Abstract
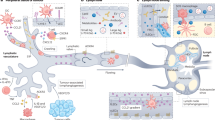
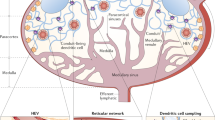
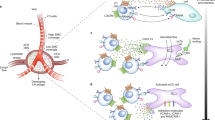
Lymph nodes are secondary lymphoid organs in which immune responses of the adaptive immune system are initiated and regulated. Distributed throughout the body and embedded in the lymphatic system, local lymph nodes are continuously informed about the state of the organs owing to a constant drainage of lymph. The tissue-derived lymph carries products of cell metabolism, proteins, carbohydrates, lipids, pathogens and circulating immune cells. Notably, there is a growing body of evidence that individual lymph nodes differ from each other in their capacity to generate immune responses. Here, we review the structure and function of the lymphatic system and then focus on the factors that lead to functional heterogeneity among different lymph nodes. We will discuss how lymph node heterogeneity impacts on cellular and humoral immune responses and the implications for vaccination, tumour development and tumour control by immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Randolph, G. J., Ivanov, S., Zinselmeyer, B. H. & Scallan, J. P. The lymphatic system: integral roles in immunity. Annu. Rev. Immunol. 35, 31–52 (2017).
Levick, J. R. & Michel, C. C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210 (2010).
Santambrogio, L. The lymphatic fluid. Int. Rev. Cell Mol. Biol. 337, 111–133 (2018).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Da Mesquita, S., Fu, Z. & Kipnis, J. The meningeal lymphatic system: a new player in neurophysiology. Neuron 100, 375–388 (2018).
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Welner, R. S. & Kincade, P. W. Stem cells on patrol. Cell 131, 842–844 (2007).
Collado-Diaz, V., Medina-Sanchez, J. D., Gkountidi, A. O. & Halin, C. Imaging leukocyte migration through afferent lymphatics. Immunol. Rev. 306, 43–57 (2022).
von Andrian, U. H. & Mackay, C. R. T-cell function and migration — two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Girard, J. P., Moussion, C. & Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012).
Gray, H. Gray’s Anatomy: the Anatomical Basis of Clinical Practice (Elsevier Health Sciences, 2015).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Qi, H., Kastenmüller, W. & Germain, R. N. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu. Rev. Cell Dev. Biol. 30, 141–167 (2014).
Acton, S. E., Onder, L., Novkovic, M., Martinez, V. G. & Ludewig, B. Communication, construction, and fluid control: lymphoid organ fibroblastic reticular cell and conduit networks. Trends Immunol. 42, 782–794 (2021).
Iannacone, M. et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 465, 1079–1083 (2010).
Zhang, Y. et al. Migratory and adhesive cues controlling innate-like lymphocyte surveillance of the pathogen-exposed surface of the lymph node. eLife 5, e18156 (2016).
Muntjewerff, E. M., Meesters, L. D. & van den Bogaart, G. Antigen cross-presentation by macrophages. Front. Immunol. 11, 1276 (2020).
Bajénoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Jalkanen, S. & Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 20, 566–578 (2020).
Itano, A. A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009).
Clement, C. C. et al. Protein expression profiles of human lymph and plasma mapped by 2D-DIGE and 1D SDS-PAGE coupled with nanoLC-ESI-MS/MS bottom-up proteomics. J. Proteom. 78, 172–187 (2013).
Palframan, R. T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Huang, J. Y., Lyons-Cohen, M. R. & Gerner, M. Y. Information flow in the spatiotemporal organization of immune responses. Immunol. Rev. 306, 93–107 (2022).
Gasteiger, G., Ataide, M. & Kastenmüller, W. Lymph node — an organ for T-cell activation and pathogen defense. Immunol. Rev. 271, 200–220 (2016).
Duckworth, B. C. & Groom, J. R. Conversations that count: cellular interactions that drive T cell fate. Immunol. Rev. 300, 203–219 (2021).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J. Exp. Med. 196, 65–75 (2002).
Faveeuw, C., Di Mauro, M. E., Price, A. A. & Ager, A. Roles of α4 integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int. Immunol. 12, 241–251 (2000).
Bajénoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003).
Druzd, D. et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46, 120–132 (2017).
Braun, A. et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 12, 879–887 (2011).
Ugur, M. et al. Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks. Immunity 56, 1778–1793.e10 (2023).
Fletcher, A. L. et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front. Immunol. 2, 35 (2011).
Pezoldt, J. et al. Neonatally imprinted stromal cell subsets induce tolerogenic dendritic cells in mesenteric lymph nodes. Nat. Commun. 9, 3903 (2018).
Hammerschmidt, S. I. et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205, 2483–2490 (2008). This study shows that lymph node stromal cells instruct tissue-homing signals in T cells in a lymph node-specific manner.
Cording, S. et al. The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 7, 359–368 (2014). This study shows that stromal cells from lymph nodes draining distinct sites differ in their capacity to induce regulatory T cells.
Wolvers, D. A. et al. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J. Immunol. 162, 1994–1998 (1999).
Podgrabinska, S. et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol. 183, 1767–1779 (2009).
Ulvmar, M. H. & Mäkinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc. Res. 111, 310–321 (2016).
Miyasaka, M. A short review on lymphatic endothelial cell heterogeneity. Inflamm. Regen. 41, 21–23 (2021).
Pegu, A. et al. Human lymphatic endothelial cells express multiple functional TLRs. J. Immunol. 180, 3399–3405 (2008).
Steinman, R. M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 (2012).
Cabeza-Cabrerizo, M. et al. Dendritic cells revisited. Annu. Rev. Immunol. 39, 131–166 (2021).
Joffre, O., Nolte, M. A., Spörri, R. & Sousa, C. R. E. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227, 234–247 (2009).
Ardouin, L. et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity 45, 305–318 (2016).
Worbs, T., Hammerschmidt, S. I. & Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 17, 30–48 (2017).
Allan, R. S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Steinman, R. M. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice: I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142–1162 (1973).
Spalding, D. M. & Griffin, J. A. Different pathways of differentiation of pre-B cell lines are induced by dendritic cells and T cells from different lymphoid tissues. Cell 44, 507–515 (1986).
Mora, J. R. et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006). This study identifies retinoic acid as part of a molecular mechanism of imprinting of gut-homing IgA-secreting cells.
Everson, M. P. et al. Dendritic cells from different tissues induce production of different T cell cytokine profiles. J. Leukoc. Biol. 59, 494–498 (1996).
Esterházy, D. et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130 (2019). This work demonstrates that the gut-draining lymph nodes are immunologically adapted to the particular intestinal segment they drain and, accordingly, preferentially elicit inflammatory or tolerogenic immune responses.
Brown, H., Komnick, M. R., Brigleb, P. H., Dermody, T. S. & Esterházy, D. Lymph node sharing between pancreas, gut, and liver leads to immune crosstalk and regulation of pancreatic autoimmunity. Immunity https://doi.org/10.1016/j.immuni.2023.07.008 (2023). This study demonstrates how lymph node co-drainage of tissues impacts the tolerance towards self-antigens.
Campbell, D. J. & Butcher, E. C. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195, 135–141 (2002). This landmark study shows a differential imprinting of homing receptors in CD4 T cells in different lymph nodes.
Johansson-Lindbom, B. et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969 (2003).
Mora, J. R. et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 424, 88–93 (2003). This landmark study shows a differential imprinting of homing receptors in CD8 T cells in different lymph nodes.
Mani, V. et al. Migratory DCs activate TGF-b to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366, https://doi.org/10.1126/science.aav5728 (2019). This study shows that tissue-derived migratory dendritic cells pre-condition naive CD8+ T cells to adopt a tissue-residency program after infection.
Lantz, O. & Bendelac, A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 180, 1077–1106 (1994).
Tilloy, F. et al. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted α/β T cell subpopulation in mammals. J. Exp. Med. 189, 1907–1921 (1999).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003); erratum 423, 1018 (2003).
Bendelac, A. et al. CD1 recognition by mouse NK1+ T lymphocytes. Science 268, 863–865 (1995).
Gumperz, J. E. et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12, 211–221 (2000).
Godfrey, D. I. et al. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin. Immunol. 22, 61–67 (2010).
Deseke, M. & Prinz, I. Ligand recognition by the γδ TCR and discrimination between homeostasis and stress conditions. Cell. Mol. Immunol. 17, 914–924 (2020).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Herrmann, T. & Karunakaran, M. M. Butyrophilins: γδ T cell receptor ligands, immunomodulators and more. Front. Immunol. 13, 876493 (2022).
Ribot, J. C., Lopes, N. & Silva-Santos, B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 21, 221–232 (2021).
Schneider, D. F. et al. A novel role for NKT cells in cutaneous wound repair. J. Surg. Res. 168, 325–333.e1 (2011).
Tanno, H. et al. Contribution of invariant natural killer T cells to skin wound healing. Am. J. Pathol. 185, 3248–3257 (2015).
Legoux, F., Salou, M. & Lantz, O. MAIT cell development and functions: the microbial connection. Immunity 53, 710–723 (2020).
Zhang, Y. et al. Mucosal-associated invariant T cells restrict reactive oxidative damage and preserve meningeal barrier integrity and cognitive function. Nat. Immunol. 23, 1714–1725 (2022).
Constantinides, M. G. & Belkaid, Y. Early-life imprinting of unconventional T cells and tissue homeostasis. Science 374, eabf0095 (2021).
Constantinides, M. G. et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624 (2019).
Howson, L. J. et al. Absence of mucosal-associated invariant T cells in a person with a homozygous point mutation in MR1. Sci. Immunol. 5, eabc9492 (2020).
Ataide, M. A. et al. Lymphatic migration of unconventional T cells promotes site-specific immunity in distinct lymph nodes. Immunity 55, 1813–1828.e9 (2022). This study demonstrates that lymph nodes draining different barrier tissues mount distinct innate and adaptive immune responses owing to their unconventional T cell composition.
Fan, X. & Rudensky, A. Y. Hallmarks of tissue-resident lymphocytes. Cell 164, 1198–1211 (2016).
Salou, M. et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 216, 133–151 (2019).
Tan, L. et al. Single-cell transcriptomics identifies the adaptation of Scart1+ Vγ6+ T cells to skin residency as activated effector cells. Cell Rep. 27, 3657–3671.e4 (2019).
Gray, E. E. et al. Deficiency in IL-17-committed Vγ4+ γδ T cells in a spontaneous Sox13-mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat. Immunol. 14, 584–592 (2013).
Nakamizo, S. et al. Dermal Vγ4+ γδ T cells possess a migratory potency to the draining lymph nodes and modulate CD8+ T-cell activity through TNF-α production. J. Invest. Dermatol. 135, 1007–1015 (2015).
Gaya, M. et al. Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell 172, 517–533.e20 (2018).
Williams, M. A. & Bevan, M. J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192 (2007).
Buchholz, V. R., Schumacher, T. N. M. & Busch, D. H. T cell fate at the single-cell level. Annu. Rev. Immunol. 34, 65–92 (2016).
Zhu, J., Yamane, H. & Paul, W. E. Differentiation of effector CD4+ T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Ugur, M., Kaminski, A. & Pabst, O. Lymph node γδ and αβ CD8+ T cells share migratory properties. Sci. Rep. 8, 8986 (2018).
Mandl, J. N. et al. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naïve CD4+ and CD8+ T cells. Proc. Natl Acad. Sci. USA 109, 18036–18041 (2012).
Ugur, M., Schulz, O., Menon, M. B., Krueger, A. & Pabst, O. Resident CD4+ T cells accumulate in lymphoid organs after prolonged antigen exposure. Nat. Commun. 5, 4821 (2014).
Fazilleau, N. et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol. 8, 753–761 (2007).
Cucak, H., Yrlid, U., Reizis, B., Kalinke, U. & Johansson-Lindbom, B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity 31, 491–501 (2009).
Beura, L. K. et al. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 216, 1214–1229 (2019).
Stolley, J. M. et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 217, e20192197 (2020).
Beura, L. K. et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48, 327–338.e5 (2018).
Campbell, C. & Rudensky, A. Roles of regulatory T cells in tissue pathophysiology and metabolism. Cell Metab. 31, 18–25 (2020).
Dikiy, S. & Rudensky, A. Y. Principles of regulatory T cell function. Immunity 56, 240–255 (2023).
Lee, H. M., Bautista, J. L. & Hsieh, C. S. Thymic and peripheral differentiation of regulatory T cells. Adv. Immunol. 112, 25–71 (2011).
Vaeth, M. et al. Tissue resident and follicular Treg cell differentiation is regulated by CRAC channels. Nat. Commun. 10, 1183 (2019).
Miragaia, R. J. et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 50, 493–504.e7 (2019).
Kaminski, A. et al. Resident regulatory T cells reflect the immune history of individual lymph nodes. Sci. Immunol. https://doi.org/10.1126/sciimmunol.adj5789 (2023).This study identifies lymph node-resident Treg cells that possess lymph node-specific TCR repertoires as a consequence of the local immune history.
Josefowicz, S. Z., Lu, L.-F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Zagorulya, M. et al. Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. Immunity 56, 386–405.e10 (2023). This study shows that lung-draining versus skin-draining lymph nodes differ in their ability to elicit an antitumor immune response owing to their Treg cell composition.
Oyler-Yaniv, A. et al. A tunable diffusion-consumption mechanism of cytokine propagation enables plasticity in cell-to-cell communication in the immune system. Immunity 46, 609–620 (2017).
Chinen, T. et al. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 17, 1322–1333 (2016).
Simeonov, D. R. et al. Non-coding sequence variation reveals fragility within interleukin 2 feedback circuitry and shapes autoimmune disease risk. Preprint at bioRxiv https://doi.org/10.1101/2023.06.17.545426 (2023).
Wong, H. S. et al. A local regulatory T cell feedback circuit maintains immune homeostasis by pruning self-activated T cells. Cell 184, 3981–3997.e22 (2021).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Cyster, J. G. et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176, 181–193 (2000).
Fazilleau, N., McHeyzer-Williams, L. J., Rosen, H. & McHeyzer-Williams, M. G. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10, 375–384 (2009).
Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008).
Grewal, I. S. & Flavell, R. A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16, 111–135 (1998).
Lim, H. W., Hillsamer, P. & Kim, C. H. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J. Clin. Invest. 114, 1640–1649 (2004).
Aloulou, M. et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat. Commun. 7, 10579 (2016).
Botta, D. et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat. Immunol. 18, 1249–1260 (2017).
Clement, R. L. et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat. Immunol. 20, 1360–1371 (2019).
Sheng, J. et al. Fate mapping analysis reveals a novel murine dermal migratory Langerhans-like cell population. eLife 10, e65412 (2021).
Mackley, E. C. et al. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat. Commun. 6, 5862 (2015).
Yamano, T. et al. Aire-expressing ILC3-like cells in the lymph node display potent APC features. J. Exp. Med. 216, 1027–1037 (2019).
Abramson, J., Dobeš, J., Lyu, M. & Sonnenberg, G. F. The emerging family of RORγt+ antigen-presenting cells. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-023-00906-5 (2023).
Lyu, M. et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 610, 744–751 (2022).
Akagbosu, B. et al. Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota. Nature 610, 752–760 (2022).
Kedmi, R. et al. A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nature 610, 737–743 (2022).
Wang, J. et al. Single-cell multiomics defines tolerogenic extrathymic Aire-expressing populations with unique homology to thymic epithelium. Sci. Immunol. 6, eabl5053 (2021).
Waffarn, E. E. et al. Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat. Commun. 6, 8991 (2015).
Wiig, H. & Swartz, M. A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 (2012).
Aukland, K. & Reed, R. K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol. Rev. 73, 1–78 (1993).
Clement, C. C. et al. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLoS One 5, e9863 (2010). The study provides a comparative, quantitative analysis of peptide fragments found in human lymph.
Fraser, J. R. E., Laurent, T. C. & Laurent, U. B. G. Hyaluronan: its nature, distribution, functions and turnover. J. Intern. Med. 242, 27–33 (1997).
Tengblad, A. et al. Concentration and relative molecular mass of hyaluronate in lymph and blood. Biochem. J. 236, 521–525 (1986).
Fraser, J. R. E., Kimpton, W. G., Laurent, T. C., Cahill, R. N. P. & Vakakis, N. Uptake and degradation of hyaluronan in lymphatic tissue. Biochem. J. 256, 153–158 (1988).
Srinivasan, S., Vannberg, F. O. & Dixon, J. B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 6, 24436 (2016).
Hood, J. L. The association of exosomes with lymph nodes. Semin. Cell Dev. Biol. 67, 29–38 (2017).
Zhang, W. et al. Exosomes in pathogen infections: a bridge to deliver molecules and link functions. Front. Immunol. 9, 90 (2018).
Shannon, A. D., Quin, J. W. & Courtice, F. C. Lysosomal enzyme activities in sheep plasma and lymph. Res. Vet. Sci. 22, 209–215 (1977).
Reichl, D., Hathaway, C. B., Sterchi, J. M. & Miller, N. E. Lipoproteins of human peripheral lymph. Apolipoprotein AI-containing lipoprotein with alpha-2 electrophoretic mobility. Eur. J. Clin. Invest. 21, 638–643 (1991).
Jacobs, F. A. & Largis, E. E. Transport of amino acids via the mesenteric lymph duct in rats. Proc. Soc. Exp. Biol. Med. 130, 692–696 (1969).
Nanjee, M. N., Cooke, C. J., Olszewski, W. L. & Miller, N. E. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans: associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J. Lipid Res. 41, 1317–1327 (2000).
O’Sullivan, D., Sanin, D. E., Pearce, E. J. & Pearce, E. L. Metabolic interventions in the immune response to cancer. Nat. Rev. Immunol. 19, 324–335 (2019).
Ohhashi, T., Kawai, Y., Maejima, D., Hayashi, M. & Watanabe-Asaka, T. Physiological roles of lymph flow-mediated nitric oxide in lymphatic system. Lymphat. Res. Biol. 21, 253–261 (2023).
Olszewski, W. L. The lymphatic system in body homeostasis: physiological conditions. Lymphat. Res. Biol. 1, 11–21 (2003).
Wu, H., Denna, T. H., Storkersen, J. N. & Gerriets, V. A. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacol. Res. 140, 100–114 (2019).
Munn, D. H. & Mellor, A. L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34, 137–143 (2013).
Fletcher, M. et al. L-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res. 75, 275–283 (2015).
Geiger, R. et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016).
Steggerda, S. M. et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer 5, 101 (2017).
Klysz, D. et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 8, ra97 (2015).
Mondanelli, G. et al. A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity 46, 233–244 (2017).
Svoronos, N. et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov. 7, 72–85 (2017).
Vasanthakumar, A. et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature 579, 581–585 (2020).
Hiltensperger, M. et al. Skin and gut imprinted helper T cell subsets exhibit distinct functional phenotypes in central nervous system autoimmunity. Nat. Immunol. 22, 880–892 (2021). This study shows that helper T cells primed in different lymph nodes are phenotypically distinct and home to distinct areas of tissues during inflammation.
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606.e3 (2019).
Clement, C. C. et al. Quantitative profiling of the lymph node clearance capacity. Sci. Rep. 8, 11253 (2018).
Zawieja, D. C. et al. Lymphatic cannulation for lymph sampling and molecular delivery. J. Immunol. 203, 2339–2350 (2019).
Reichl, D., Simons, L. A., Myant, N. B., Pflug, J. J. & Mills, G. L. The lipids and lipoproteins of human peripheral lymph, with observations on the transport of cholesterol from plasma and tissues into lymph. Clin. Sci. Mol. Med. 45, 313–329 (1973).
Sloop, C. H., Castle, C. K., Lefevre, M. & Wong, L. Comparison of the lipid and apolipoprotein composition of skeletal muscle and peripheral lymph in control dogs and in dogs fed a high fat, high cholesterol, hypothyroid-inducing diet. Biochim. Biophys. Acta 1169, 196–201 (1993).
Julien, P., Fong, B. & Angel, A. Cardiac and peripheral lymph lipoproteins in dogs fed cholesterol and saturated fat. Arteriosclerosis 4, 435–442 (1984).
Santambrogio, L. & Rammensee, H. G. Contribution of the plasma and lymph degradome and peptidome to the MHC ligandome. Immunogenetics 71, 203–216 (2019).
Clement, C. C. et al. The dendritic cell major histocompatibility complex II (MHC II) peptidome derives from a variety of processing pathways and includes peptides with a broad spectrum of HLA-DM sensitivity. J. Biol. Chem. 291, 5576–5595 (2016).
Houston, S. A. et al. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 9, 468–478 (2016). This study shows that migratory dendritic cells from the small intestine-draining lymph nodes and colon-draining lymph nodes differ phenotypically and differentially imprint T cells for tissue-specific homing.
Yang, W. & Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 18, 866–877 (2021).
Gutiérrez-Vázquez, C. & Quintana, F. J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48, 19–33 (2018).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Nguyen, L. P. & Bradfield, C. A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 21, 102–116 (2008).
Li, S. et al. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group 2 innate lymphoid cell function. Immunity 49, 915–928.e5 (2018).
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Chng, S. H. et al. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Sci. Rep. 6, 23820 (2016).
Schiering, C. et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 542, 242–245 (2017).
Cifarelli, V. & Eichmann, A. The intestinal lymphatic system: functions and metabolic implications. Cell. Mol. Gastroenterol. Hepatol. 7, 503–513 (2019).
D’Ambrosio, D. N., Clugston, R. D. & Blaner, W. S. Vitamin A metabolism: an update. Nutrients 3, 63–103 (2011).
Eroglu, A. & Harrison, E. H. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J. Lipid Res. 54, 1719–1730 (2013).
Sun, C. M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Kim, C. H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 18, 1161–1171 (2021).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Bachem, A. et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297.e5 (2019).
Kim, M., Qie, Y., Park, J. & Kim, C. H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20, 202–214 (2016).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
Kobayashi, T., Chanmee, T. & Itano, N. Hyaluronan: metabolism and function. Biomolecules 10, 1525 (2020).
Lee-Sayer, S. S. M. et al. The where, when, how and why of hyaluronan binding by immune cells. Front. Immunol. 6, 150 (2015).
Rundqvist, H. et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife 9, e59996 (2020).
Quinn, W. J. et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. 33, 108500 (2020).
Feng, Q. et al. Lactate increases stemness of CD8+ T cells to augment anti-tumor immunity. Nat. Commun. 13, 4981 (2022).
Riedel, A. et al. Tumor-derived lactic acid modulates activation and metabolic status of draining lymph node stroma. Cancer Immunol. Res. 10, 482–497 (2022).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Uren, R. F., Howman-Giles, R. & Thompson, J. F. Patterns of lymphatic drainage of the skin in patients with melanoma. J. Nucl. Med. 44, 570–582 (2003).
Connolly, K. A. et al. A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol. 6, eabg7836 (2021).
Schenkel, J. M. et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. Immunity 54, 2338–2353.e6 (2021).
Thomas, S. N., Vokali, E., Lund, A. W., Hubbell, J. A. & Swartz, M. A. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824 (2014).
Koster, B. D. et al. Local adjuvant treatment with low-dose CpG-B offers durable protection against disease recurrence in clinical stage I-II melanoma: data from two randomized phase II trials. Clin. Cancer Res. 23, 5679–5686 (2017).
Ji, P. et al. Smart exosomes with lymph node homing and immune-amplifying capacities for enhanced immunotherapy of metastatic breast cancer. Mol. Ther. Nucleic Acids 26, 987–996 (2021).
Dammeijer, F. et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell 38, 685–700.e8 (2020).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Lund, A. W. et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 1, 191–199 (2012).
Farnsworth, R. H., Lackmann, M., Achen, M. G. & Stacker, S. A. Vascular remodeling in cancer. Oncogene 33, 3496–3505 (2014).
Dubrot, J. et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J. Exp. Med. 211, 1153–1166 (2014).
Riedel, A., Shorthouse, D., Haas, L., Hall, B. A. & Shields, J. Tumor-induced stromal reprogramming drives lymph node transformation. Nat. Immunol. 17, 1118–1127 (2016).
Gillot, L., Baudin, L., Rouaud, L., Kridelka, F. & Noël, A. The pre-metastatic niche in lymph nodes: formation and characteristics. Cell. Mol. Life Sci. 78, 5987–6002 (2021).
Cochran, A. J. et al. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 6, 659–670 (2006).
Mannino, M. H. et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 367, 103–107 (2015).
Batlle, E. & Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Ito, M. et al. Tumor-derived TGFβ-1 induces dendritic cell apoptosis in the sentinel lymph node. J. Immunol. 176, 5637–5643 (2006).
Lee, J. H. et al. Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin. Cancer Res. 11, 107–112 (2005).
Botella-Estrada, R. et al. Cytokine expression and dendritic cell density in melanoma sentinel nodes. Melanoma Res. 15, 99–106 (2005).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Buzas, E. I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 23, 236–250 (2022).
Hu, L., Wickline, S. A. & Hood, J. L. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn. Reson. Med. 74, 266–271 (2015).
Pucci, F. et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352, 242–246 (2016).
Taylor, D. D. & Gerçel-Taylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 92, 305–311 (2005).
Czystowska-Kuzmicz, M. et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 10, 3000 (2019).
Liu, C. et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 176, 1375–1385 (2006).
Cochran, A. J., Pihl, E., Wen, D. R., Hoon, D. S. & Korn, E. L. Zoned immune suppression of lymph nodes draining malignant melanoma: histologic and immunohistologic studies. J. Natl Cancer Inst. 78, 399–405 (1987).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Backlund, C., Jalili-Firoozinezhad, S., Kim, B. & Irvine, D. J. Biomaterials-mediated engineering of the immune system. Annu. Rev. Immunol. 41, 153–179 (2023).
Lavelle, E. C. & Ward, R. W. Mucosal vaccines — fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250 (2022).
Regev, A. et al. The Human Cell Atlas white paper. Preprint at arXiv https://doi.org/10.48550/arXiv.1810.05192 (2018).
Petrova, T. V. & Koh, G. Y. Biological functions of lymphatic vessels. Science 369, eaax4063 (2020).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Oliver, G., Kipnis, J., Randolph, G. J. & Harvey, N. L. The lymphatic vasculature in the 21st century: novel functional roles in homeostasis and disease. Cell 182, 270–296 (2020).
Randolph, G. J. & Miller, N. E. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Invest. 124, 929–935 (2014).
Lewis, S. M., Williams, A. & Eisenbarth, S. C. Structure and function of the immune system in the spleen. Sci. Immunol. 4, eaau6085 (2019).
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000).
Chauveau, A. et al. Visualization of T cell migration in the spleen reveals a network of perivascular pathways that guide entry into T zones. Immunity 52, 794–807.e7 (2020).
Bronte, V. & Pittet, M. J. The spleen in local and systemic regulation of immunity. Immunity 39, 806–818 (2013).
Reboldi, A. & Cyster, J. G. Peyer’s patches: organizing B-cell responses at the intestinal frontier. Immunol. Rev. 271, 230–245 (2016).
Heel, K. A., McCauley, R. D., Papadimitriou, J. M. & Hall, J. C. Review: Peyer’s patches. J. Gastroenterol. Hepatol. 12, 122–136 (1997).
Cornes, J. S. Peyer’s patches in the human gut. Proc. R. Soc. Med. 58, 716 (1965).
Cerutti, A. & Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 28, 740–750 (2008).
Cyster, J. G. & Von Andrian, U. H. Dynamics of B cell migration to and within secondary lymphoid organs. in Molecular Biology of B Cells (eds Honjo, T., Alt, F. W. & Neuberger, M. S.) 203–221 (Elsevier, 2004).
Travis, M. A. et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 (2007).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Sheikh-Mohamed, S. et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 15, 799–808 (2022).
Poon, M. M. L. et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 6, eabl9105 (2021).
Tang, J. et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 7, eadd4853 (2022).
Knisely, J. M. et al. Mucosal vaccines for SARS-CoV-2: scientific gaps and opportunities — workshop report. NPJ Vaccines 8, 53 (2023).
Liao, M. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020).
Grau-Expósito, J. et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 12, 3010 (2021).
Onodera, T. et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl Acad. Sci. USA 109, 2485–2490 (2012).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Son, Y. M. et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci. Immunol. 6, eabb6852 (2021).
Kingstad-Bakke, B. et al. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc. Natl Acad. Sci. USA 119, e2118312119 (2022).
Nouailles, G. et al. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat. Microbiol. 8, 860–874 (2023).
Lapuente, D. et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat. Commun. 12, 6871 (2021).
Mao, T. et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 378, eabo2523 (2022).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e13 (2020).
King, R. G. et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge. Vaccines 9, 881 (2021).
Hassan, A. O. et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 36, 109452 (2021).
Afkhami, S. et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185, 896–915.e19 (2022).
van Doremalen, N. et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 13, eabh0755 (2021).
Madhavan, M. et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: an open-label partially-randomised ascending dose phase I trial. eBioMedicine 85, 104298 (2022).
Acknowledgements
The authors thank the WÜSI principal investigators, A. Riedel, M. Bajénoff and the Kastenmüller Lab for the critical reading of the manuscript. Our research is supported by the European Research Council (ERC) (819329- STEP2), the German Research Foundation (DFG) (SFB 1583- DECIDE and GRK2581 ‘SPHINGOINF’) and the Max Planck Society (Max Planck Research Groups).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks A. Andrusaite, V. Cerovic, S. Milling and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Affinity maturation and antibody class switching
-
Affinity maturation and antibody class switching are two important mechanisms B cells use to improve the efficacy of the antibody response. Once B cells have recognized their cognate antigen through the B cell receptor (BCR), they start producing IgM antibodies and this is known as the primary response. To increase the binding affinity of the BCR to cognate antigen, B cells introduce mutations into the complementarity-determining region of the BCR via a process known as somatic hypermutation (SHM). SHM produces B cells with varying affinities of BCRs, and only the ones with the strongest affinity are positively selected by follicular helper T cells in germinal centres. During antibody class switching, changes occur in the heavy-chain domain of the antibody, creating antibodies with similar affinity but different effector functions. This process involves recombining the exon clusters on the IgH locus, enabling antibody isotype switching (for example, from IgM to IgG, IgE or IgA).
- Aryl hydrocarbon receptor
-
(AhR). A ligand-activated transcription factor, which upon ligand binding translocates from the cytoplasm to the nucleus, forms a complex with aryl hydrocarbon receptor nuclear translocator and induces transcription of target genes. In its inactive state, it forms a protein dimer complex with HSP90, XAP2, p23 and SRC. AhR has diverse roles in adaptive immune responses, such as promoting Th17 cell induction or Treg cell stabilization.
- Central Treg cells
-
Treg cells can be classified either on their developmental origin — as tTreg and pTreg cells — or based on their migratory pattern — as central Treg and effector Treg cells. Central Treg cells primarily recirculate in the blood and SLOs. They are characterized by a CD4+CD25+FOXP3+CD44lowCD62LhighCCR7+ phenotype and primarily localize to the T cell zone in the spleen and lymph nodes. They require IL-2 for their homoeostasis and survival and serve as a pool of long-lived recirculating Treg cells in SLOs.
- Effector Treg cells
-
Effector Treg cells develop from central Treg cells in a BATF-dependent manner and require ICOS signalling for their survival. Even though inflammatory signals primarily drive the differentiation of effector Treg cells from central Treg cells, constant T cell receptor stimulation is required for the maintenance of effector Treg cells. These Treg cells have a CD4+CD25+FOXP3+CD44highCD62LlowCCR7− phenotype and primarily localize to non-lymphoid tissues.
- Exosomes
-
Exosomes are single-membraned extracellular vesicles that are produced by cells in the endoplasmic reticulum and can carry different types of cargo from nucleic acids to proteins or metabolites. They are critical components in cellular communications both over short and long distances.
- Hyaluronic acid
-
Hyaluronic acid is a large polysaccharide formed of glucuronic acid and glucosamine, with an approximate relative molecular weight of 105–107 (ratio of the molecular weights of hyaluronic acid and carbon). It is produced by distinct cell types and is the main component of the extracellular matrix.
- Sphingosine-1-phosphate
-
(S1P). A sphingolipid that is formed from ceramide by the action of ceramidase and sphingosine kinases (SK1 and SK2). S1P acts as a crucial mediator in lymphocyte trafficking, vascular development and heart development via its receptors S1PR1–S1PR5.
- Tumour secretome
-
The tumour secretome is the entire collection of macromolecules — including both soluble proteins and insoluble vesicles — that take part in cell–cell communication in the tumour (for example, growth factors, lipids and exosomes).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cruz de Casas, P., Knöpper, K., Dey Sarkar, R. et al. Same yet different — how lymph node heterogeneity affects immune responses. Nat Rev Immunol 24, 358–374 (2024). https://doi.org/10.1038/s41577-023-00965-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00965-8