Abstract
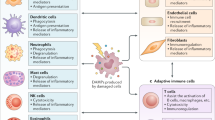
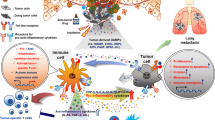
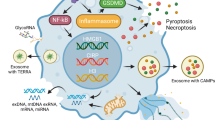
Damage-associated molecular patterns (DAMPs) are endogenous molecules that are released from host cells as a result of cell death or damage. The release of DAMPs in tissues is associated with loss of tissue homeostasis. Sensing of DAMPs by innate immune receptors triggers inflammation, which can be beneficial in initiating the processes that restore tissue homeostasis but can also drive inflammatory diseases. In recent years, the sensing of intracellular DAMPs has received extensive attention in the field of sterile inflammation. However, emerging studies have shown that DAMPs that originate from neighbouring cells, and even from distal tissues or organs, also mediate sterile inflammatory responses. This multi-level sensing of DAMPs is crucial for intercellular, trans-tissue and trans-organ communication. Here, we summarize how DAMP-sensing receptors detect DAMPs from intracellular, intercellular or distal tissue and organ sources to mediate sterile inflammation. We also discuss the possibility of targeting DAMPs or their corresponding receptors to treat inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Janeway, C. A. Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Land, W. Allograft injury mediated by reactive oxygen species: from conserved proteins of Drosophila to acute and chronic rejection of human transplants. Part III: interaction of (oxidative) stress-induced heat shock proteins with toll-like receptor-bearing cells of innate immunity and its consequences for the development of acute and chronic allograft rejection. Transplant. Rev. 17, 67–86 (2003). This paper represents the first description of the concept of damage-associated molecular patterns (DAMPs).
Chen, G. Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112 (2020).
Roh, J. S. & Sohn, D. H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 18, e27 (2018).
Kaur, J., Singh, H. & Naqvi, S. Intracellular DAMPs in neurodegeneration and their role in clinical therapeutics. Mol. Neurobiol. 60, 3600–3616 (2023).
Wang, X. & Labzin, L. I. Inflammatory cell death: how macrophages sense neighbouring cell infection and damage. Biochem. Soc. Trans. 51, 303–313 (2023).
Shim, Y.-R. & Jeong, W.-I. Recent advances of sterile inflammation and inter-organ cross-talk in alcoholic liver disease. Exp. Mol. Med. 52, 772–780 (2020).
Bai, J. & Liu, F. Nuclear cGAS: sequestration and beyond. Protein Cell 13, 90–101 (2021).
Briard, B., Place, D. E. & Kanneganti, T.-D. DNA sensing in the innate immune response. Physiology 35, 112–124 (2020).
Chen, M., Linstra, R. & van Vugt, M. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer 1877, 188661 (2022).
Volkman, H. E., Cambier, S., Gray, E. E. & Stetson, D. B. Tight nuclear tethering of cGAS is essential for preventing autoreactivity. eLife 8, e47491 (2019).
Gentili, M. et al. The N-terminal domain of cGAS determines preferential association with centromeric DNA and innate immune activation in the nucleus. Cell Rep. 26, 2377–2393.e2313 (2019).
Michalski, S. et al. Structural basis for sequestration and autoinhibition of cGAS by chromatin. Nature 587, 678–682 (2020).
Pathare, G. R. et al. Structural mechanism of cGAS inhibition by the nucleosome. Nature 587, 668–672 (2020).
Zhao, B. et al. The molecular basis of tight nuclear tethering and inactivation of cGAS. Nature 587, 673–677 (2020).
Boyer, J. A. et al. Structural basis of nucleosome-dependent cGAS inhibition. Science 370, 450–454 (2020).
Kujirai, T. et al. Structural basis for the inhibition of cGAS by nucleosomes. Science 370, 455–458 (2020). This paper, together with Michalski et al. (2020), Pathare et al. (2020), Zhao et al. (2020) and Boyer et al. (2020), provides the key mechanism of how nucleosomes inhibit nuclear cGAS activation, explaining why nuclear cGAS does not recognize genomic self DNA.
Chen, H. et al. cGAS suppresses genomic instability as a decelerator of replication forks. Sci. Adv. 6, eabb8941 (2020).
Liu, H. et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563, 131–136 (2018).
Zhen, Z. et al. Nuclear cGAS restricts L1 retrotransposition by promoting TRIM41-mediated ORF2p ubiquitination and degradation. Nat. Commun. 14, 8217 (2023).
Wang, L., Wen, M. & Cao, X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science 365, eaav0758 (2019). This paper identifies hnRNPA2B1 as a nuclear receptor that recognizes DNA viruses and fulfils a crucial role in the antiviral innate immune response.
Zhang, X., Flavell, R. A. & Li, H.-B. hnRNPA2B1: a nuclear DNA sensor in antiviral Immunity. Cell Res. 29, 879–880 (2019).
Liu, Y. et al. Structural insight into hnRNP A2/B1 homodimerization and DNA recognition. J. Mol. Biol. 435, 167920 (2023).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Coquel, F. et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature 557, 57–61 (2018).
Di Micco, A. et al. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc. Natl Acad. Sci. USA 113, E4671–E4680 (2016).
Krupina, K., Goginashvili, A. & Cleveland, D. W. Causes and consequences of micronuclei. Curr. Opin. Cell Biol. 70, 91–99 (2021).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Li, T. & Chen, Z. J. The cGAS–cGAMP–STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 215, 1287–1299 (2018).
Martin, S. K., Tomida, J. & Wood, R. D. Disruption of DNA polymerase zeta engages an innate immune response. Cell Rep. 34, 108775 (2021).
Bartsch, K. et al. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum. Mol. Genet. 26, 3960–3972 (2017).
Gratia, M. et al. Bloom syndrome protein restrains innate immune sensing of micronuclei by cGAS. J. Exp. Med. 216, 1199–1213 (2019).
Orr, B. et al. An anaphase surveillance mechanism prevents micronuclei formation from frequent chromosome segregation errors. Cell Rep. 37, 109783 (2021).
Mohr, L. et al. ER-directed TREX1 limits cGAS activation at micronuclei. Mol. Cell 81, 724–738.e729 (2021).
Li, T. et al. Phosphorylation and chromatin tethering prevent cGAS activation during mitosis. Science 371, eabc5386 (2021). This paper demonstrates that cGAS is hyperphosphorylated and tethered to chromatin during mitosis to prevent its activation and autoimmune reaction.
Zhong, L. et al. Phosphorylation of cGAS by CDK1 impairs self-DNA sensing in mitosis. Cell Discov. 6, 26 (2020).
Guey, B. et al. BAF restricts cGAS on nuclear DNA to prevent innate immune activation. Science 369, 823–828 (2020).
Rice, G. I. et al. Mutations in ADAR1 cause Aicardi–Goutieres syndrome associated with a type I interferon signature. Nat. Genet. 44, 1243–1248 (2012).
Inoue, M. et al. An Aicardi–Goutieres syndrome-causative point mutation in adar1 gene invokes multiorgan inflammation and late-onset encephalopathy in mice. J. Immunol. 207, 3016–3027 (2021).
Guo, X. et al. Aicardi–Goutieres syndrome-associated mutation at ADAR1 gene locus activates innate immune response in mouse brain. J. Neuroinflammation 18, 169 (2021).
de Reuver, R. et al. ADAR1 interaction with Z-RNA promotes editing of endogenous double-stranded RNA and prevents MDA5-dependent immune activation. Cell Rep. 36, 109500 (2021).
Liddicoat, B. J. et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 349, 1115–1120 (2015).
Tang, Q. et al. Adenosine-to-inosine editing of endogenous Z-form RNA by the deaminase ADAR1 prevents spontaneous MAVS-dependent type I interferon responses. Immunity 54, 1961–1975.e1965 (2021).
Maurano, M. et al. Protein kinase R and the integrated stress response drive immunopathology caused by mutations in the RNA deaminase ADAR1. Immunity 54, 1948–1960.e1945 (2021).
Pestal, K. et al. Isoforms of RNA-editing enzyme ADAR1 independently control nucleic acid sensor MDA5-driven autoimmunity and multi-organ development. Immunity 43, 933–944 (2015).
Mannion, N. M. et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 9, 1482–1494 (2014).
Bajad, P. et al. An internal deletion of ADAR rescued by MAVS deficiency leads to a minute phenotype. Nucleic Acids Res. 48, 3286–3303 (2020).
de Reuver, R. et al. ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature 607, 784–789 (2022).
Hubbard, N. W. et al. ADAR1 mutation causes ZBP1-dependent immunopathology. Nature 607, 769–775 (2022).
Jiao, H. et al. ADAR1 averts fatal type I interferon induction by ZBP1. Nature 607, 776–783 (2022). This paper, together with de Reuver et al. (2022) and Hubbard et al. (2022), demonstrates that endogenous Alu duplex RNA accumulated as a result of Adar1 mutations can be recognized by ZBP1 and contributes to pathological features of Aicardi–Goutieres syndrome.
Wang, R. et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 580, 386–390 (2020).
Devos, M. et al. Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J. Exp. Med. 217, e20191913 (2020).
Li, J. et al. cGAS inhibition alleviates Alu RNA-induced immune responses and cytotoxicity in retinal pigmented epithelium. Cell Biosci. 12, 116 (2022).
Slavik, K. M. et al. cGAS-like receptors sense RNA and control 3′2′-cGAMP signalling in Drosophila. Nature 597, 109–113 (2021).
Wang, S. B. et al. DDX17 is an essential mediator of sterile NLRC4 inflammasome activation by retrotransposon RNAs. Sci. Immunol. 6, eabi4493 (2021).
Song, Y., Zhou, Y. & Zhou, X. The role of mitophagy in innate immune responses triggered by mitochondrial stress. Cell Commun. Signal. 18, 186 (2020).
Riley, J. S. & Tait, S. W. Mitochondrial DNA in inflammation and Immunity. EMBO Rep. 21, e49799 (2020).
Zhong, W. et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging Cell 21, e13622 (2022).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047 (2018). This paper demonstrates that mtDNA leaked into the cytoplasm through BAK/BAX macropores is able to be recognized by cGAS to initiate the innate immune response.
Yu, C.-H. et al. TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell 183, 636–649.e618 (2020).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
Miao, R. et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 56, 2523–2541.e2528 (2023).
West, A. P. & Shadel, G. S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 17, 363–375 (2017).
Zhang, Z. et al. Mitochondrial DNA-LL-37 complex promotes atherosclerosis by escaping from autophagic recognition. Immunity 43, 1137–1147 (2015).
Bao, D. et al. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene 38, 5007–5020 (2019).
Li, J. et al. Electronic cigarettes induce mitochondrial DNA damage and trigger TLR9 (Toll-like receptor 9)-mediated atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 41, 839–853 (2021).
Irazoki, A. et al. Disruption of mitochondrial dynamics triggers muscle inflammation through interorganellar contacts and mitochondrial DNA mislocation. Nat. Commun. 14, 108 (2023).
Bae, J. H. et al. Circulating cell-free mtDNA contributes to AIM2 inflammasome-mediated chronic inflammation in patients with type 2 diabetes. Cells 8, 328 (2019).
Xu, L. et al. Mitochondrial DNA enables AIM2 inflammasome activation and hepatocyte pyroptosis in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G1034–G1044 (2021).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018).
Maekawa, H. et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 29, 1261–1273.e1266 (2019).
Huang, L. S. et al. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity 52, 475–486.e475 (2020).
Chen, D. et al. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc. Natl Acad. Sci. USA 115, 3930–3935 (2018).
Szczesny, B. et al. Mitochondrial DNA damage and subsequent activation of Z-DNA binding protein 1 links oxidative stress to inflammation in epithelial cells. Sci. Rep. 8, 914 (2018).
Wang, W. Y. et al. Z-DNA/RNA binding protein 1 senses mitochondrial dna to induce receptor-interacting protein kinase-3/mixed lineage kinase domain-like-driven necroptosis in developmental sevoflurane neurotoxicity. NeuroScience 507, 99–111 (2022).
Saada, J. et al. Oxidative stress induces Z-DNA-binding protein 1-dependent activation of microglia via mtDNA released from retinal pigment epithelial cells. J. Biol. Chem. 298, 101523 (2022).
Bordon, Y. Double (mtRNA) trouble. Nat. Rev. Immunol. 18, 543 (2018).
Matilainen, S. et al. Defective mitochondrial RNA processing due to PNPT1 variants causes Leigh syndrome. Hum. Mol. Genet. 26, 3352–3361 (2017).
Szewczyk, M. et al. Human REXO2 controls short mitochondrial RNAs generated by mtRNA processing and decay machinery to prevent accumulation of double-stranded RNA. Nucleic Acids Res. 48, 5572–5590 (2020).
Kim, Y. et al. PKR senses nuclear and mitochondrial signals by interacting with endogenous double-stranded RNAs. Mol. Cell 71, 1051–1063.e1056 (2018).
Dhir, A. et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 560, 238–242 (2018).
Tigano, M., Vargas, D. C., Tremblay-Belzile, S., Fu, Y. & Sfeir, A. Nuclear sensing of breaks in mitochondrial DNA enhances immune surveillance. Nature 591, 477–481 (2021).
Doke, T. et al. NAD+ precursor supplementation prevents mtRNA/RIG-I-dependent inflammation during kidney injury. Nat. Metab. 5, 414–430 (2023).
Bauernfried, S., Scherr, M. J., Pichlmair, A., Duderstadt, K. E. & Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 371, eabd0811 (2021). This paper reveals that human NLRP1 acts as a direct sensor of dsRNA.
Shen, C. et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell 184, 5759–5774.e5720 (2021).
Nassour, J. et al. Telomere-to-mitochondria signalling by ZBP1 mediates replicative crisis. Nature 614, 767–773 (2023).
Davidson, S. et al. Protein kinase R is an innate immune sensor of proteotoxic stress via accumulation of cytoplasmic IL-24. Sci. Immunol. 7, eabi6763 (2022).
Wan, L. et al. Translation stress and collided ribosomes are co-activators of cGAS. Mol. Cell 81, 2808–2822.e2810 (2021).
Read, A. & Schroder, M. The unfolded protein response: an overview. Biology 10, 384 (2021).
Mohamed, E. et al. The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immunity 52, 668–682.e667 (2020).
Stengel, S. T. et al. Activating transcription factor 6 mediates inflammatory signals in intestinal epithelial cells upon endoplasmic reticulum stress. Gastroenterology 159, 1357–1374.e1310 (2020).
Billingham, L. K. et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 23, 692–704 (2022).
Braga, T. T. et al. Sensing soluble uric acid by Naip1–Nlrp3 platform. Cell Death Dis. 12, 158 (2021).
Ismail, R. et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J. Neuroinflammation 17, 151 (2020).
Rhoads, J. P. et al. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcγR cooperation and is dependent on CARD9. J. Immunol. 198, 2105–2114 (2017).
Zhivaki, D. & Kagan, J. C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat. Rev. Immunol. 22, 322–330 (2022).
Zanoni, I. et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236 (2016). This paper reports that microbial products and self-encoded oxidized phospholipids (oxPAPC) can bind to caspase 11 to induce IL-1β release, but do not cause pyroptosis.
Di Gioia, M. et al. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat. Immunol. 21, 42–53 (2020).
Que, X. et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306 (2018).
Hooftman, A. et al. Macrophage fumarate hydratase restrains mtRNA-mediated interferon production. Nature 615, 490–498 (2023).
Zecchini, V. et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature 615, 499–506 (2023).
Venereau, E., Ceriotti, C. & Bianchi, M. E. DAMPs from cell death to new life. Front. Immunol. 6, 422 (2015).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Jang, G. Y. et al. Interactions between tumor-derived proteins and Toll-like receptors. Exp. Mol. Med. 52, 1926–1935 (2020).
Park, H. D. et al. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene 30, 201–211 (2011).
Kim, Y. S. et al. A novel function of API5 (apoptosis inhibitor 5), TLR4-dependent activation of antigen presenting cells. Oncoimmunology 7, e1472187 (2018).
Park, H. J. et al. A novel TLR4 binding protein, 40S ribosomal protein S3, has potential utility as an adjuvant in a dendritic cell-based vaccine. J. Immunother. Cancer 7, 60 (2019).
Hanc, P. et al. Structure of the complex of F-actin and DNGR-1, a C-type lectin receptor involved in dendritic cell cross-presentation of dead cell-associated antigens. Immunity 42, 839–849 (2015).
Canton, J. et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat. Immunol. 22, 140–153 (2021).
Kraakman, M. J. et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J. Clin. Invest. 127, 2133–2147 (2017).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Lai, J. J., Cruz, F. M. & Rock, K. L. Immune sensing of cell death through recognition of histone sequences by C-type lectin-receptor-2d causes inflammation and tissue injury. Immunity 52, 123–135.e126 (2020).
Wang, X. et al. GPR34-mediated sensing of lysophosphatidylserine released by apoptotic neutrophils activates type 3 innate lymphoid cells to mediate tissue repair. Immunity 54, 1123–1136.e1128 (2021). This paper shows that GPR34 expressed on ILC3s can sense LysoPS released by apoptotic neutrophils to promote tissue repair.
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Cullen, D., Wofford, K. & Loane, D. Acute drivers of neuroinflammation in traumatic brain injury. Neural Regen. Res. 14, 1481 (2019).
Burnstock, G. P2X ion channel receptors and inflammation. Purinergic Signal. 12, 59–67 (2016).
Roth, T. L. et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228 (2014).
Shimizu, T. et al. Direct activation of microglia by beta-glucosylceramide causes phagocytosis of neurons that exacerbates Gaucher disease. Immunity 56, 307–319.e308 (2023).
Lv, L. L. et al. SAP130 released by damaged tubule drives necroinflammation via miRNA-219c/Mincle signaling in acute kidney injury. Cell Death Dis. 12, 866 (2021).
de Rivero Vaccari, J. C. et al. Mincle signaling in the innate immune response after traumatic brain injury. J. Neurotrauma 32, 228–236 (2015).
Fischer, S. et al. Self-extracellular RNA promotes pro-inflammatory response of astrocytes to exogenous and endogenous danger signals. J. Neuroinflammation 18, 252 (2021).
Wheeler, D. S. et al. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir. Res. 10, 31 (2009).
Zhang, W. et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J. Am. Heart Assoc. 4, e001993 (2015).
Yang, Y. et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 7, e2234 (2016).
Wong, P., Laxton, V., Srivastava, S., Chan, Y. W. & Tse, G. The role of gap junctions in inflammatory and neoplastic disorders (Review). Int. J. Mol. Med. 39, 498–506 (2017).
Kasper, C. A. et al. Cell-cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity 33, 804–816 (2010).
Patel, S. J., King, K. R., Casali, M. & Yarmush, M. L. DNA-triggered innate immune responses are propagated by gap junction communication. Proc. Natl Acad. Sci. USA 106, 12867–12872 (2009).
Ablasser, A. et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503, 530–534 (2013).
Luther, J. et al. Hepatic gap junctions amplify alcohol liver injury by propagating cGAS-mediated IRF3 activation. Proc. Natl Acad. Sci. USA 117, 11667–11673 (2020).
Murao, A., Aziz, M., Wang, H., Brenner, M. & Wang, P. Release mechanisms of major DAMPs. Apoptosis 26, 152–162 (2021).
Nabet, B. Y. et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 170, 352–366.e313 (2017).
Lian, Q. et al. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 27, 784–800 (2017).
Malkin, E. Z. & Bratman, S. V. Bioactive DNA from extracellular vesicles and particles. Cell Death Dis. 11, 584 (2020).
Diamond, J. M. et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol. Res. 6, 910–920 (2018).
Kitai, Y. et al. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J. Immunol. 198, 1649–1659 (2017).
Torralba, D. et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 9, 2658 (2018).
Antiochos, B. et al. The DNA sensors AIM2 and IFI16 are SLE autoantigens that bind neutrophil extracellular traps. eLife 11, e72103 (2022).
Apel, F. et al. The cytosolic DNA sensor cGAS recognizes neutrophil extracellular traps. Sci. Signal. 14, eaax7942 (2021).
McKee, C. A. & Lukens, J. R. Emerging roles for the immune system in traumatic brain injury. Front. Immunol. 7, 556 (2016).
Roth, S. et al. Brain-released alarmins and stress response synergize in accelerating atherosclerosis progression after stroke. Sci. Transl. Med. 10, eaao1313 (2018).
Liu, Q. et al. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat. Immunol. 20, 1023–1034 (2019).
Tammaro, A. et al. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol. Ther. 177, 81–95 (2017).
Kerr, N. A. et al. Human lung cell pyroptosis following traumatic brain injury. Cells 8, 69 (2019).
Kerr, N. A. et al. Traumatic brain injury-induced acute lung injury: evidence for activation and inhibition of a neural-respiratory-inflammasome axis. J. Neurotrauma 35, 2067–2076 (2018).
Harvey, S. B. et al. O-glycoside biomarker of apolipoprotein C3: responsiveness to obesity, bariatric surgery, and therapy with metformin, to chronic or severe liver disease and to mortality in severe sepsis and graft vs host disease. J. Proteome Res. 8, 603–612 (2009).
Zewinger, S. et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 21, 30–41 (2020).
Gong, T. & Zhou, R. ApoC3: an ‘alarmin’ triggering sterile inflammation. Nat. Immunol. 21, 9–11 (2020).
Orecchioni, M. et al. Olfactory receptor 2 in vascular macrophages drives atherosclerosis by NLRP3-dependent IL-1 production. Science 375, 214–221 (2022).
Del Fresno, C. et al. DNGR-1 in dendritic cells limits tissue damage by dampening neutrophil recruitment. Science 362, 351–356 (2018).
Dutra, F. F. et al. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl Acad. Sci. USA 111, E4110–E4118 (2014).
Chen, G. et al. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 123, 3818–3827 (2014).
Sundaram, B. et al. NLRP12-PANoptosome activates PANoptosis and pathology in response to heme and PAMPs. Cell 186, 2783–2801.e2720 (2023).
Xu, P. et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 10, 555 (2019).
Huang, Y. et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10, e8689 (2018).
Giampazolias, E. et al. Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer Immunity. Cell 184, 4016–4031.e4022 (2021).
Vinogradova, E. V. et al. An activity-guided map of electrophile-cysteine interactions in primary human T cells. Cell 182, 1009–1026.e1029 (2020).
National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05130892?term=NCT05130892 (2023).
National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/search?term=NCT02180698 (2019).
Shichita, T. et al. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat. Med. 23, 723–732 (2017).
Maehara, N. et al. AIM/CD5L attenuates DAMPs in the injured brain and thereby ameliorates ischemic stroke. Cell Rep. 36, 109693 (2021).
Yamada, Y. et al. The release of high mobility group box 1 in apoptosis is triggered by nucleosomal DNA fragmentation. Arch. Biochem. Biophys. 506, 188–193 (2011).
Chen, R., Kang, R., Fan, X. G. & Tang, D. Release and activity of histone in diseases. Cell Death Dis. 5, e1370 (2014).
Chekeni, F. B. et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010).
Reich, C. F. & Pisetsky, D. S. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp. Cell Res. 315, 760–768 (2009).
Kaczmarek, A., Vandenabeele, P. & Krysko, D. V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–223 (2013).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Mazlo, A. et al. Types of necroinflammation, the effect of cell death modalities on sterile inflammation. Cell Death Dis. 13, 423 (2022).
Deng, T., Tang, C., Zhang, G. & Wan, X. DAMPs released by pyroptotic cells as major contributors and therapeutic targets for CAR-T-related toxicities. Cell Death Dis. 12, 129 (2021).
Wei, X. et al. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 19, 971–992 (2022).
Liu, J., Kang, R. & Tang, D. ESCRT-III-mediated membrane repair in cell death and tumor resistance. Cancer Gene Ther. 28, 1–4 (2020).
Wiernicki, B. et al. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor Immunity. Nat. Commun. 13, 3676 (2022).
Shi, L., Liu, Y., Li, M. & Luo, Z. Emerging roles of ferroptosis in the tumor immune landscape: from danger signals to anti‐tumor Immunity. FEBS J. 289, 3655–3665 (2021).
Kobayashi, H., Yoshimoto, C., Matsubara, S., Shigetomi, H. & Imanaka, S. A comprehensive overview of recent developments on the mechanisms and pathways of ferroptosis in cancer: the potential implications for therapeutic strategies in ovarian cancer. Cancer Drug Resist. 6, 547–566 (2023).
Tsvetkov, P. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375, 1254–1261 (2022). This paper is the first to identify that cuproptosis is a form of programmed cell death driven by copper ions, which is mainly characterized by copper accumulation and proteotoxic stress.
Chen, L., Min, J. & Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 7, 378 (2022).
Chen, Y. et al. A broad cuproptosis landscape in inflammatory bowel disease. Front. Immunol. 13, 1031539 (2022).
Huang, Q.-X. et al. Metal-organic framework nanoagent induces cuproptosis for effective immunotherapy of malignant glioblastoma. Nano Today 51, 101911 (2023).
Liu, J., Liu, Y., Wang, Y., Kang, R. & Tang, D. HMGB1 is a mediator of cuproptosis-related sterile inflammation. Front. Cell Dev. Biol. 10, 996307 (2022).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021). This paper identifies NINJ1 as a mediator of plasma membrane rupture, allowing DAMPs to be released to propagate the inflammatory response.
Kayagaki, N. et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature 618, 1072–1077 (2023).
Degen, M. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 618, 1065–1071 (2023).
Borges, J. P. et al. Glycine inhibits NINJ1 membrane clustering to suppress plasma membrane rupture in cell death. eLife 11, e78609 (2022).
Acknowledgements
R.Z. was supported by grants from the National Key Research and Development Program of China (2019YFA0508500), the National Natural Science Foundation of China (81821001, 82130107, 82330052) and the CAS Project for Young Scientists in Basic Research (YSBR-074). W.J. was supported by the National Key Research and Development Program of China (2020YFA0509101), the National Natural Science Foundation of China (U20A20359). Y.H. was supported by the Research Start-up Fund (2024KYQD004) of the Institute of Health and Medicine, Hefei Comprehensive National Science Center, the National Natural Science Foundation of China (82202038) and the Natural Science Foundation of Jiangsu Province (BK20221085).
Author information
Authors and Affiliations
Contributions
All authors contributed to writing and editing the review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- 2′-3′-cyclic GMP–AMP
-
(2′-3′-cGAMP). An adaptor protein that is a key component of the innate cellular immune response to pathogenic cytoplasmic DNA.
- Acute kidney injury
-
A common clinical syndrome characterized by rapid decline in renal function and accumulation of metabolic waste.
- Aicardi–Goutieres syndrome
-
A rare inherited disease that results in severe intellectual and physical disability, usually caused by mutations in several genes associated with the innate immune response.
- Alu elements
-
A scattered set of related sequences in the human genome, each about 300 bp long. This sequence is so-named because of the presence of Alu restriction enzyme cleavage sites at each end of a single member. A large number of different types of Alu element exist in the primate genome.
- Amyotrophic lateral sclerosis
-
A rare progressive neurological disease. Patients lose the ability to initiate and control voluntary movements as their motor neurons degenerate and die.
- Atrophic macular degeneration
-
An eye disease that results in loss of central vision. It is caused primarily by macular damage to the retina.
- Danger theory
-
The danger theory was proposed by Polly Matzinger in 1994. It states that danger signals from the body’s own cells can elicit an immune response and that the immune system is more interested in the detection of, and protection from, danger than in the distinction between self and non-self.
- Exosomes
-
Small vesicles with a diameter of about 30–150 nm secreted by living cells, which typically have a lipid bilayer structure. They can carry various proteins, lipids, RNA and other important information and have an important role in the transfer of material and information between cells.
- Interferon-stimulated genes
-
(ISGs). A set of genes induced by interferons that fulfil a crucial role in host resistance to viral infections.
- Micronuclei
-
Small nuclear structures that contain DNA. They are spatially separated from the main nucleus and usually form as a result of chromosome mis-segregation and mutagenesis.
- Multiple sclerosis
-
An autoimmune disease in which the immune system mistakenly attacks healthy cells that produce myelin, causing damage to nerve fibres in the central nervous system and disrupting the transmission of nerve signals.
- Neutrophil extracellular traps
-
(NETs). Reticular fibrous structures formed by the release of components from the nucleus of a neutrophil to the outside of the cell.
- Nonalcoholic fatty liver disease
-
(NAFLD). A syndrome characterized by diffuse hepatocyte macrovesicular steatosis, which is not caused by ethanol and other definite liver injury factors.
- PANoptosome
-
A cytoplasmic multimeric protein complex that drives PANoptosis, a distinct form of programmed inflammatory cell death that is implicated in various human diseases, including autoinflammatory diseases, metabolic diseases, neurodegenerative diseases and cancer.
- Proteasome-associated autoinflammatory disease
-
(PRAAS). An autoinflammatory disease caused by genetic mutations that result in defective proteasome activity.
- Systemic lupus erythematosus
-
An autoimmune disease in which the immune system attacks its own tissues and causes widespread inflammation and tissue damage.
- Unfolded protein response
-
(UPR). Cells can sense the accumulation of misfolded or unfolded proteins in the endoplasmic reticulum and alleviate endoplasmic reticulum stress through three main signalling pathways. The signalling pathways that mediate this regulation are called the UPR.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Jiang, W. & Zhou, R. DAMP sensing and sterile inflammation: intracellular, intercellular and inter-organ pathways. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01027-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01027-3