011).
Mortera-Blanco, T., Mantalaris, A., Bismarck, A., Aqel, N. & Panoskaltsis, N. Long-term cytokine-free expansion of cord blood mononuclear cells in three-dimensional scaffolds. Biomaterials 32, 9263–9270 (2011).
Nichols, J. E. et al. In vitro analog of human bone marrow from 3D scaffolds with biomimetic inverted colloidal crystal geometry. Biomaterials 30, 1071–1079 (2009).
Raic, A., Rodling, L., Kalbacher, H. & Lee-Thedieck, C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials 35, 929–940 (2014).
Feng, Q., Chai, C., Jiang, X. S., Leong, K. W. & Mao, H. Q. Expansion of engrafting human hematopoietic stem/progenitor cells in three-dimensional scaffolds with surface-immobilized fibronectin. J. Biomed. Mater. Res. A 78, 781–791 (2006).
Nilsson, S. K. et al. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J. Histochem. Cytochem. 46, 371–377 (1998).
Choi, J. S. & Harley, B. A. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 3, e1600455 (2017).
Kotov, N. A. et al. Inverted colloidal crystals as three-dimensional cell scaffolds. Langmuir 20, 7887–7892 (2004).
Huang, X. et al. Co-cultured hBMSCs and HUVECs on human bio-derived bone scaffolds provide support for the long-term ex vivo culture of HSC/HPCs. J. Biomed. Mater. Res. A 104, 1221–1230 (2016).
Ronaldson-Bouchard, K. & Vunjak-Novakovic, G. Organs-on-a-Chip: a fast track for engineered human tissues in drug development. Cell Stem Cell 22, 310–324 (2018).
Mahadik, B. P., Wheeler, T. D., Skertich, L. J., Kenis, P. J. & Harley, B. A. Microfluidic generation of gradient hydrogels to modulate hematopoietic stem cell culture environment. Adv. Healthc. Mater. 3, 449–458 (2014).
Torisawa, Y. S. et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 11, 663–669 (2014). In this study, the authors report an engineered bone-marrow-on-chip that recapitulates organ-level toxicity responses and protective effects of radiation countermeasure drugs.
Rodling, L. et al. 3D models of the hematopoietic stem cell niche under steady-state and active conditions. Sci. Rep. 7, 4625 (2017).
Bourgine, P. E. et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc. Natl Acad. Sci. USA 115, E5688–E5695 (2018).
Di Buduo, C. A. et al. Modular flow chamber for engineering bone marrow architecture and function. Biomaterials 146, 60–71 (2017).
Di Buduo, C. A. et al. Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood 125, 2254–2264 (2015).
Shepherd, J. H. et al. Structurally graduated collagen scaffolds applied to the ex vivo generation of platelets from human pluripotent stem cell-derived megakaryocytes: enhancing production and purity. Biomaterials 182, 135–144 (2018).
Tozzi, L. et al. Multi-channel silk sponge mimicking bone marrow vascular niche for platelet production. Biomaterials 178, 122–133 (2018).
Braham, M. V. J. et al. Endosteal and perivascular subniches in a 3D bone marrow model for multiple myeloma. Tissue Eng. Part C Methods 24, 300–312 (2018).
Braham, M. V. J. et al. Cellular immunotherapy on primary multiple myeloma expanded in a 3D bone marrow niche model. Oncoimmunology 7, e1434465 (2018).
Reagan, M. R. et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood 124, 3250–3259 (2014).
Shih, Y. R. et al. In vivo engineering of bone tissues with hematopoietic functions and mixed chimerism. Proc. Natl Acad. Sci. USA 114, 5419–5424 (2017).
Shah, N. J. et al. An injectable bone marrow-like scaffold enhances T cell immunity after hematopoietic stem cell transplantation. Nat. Biotechnol. https://doi.org/10.1038/s41587-019-0017-2 (2019).
Fan, Y. et al. Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Mol. Ther. 23, 1262–1277 (2015).
Tajima, A., Pradhan, I., Trucco, M. & Fan, Y. Restoration of thymus function with bioengineered thymus organoids. Curr. Stem Cell Rep. 2, 128–139 (2016).
Palmer, D. B. The effect of age on thymic function. Front. Immunol. 4, 316 (2013).
Neelapu, S. S. et al. Kte-C19 (anti-CD19 CAR T Cells) induces complete remissions in patients with refractory diffuse large B-cell lymphoma (DLBCL): results from the pivotal phase 2 zuma-1. Blood 128, LBA-6 (2016).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Fischbach, M. A., Bluestone, J. A. & Lim, W. A. Cell-based therapeutics: the next pillar of medicine. Sci. Transl Med. 5, 179ps177 (2013).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Hare, K. J., Jenkinson, E. J. & Anderson, G. In vitro models of T cell development. Semin. Immunol. 11, 3–12 (1999).
Dallas, M. H., Varnum-Finney, B., Delaney, C., Kato, K. & Bernstein, I. D. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J. Exp. Med. 201, 1361–1366 (2005).
Dallas, M. H., Varnum-Finney, B., Martin, P. J. & Bernstein, I. D. Enhanced T cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood 109, 3579–3587 (2007).
Varnum-Finney, B. et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 23, 4313–4318 (2000).
Schmitt, T. M. et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat. Immunol. 5, 410–417 (2004).
Schmitt, T. M. & Zuniga-Pflucker, J. C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17, 749–756 (2002).
Taqvi, S., Dixit, L. & Roy, K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. J. Biomed. Mater. Res. A 79, 689–697 (2006).
Shukla, S. et al. Progenitor T cell differentiation from hematopoietic stem cells using Delta-like-4 and VCAM-1. Nature Methods 14, 531–538 (2017).
Mohtashami, M. & Zuniga-Pflucker, J. C. Three-dimensional architecture of the thymus is required to maintain delta-like expression necessary for inducing T cell development. J. Immunol. 176, 730–734 (2006).
Seet, C. S. et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods 14, 521–530 (2017). In this paper, the authors report on artificial thymic organoids for directing human HSCs along the T cell lineage.
Nitta, T., Ohigashi, I., Nakagawa, Y. & Takahama, Y. Cytokine crosstalk for thymic medulla formation. Curr. Opin. Immunol. 23, 190–197 (2011).
van Ewijk, W. et al. Thymic microenvironments, 3D versus 2D? Semin. Immunol. 11, 57–64 (1999).
Hun, M. et al. Native thymic extracellular matrix improves in vivo thymic organoid T cell output, and drives in vitro thymic epithelial cell differentiation. Biomaterials 118, 1–15 (2017).
Hussey, G. S., Dziki, J. L. & Badylak, S. F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 3, 159–173 (2018).
Shah, S. B. & Singh, A. Cellular self-assembly and biomaterials-based organoid models of development and diseases. Acta Biomater. 53, 29–45 (2017).
Tajima, A. et al. Bioengineering mini functional thymic units with EAK16-II/EAKIIH6 self-assembling hydrogel. Clin. Immunol. 160, 82–89 (2015).
Junt, T., Scandella, E. & Ludewig, B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat. Rev. Immunol. 8, 764–775 (2008).
Zappasodi, R. et al. The effect of artificial antigen-presenting cells with preclustered anti-CD28/-CD3/-LFA-1 monoclonal antibodies on the induction of ex vivo expansion of functional human antitumor T cells. Haematologica 93, 1523–1534 (2008).
Li, Y. & Kurlander, R. J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: differing impact on CD8 T cell phenotype and responsiveness to restimulation. J. Transl Med. 8, 104 (2010).
Dustin, M. L. The immunological synapse. Cancer Immunol. Res. 2, 1023–1033 (2014).
Huppa, J. B. & Davis, M. M. T cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 (2003).
Mossman, K. D., Campi, G., Groves, J. T. & Dustin, M. L. Altered TCR signaling from geometrically repatterned immunological synapses. Science 310, 1191–1193 (2005).
Thery, M. et al. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947–953 (2005).
Coyer, S. R. et al. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J. Cell Sci. 125, 5110–5123 (2012).
Coyer, S. R., Delamarche, E. & Garcia, A. J. Protein tethering into multiscale geometries by covalent subtractive printing. Adv. Mater. 23, 1550–1553 (2011).
Singh, A. et al. Adhesion strength-based, label-free isolation of human pluripotent stem cells. Nat. Methods 10, 438–444 (2013).
Dumbauld, D. W. et al. How vinculin regulates force transmission. Proc. Natl Acad. Sci. USA 110, 9788–9793 (2013).
Doh, J. & Irvine, D. J. Immunological synapse arrays: patterned protein surfaces that modulate immunological synapse structure formation in T cells. Proc. Natl Acad. Sci. USA 103, 5700–5705 (2006). In this paper, the authors report on lithographically defined patterns of T cell receptor ligands surrounded by a field of tethered ICAM1 to mimic T cell–APC interactions.
Shen, K., Thomas, V. K., Dustin, M. L. & Kam, L. C. Micropatterning of costimulatory ligands enhances CD4+ T cell function. Proc. Natl Acad. Sci. USA 105, 7791–7796 (2008).
Tan, J. L. et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl Acad. Sci. USA 100, 1484–1489 (2003).
Fu, J. et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 7, 733–736 (2010).
Basu, R. et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell 165, 100–110 (2016).
Steenblock, E. R. & Fahmy, T. M. A comprehensive platform for ex vivo T cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol. Ther. 16, 765–772 (2008).
Prakken, B. et al. Artificial antigen-presenting cells as a tool to exploit the immune ‘synapse’. Nat. Med. 6, 1406 (2000).
Zitvogel, L. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600 (1998).
Fadel, T. R. et al. Enhanced cellular activation with single walled carbon nanotube bundles presenting antibody stimuli. Nano Lett. 8, 2070–2076 (2008).
Fadel, T. R. et al. A carbon nanotube-polymer composite for T cell therapy. Nat. Nanotechnol. 9, 639–647 (2014).
Cheung, A. S., Zhang, D. K. Y., Koshy, S. T. & Mooney, D. J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 36, 160–169 (2018).
Oelke, M. et al. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig–coated artificial antigen-presenting cells. Nat. Med. 9, 619 (2003).
Cui, H. F., Vashist, S. K., Al-Rubeaan, K., Luong, J. H. & Sheu, F. S. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem. Res. Toxicol. 23, 1131–1147 (2010).
Sato, Y. et al. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol. Biosyst. 1, 176–182 (2005).
Perica, K. et al. Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano 9, 6861–6871 (2015).
Smith, M. R., Tolbert, S. V. & Wen, F. Protein-scaffold directed nanoscale assembly of T cell ligands: artificial antigen presentation with defined valency, density, and ratio. ACS Synth. Biol. 7, 1629–1639 (2018).
Koffeman, E., Keogh, E., Klein, M., Prakken, B. & Albani, S. Identification and manipulation of antigen specific T cells with artificial antigen presenting cells. Methods Mol. Med. 136, 69–86 (2007).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Lamers, C. H. et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912 (2013).
Smith, T. T. et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Invest. 127, 2176–2191 (2017).
Stephan, S. B. et al. Biopolymer implants enhance the efficacy of adoptive T cell therapy. Nat. Biotechnol. 33, 97–101 (2015).
Allen, C. D., Okada, T. & Cyster, J. G. Germinal-center organization and cellular dynamics. Immunity 27, 190–202 (2007).
De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015).
McHeyzer-Williams, M., Okitsu, S., Wang, N. & McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 12, 24–34 (2012). In this Review, the authors discuss each phase of antigen-specific B cell development and affinity maturation of memory B cells.
Grilo, A. L. & Mantalaris, A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 37, 9–16 (2019).
Yokoyama, W. M. et al. Production of monoclonal antibodies. Curr. Protoc. Immunol. 102, 2.5.1–2.5.29 (2013).
Georgiou, G. et al. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat. Biotechnol. 32, 158–168 (2014).
Rathmell, J. C. et al. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature 376, 181–184 (1995).
Wu, Y. et al. Immune complex-bearing follicular dendritic cells deliver a late antigenic signal that promotes somatic hypermutation. J. Immunol. 180, 281–290 (2008).
Pound, J. D. & Gordon, J. Maintenance of human germinal center B cells in vitro. Blood 89, 919–928 (1997).
Arpin, C. et al. Generation of memory B cells and plasma cells in vitro. Science 268, 720–722 (1995).
Nojima, T. et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat. Commun. 2, 465 (2011).
Beguelin, W. et al. EZH2 enables germinal centre formation through epigenetic silencing of CDKN1A and an Rb-E2F1 feedback loop. Nat. Commun. 8, 877 (2017). In this study, the authors develop immune organoids to reveal the epigenetic mechanism behind B cell hyperproliferation in response to infection.
Roh, K. H. et al. A synthetic stroma-free germinal center niche for efficient generation of humoral immunity ex vivo. Biomaterials 164, 106–120 (2018).
Purwada, A. & Singh, A. Immuno-engineered organoids for regulating the kinetics of B cell development and antibody production. Nat. Protoc. 12, 168–182 (2017).
Purwada, A. et al. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials 63, 24–34 (2015).
Apoorva, F. N. U. et al. Lymph node stiffness-mimicking hydrogels regulate human B cell lymphoma growth and cell surface receptor expression in a molecular subtype-specific manner. J. Biomed. Mater. Res. A 105, 1833–1844 (2017).
Cayrol, F. et al. Integrin αvβ3 acting as membrane receptor for thyroid hormones mediates angiogenesis in malignant T cells. Blood 125, 841–851 (2015).
Tian, Y. F. et al. Integrin-specific hydrogels as adaptable tumor organoids for malignant B and T cells. Biomaterials 73, 110–119 (2015).
Purwada, A. et al. Ex vivo synthetic immune tissues with T cell signals for differentiating antigen-specific, high affinity germinal center B cells. Biomaterials 198, 27–36 (2019). In this paper, the authors demonstrate that ex vivo 3D immune organoids can form antigen-specific B cells in a dish.
Purwada, A., Shah, S. B., Beguelin, W., Melnick, A. M. & Singh, A. Modular immune organoids with integrin ligand specificity differentially regulate ex vivo B cell activation. ACS Biomater. Sci. Eng. 3, 214–225 (2017).
Apoorva, F. et al. How biophysical forces regulate human B cell lymphomas. Cell Rep. 23, 499–511 (2018).
Hoch, S., Boyd, M., Malone, B., Gonye, G. & Schwaber, J. Fas-mediated apoptosis eliminates B cells that acquire self-reactivity during the germinal center response to NP. Cell. Immunol. 203, 103–110 (2000).
Meyer-Hermann, M. et al. A theory of germinal center B cell selection, division, and exit. Cell Rep. 2, 162–174 (2012).
Jones, G. W., Hill, D. G. & Jones, S. A. Understanding immune cells in tertiary lymphoid organ development: it is all starting to come together. Front. Immunol. 7, 401 (2016).
Mueller, C. G., Nayar, S., Gardner, D. & Barone, F. Cellular and vascular components of tertiary lymphoid structures. Methods Mol. Biol. 1845, 17–30 (2018).
Nerviani, A. & Pitzalis, C. Role of chemokines in ectopic lymphoid structures formation in autoimmunity and cancer. J. Leukoc. Biol. 104, 333–341 (2018).
Rangel-Moreno, J. et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat. Immunol. 12, 639–646 (2011).
Dorraji, S. E. et al. Mesenchymal stem cells and T cells in the formation of tertiary lymphoid structures in lupus nephritis. Sci. Rep. 8, 7861 (2018).
Figenschau, S. L. et al. ICAM1 expression is induced by proinflammatory cytokines and associated with TLS formation in aggressive breast cancer subtypes. Sci. Rep. 8, 11720 (2018).
Vu Van, D. et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat. Commun. 7, 10875 (2016).
Cheng, J. et al. Ectopic B cell clusters that infiltrate transplanted human kidneys are clonal. Proc. Natl Acad. Sci. USA 108, 5560–5565 (2011).
Mittal, S. et al. Lymphoid aggregates that resemble tertiary lymphoid organs define a specific pathological subset in metal-on-metal hip replacements. PLOS ONE 8, e63470 (2013).
Akhavanpoor, M. et al. Adventitial tertiary lymphoid organ classification in human atherosclerosis. Cardiovasc. Pathol. 32, 8–14 (2018).
Neyt, K., Perros, F., GeurtsvanKessel, C. H., Hammad, H. & Lambrecht, B. N. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 33, 297–305 (2012).
Yin, C., Mohanta, S., Maffia, P. & Habenicht, A. J. Editorial: tertiary lymphoid organs (TLOs): powerhouses of disease immunity. Front. Immunol. 8, 228 (2017).
Ventura Ferreira, M. S. et al. An engineered multicomponent bone marrow niche for the recapitulation of hematopoiesis at ectopic transplantation sites. J. Hematol. Oncol. 9, 4 (2016).
Kobayashi, Y. & Watanabe, T. Gel-trapped lymphorganogenic chemokines trigger artificial tertiary lymphoid organs and mount adaptive immune responses in vivo. Front. Immunol. 7, 316 (2016).
Ali, O. A., Huebsch, N., Cao, L., Dranoff, G. & Mooney, D. J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 8, 151–158 (2009).
Singh, A. et al. An injectable synthetic immune-priming center mediates efficient T cell class switching and T-helper 1 response against B cell lymphoma. J. Control. Release 155, 184–192 (2011).
Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Matsuda, N., Shimizu, T., Yamato, M. & Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 19, 3089–3099 (2007).
DeForest, C. A. & Anseth, K. S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 3, 925–931 (2011).
DeForest, C. A., Polizzotti, B. D. & Anseth, K. S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 8, 659–664 (2009). In this study, the authors demonstrate direct fabrication of biologically functionalized gels with photopatterned structures in the presence of cells — a first step towards 4D cultures.
Kloxin, A. M. et al. Responsive culture platform to examine the influence of microenvironmental geometry on cell function in 3D. Integ. Biol. 4, 1540–1549 (2012).
Hasani-Sadrabadi, M. M. et al. Mechanobiological mimicry of helper T lymphocytes to evaluate cell–biomaterials crosstalk. Adv. Mater. 30, 1706780 (2018).
Shifrut, E. et al. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell 175, 1958–1971 (2018).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Dudda, J. C. et al. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity 38, 742–753 (2013).
Shin, S. R. et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 6, 362–372 (2012).
Dvir, T. et al. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 6, 720–725 (2011).
Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 69, 522–530 (2001).
Carswell, K. S. & Papoutsakis, E. T. Extracellular pH affects the proliferation of cultured human T cells and their expression of the interleukin-2 receptor. J. Immunother. 23, 669–674 (2000).
Patel, A. et al. Highly elastomeric poly(glycerol sebacate)-co-poly(ethylene glycol) amphiphilic block copolymers. Biomaterials 34, 3970–3983 (2013).
Roybal, K. T. & Lim, W. A. Synthetic immunology: hacking immune cells to expand their therapeutic capabilities. Annu. Rev. Immunol. 35, 229–253 (2017).
Wu, C. Y., Rupp, L. J., Roybal, K. T. & Lim, W. A. Synthetic biology approaches to engineer T cells. Curr. Opin. Immunol. 35, 123–130 (2015).
Xie, M. & Fussenegger, M. Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat. Rev. Mol. Cell Biol. 19, 507–525 (2018).
La Motte-Mohs, R. N., Herer, E. & Zuniga-Pflucker, J. C. Induction of T cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood 105, 1431–1439 (2005).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221 (1999). This work demonstrates that immunological synapse formation is a multistage process, in which T cell activation is reconstituted by interacting with MHC–peptide and the adhesion ligand ICAM1.
Doh, J. & Irvine, D. J. Photogenerated polyelectrolyte bilayers from an aqueous-processible photoresist for multicomponent protein patterning. J. Am. Chem. Soc. 126, 9170–9171 (2004).
Bashour, K. T. et al. CD28 and CD3 have complementary roles in T cell traction forces. Proc. Natl Acad. Sci. USA 111, 2241–2246 (2014).
Moon, J. J. et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl Acad. Sci. USA 109, 1080–1085 (2012).
Acknowledgements
The authors acknowledge financial support from the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (1R01AI132738-01A1 awarded to A.S.), the Innovative Molecular Analysis Technology programme of the US National Cancer Institute (NIH R33-CA212968-01 awarded to A.S.), a US National Science Foundation CAREER award (DMR-1554275 awarded to A.S.), a US Department of Defense Congressionally Directed Medical Research Program (CDMRP) cancer career development award (W81XWH-17-1-0215 awarded to A.S.) and the 3 M Non-Tenured Faculty Award (awarded to A.S.).
Author information
Authors and Affiliations
Contributions
S.K., S.B.S., P.G. and A.S. wrote the article. A.S. edited and reviewed the article. All authors contributed to the discussion of the content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S., Shah, S.B., Graney, P.L. et al. Multiscale engineering of immune cells and lymphoid organs. Nat Rev Mater 4, 355–378 (2019). https://doi.org/10.1038/s41578-019-0100-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-019-0100-9
This article is cited by
-
The therapeutic potential of immunoengineering for systemic autoimmunity
Nature Reviews Rheumatology (2024)
-
Bioengineering translational models of lymphoid tissues
Nature Reviews Bioengineering (2023)
-
A cytotoxic T cell inspired oncolytic nanosystem promotes lytic cell death by lipid peroxidation and elicits antitumor immune responses
Nature Communications (2023)
-
Important Considerations in the Diagnosis and Management of Post-transplant Lymphoproliferative Disorder
Current Oncology Reports (2023)
-
Combinatorial treatment rescues tumour-microenvironment-mediated attenuation of MALT1 inhibitors in B-cell lymphomas
Nature Materials (2023)