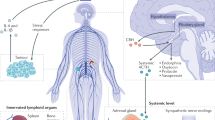
Abstract
Although there is little direct evidence supporting that stress affects cancer incidence, it does influence the evolution, dissemination and therapeutic outcomes of neoplasia, as shown in human epidemiological analyses and mouse models. The experience of and response to physiological and psychological stressors can trigger neurological and endocrine alterations, which subsequently influence malignant (stem) cells, stromal cells and immune cells in the tumour microenvironment, as well as systemic factors in the tumour macroenvironment. Importantly, stress-induced neuroendocrine changes that can regulate immune responses have been gradually uncovered. Numerous stress-associated immunomodulatory molecules (SAIMs) can reshape natural or therapy-induced antitumour responses by engaging their corresponding receptors on immune cells. Moreover, stress can cause systemic or local metabolic reprogramming and change the composition of the gastrointestinal microbiota which can indirectly modulate antitumour immunity. Here, we explore the complex circuitries that link stress to perturbations in the cancer-immune dialogue and their implications for therapeutic approaches to cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Braslow, J. T. & Marder, S. R. History of psychopharmacology. Annu. Rev. Clin. Psychol. 15, 25–50 (2019).
Cruz-Pereira, J. S. et al. Depression’s unholy trinity: dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 71, 49–78 (2020).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000). This review elegantly summarizes current knowledge about the links among depression pathogenesis, immunity and the microbiome, as well as possible therapeutic interventions.
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Veiga-Fernandes, H. & Artis, D. Neuronal-immune system cross-talk in homeostasis. Science 359, 1465–1466 (2018).
Godinho-Silva, C., Cardoso, F. & Veiga-Fernandes, H. Neuro-immune cell units: a new paradigm in physiology. Annu. Rev. Immunol. 37, 19–46 (2019).
Huang, S. et al. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184, 441–459.e25 (2021). This study discovers that lymph node-innervating sensory neurons can interact with several predicted cell types and change their transcriptome, and the unexpected sensory neuro-immune circuit exhibits the capacity to monitor the inflammatory state in the lymph node.
Kabata, H. & Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Invest. 130, 1475–1482 (2019).
Chen, C.-S., Barnoud, C. & Scheiermann, C. Peripheral neurotransmitters in the immune system. Curr. Opin. Physiol. 19, 73–79 (2021).
Wang, A., Luan, H. H. & Medzhitov, R. An evolutionary perspective on immunometabolism. Science 363, eaar3932 (2019).
Guyot, M. et al. Apical splenic nerve electrical stimulation discloses an anti-inflammatory pathway relying on adrenergic and nicotinic receptors in myeloid cells. Brain Behav. Immun. 80, 238–246 (2019).
Al-Shalan, H. A. M., Hu, D., Nicholls, P. K., Greene, W. K. & Ma, B. Immunofluorescent characterization of innervation and nerve-immune cell neighborhood in mouse thymus. Cell Tissue Res. 378, 239–254 (2019).
Jung, W. C., Levesque, J. P. & Ruitenberg, M. J. It takes nerve to fight back: the significance of neural innervation of the bone marrow and spleen for immune function. Semin. Cell Dev. Biol. 61, 60–70 (2017).
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020). This well-designed study identifies a specific brain–spleen neural connection in mice that enhances humoral responses in response to an elevated platform regimen.
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008).
Miyajima, M. et al. Metabolic shift induced by systemic activation of T cells in PD-1-deficient mice perturbs brain monoamines and emotional behavior. Nat. Immunol. 18, 1342–1352 (2017). This paper reveals how the key immune checkpoint molecule PD1 is involved in regulating systemic metabolism, the biosynthesis of neurotransmitters and behaviours.
Fan, K. Q. et al. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179, 864–879.e19 (2019). This interesting study suggests that stress-induced abnormal mitochondrial fission and purine synthesis in CD4+ T cells influence oligodendrocytes in the amygdala, which is a prerequisite for the onset of anxiety.
Fridman, W. H., Zitvogel, L., Sautes-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Faulkner, S., Jobling, P., March, B., Jiang, C. C. & Hondermarck, H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 9, 702–710 (2019). This comprehensive review summarizes recent progress that deals with the potential link between tumour-induced innervation within the TME and cancer initiation, progression and metastasis.
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013).
Zahalka, A. H. et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017).
Kamiya, A. et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 22, 1289–1305 (2019).
Cervantes-Villagrana, R. D., Albores-Garcia, D., Cervantes-Villagrana, A. R. & Garcia-Acevez, S. J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal. Transduct. Target. Ther. 5, 99 (2020).
Wei, E. K., Wolin, K. Y. & Colditz, G. A. Time course of risk factors in cancer etiology and progression. J. Clin. Oncol. 28, 4052–4057 (2010).
Feng, Z. et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Natl Acad. Sci. USA 109, 7013–7018 (2012).
Jang, H. J., Boo, H. J., Lee, H. J., Min, H. Y. & Lee, H. Y. Chronic stress facilitates lung tumorigenesis by promoting exocytosis of IGF2 in lung epithelial cells. Cancer Res. 76, 6607–6619 (2016).
Schoemaker, M. J. et al. Psychological stress, adverse life events and breast cancer incidence: a cohort investigation in 106,000 women in the United Kingdom. Breast Cancer Res. 18, 72 (2016).
Wang, Y. H. et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol. Psychiatry 25, 1487–1499 (2020). This systematic review and meta-analysis contains 51 eligible cohort studies involving 2,611,907 participants which test the association between ‘anxiety and depression’ and the risk of cancer incidence, cancer-specific mortality and all-cause mortality in patients with cancer.
Butow, P. et al. Does stress increase risk of breast cancer? A 15-year prospective study. Psychooncology 27, 1908–1914 (2018).
Tomiyama, A. J. Stress and obesity. Annu. Rev. Psychol. 70, 703–718 (2019).
Song, H. et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA 319, 2388–2400 (2018).
Buchanan, T. W. & Lovallo, W. R. The role of genetics in stress effects on health and addiction. Curr. Opin. Psychol. 27, 72–76 (2019).
Anacker, C. et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol. Psychiatry 79, 840–849 (2016).
Misiewicz, Z. et al. Multi-omics analysis identifies mitochondrial pathways associated with anxiety-related behavior. PLoS Genet. 15, e1008358 (2019).
Mitchell, A. J. et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 12, 160–174 (2011).
Cordova, M. J., Riba, M. B. & Spiegel, D. Post-traumatic stress disorder and cancer. Lancet Psychiatry 4, 330–338 (2017).
Mehnert, A. & Koch, U. Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: a prospective study. Psychooncology 16, 181–188 (2007).
Horowitz, M., Neeman, E., Sharon, E. & Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 12, 213–226 (2015).
Batty, G. D., Russ, T. C., Stamatakis, E. & Kivimaki, M. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ 356, j108 (2017).
Palesh, O. et al. Stress history and breast cancer recurrence. J. Psychosom. Res. 63, 233–239 (2007).
Wang, X. et al. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: a systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 25, 3186–3197 (2020).
Moreno-Smith, M., Lutgendorf, S. K. & Sood, A. K. Impact of stress on cancer metastasis. Future Oncol. 6, 1863–1881 (2010).
Chida, Y., Hamer, M., Wardle, J. & Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 5, 466–475 (2008).
Powell, N. D. et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl Acad. Sci. USA 110, 16574–16579 (2013).
Irwin, M. R. & Cole, S. W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632 (2011).
Lu, D. et al. Stress-related signaling pathways in lethal and nonlethal prostate cancer. Clin. Cancer Res. 22, 765–772 (2016).
Thaker, P. H. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 12, 939–944 (2006).
Zhang, X. et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 10, 788 (2019).
Le, C. P. et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 7, 10634 (2016).
Saul, A. N. et al. Chronic stress and susceptibility to skin cancer. J. Natl Cancer Inst. 97, 1760–1767 (2005).
Ben-Eliyahu, S., Page, G. G., Yirmiya, R. & Shakhar, G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer 80, 880–888 (1999).
Yang, H. et al. Stress–glucocorticoid–TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 25, 1428–1441 (2019). This study provides strong evidence that psychological stress compromises the efficacy of immune-dependent cancer therapies, by elevating the endogenous glucocorticoid tonus and stimulating TSC22D3 expression in tumour-infiltrating dendritic cells.
Kokolus, K. M. et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl Acad. Sci. USA 110, 20176–20181 (2013).
Cao, L. et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142, 52–64 (2010).
He, L. et al. Glucocorticoid receptor signaling activates TEAD4 to promote breast cancer progression. Cancer Res. 79, 4399–4411 (2019).
Obradovic, M. M. S. et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019).
Melhem, A. et al. Administration of glucocorticoids to ovarian cancer patients is associated with expression of the anti-apoptotic genes SGK1 and MKP1/DUSP1 in ovarian tissues. Clin. Cancer Res. 15, 3196–3204 (2009).
Petrella, A. et al. Dexamethasone inhibits TRAIL-induced apoptosis of thyroid cancer cells via Bcl-xL induction. Eur. J. Cancer 42, 3287–3293 (2006).
Sorrentino, G. et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat. Commun. 8, 14073 (2017).
Flaherty, R. L. et al. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 19, 35 (2017).
Cui, B. et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Invest. 129, 1030–1046 (2019).
Sastry, K. S. et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J. Biol. Chem. 282, 14094–14100 (2007).
Nagaraja, A. S. et al. Adrenergic-mediated increases in INHBA drive CAF phenotype and collagens. JCI Insight 2, e93076 (2017).
Yang, E. V. et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 66, 10357–10364 (2006).
Sood, A. K. et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 12, 369–375 (2006).
Kilpatrick, L. E. et al. Complex formation between VEGFR2 and the β2-adrenoceptor. Cell Chem. Biol. 26, 830–841.e9 (2019).
Allen, J. K. et al. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res. 78, 3233–3242 (2018).
Ulrich-Lai, Y. M. & Herman, J. P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (2009).
Picard, M. et al. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl Acad. Sci. USA 112, E6614–6623 (2015).
Cole, S. W. & Sood, A. K. Molecular pathways: β-adrenergic signaling in cancer. Clin. Cancer Res. 18, 1201–1206 (2012).
Wu, D., Katz, A., Lee, C. H. & Simon, M. I. Activation of phospholipase C by α1-adrenergic receptors is mediated by the α subunits of Gq family. J. Biol. Chem. 267, 25798–25802 (1992).
Wick, G., Hu, Y., Schwarz, S. & Kroemer, G. Immunoendocrine communication via the hypothalamo-pituitary–adrenal axis in autoimmune diseases. Endocr. Rev. 14, 539–563 (1993).
Weikum, E. R., Knuesel, M. T., Ortlund, E. A. & Yamamoto, K. R. Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 18, 159–174 (2017).
Ping, Y. Q. et al. Structures of the glucocorticoid-bound adhesion receptor GPR97–Go complex. Nature 589, 620–626 (2021).
Wang, J. J. et al. Gpr97 is essential for the follicular versus marginal zone B-lymphocyte fate decision. Cell Death Dis. 4, e853 (2013).
Chu, T. Y. et al. GPR97 triggers inflammatory processes in human neutrophils via a macromolecular complex upstream of PAR2 activation. Nat. Commun. 13, 6385 (2022).
Graeff, F. G., Guimaraes, F. S., De Andrade, T. G. & Deakin, J. F. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 54, 129–141 (1996).
Mineur, Y. S. et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl Acad. Sci. USA 110, 3573–3578 (2013).
Picciotto, M. R., Higley, M. J. & Mineur, Y. S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129 (2012).
Lydiard, R. B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 64, 21–27 (2003).
Nuss, P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 11, 165–175 (2015).
Panula, P. & Nuutinen, S. The histaminergic network in the brain: basic organization and role in disease. Nat. Rev. Neurosci. 14, 472–487 (2013).
de Almeida, D. O., Ferreira, H. S., Pereira, L. B. & Fregoneze, J. B. Hypertensive response to stress: the role of histaminergic H1 and H2 receptors in the medial amygdala. Physiol. Behav. 144, 95–102 (2015).
Bali, A., Randhawa, P. K. & Jaggi, A. S. Stress and opioids: role of opioids in modulating stress-related behavior and effect of stress on morphine conditioned place preference. Neurosci. Biobehav. Rev. 51, 138–150 (2015).
Valentino, R. J. & Van Bockstaele, E. Endogenous opioids: the downside of opposing stress. Neurobiol. Stress. 1, 23–32 (2015).
Pecina, M. et al. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol. Psychiatry 24, 576–587 (2019).
Oshaghi, M., Kourosh-Arami, M. & Roozbehkia, M. Role of neurotransmitters in immune-mediated inflammatory disorders: a crosstalk between the nervous and immune systems. Neurol. Sci. 44, 99–113 (2023).
Herman, J. P. et al. Regulation of the hypothalamic–pituitary–adrenocortical stress response. Compr. Physiol. 6, 603–621 (2016).
Lanfumey, L., Mongeau, R., Cohen-Salmon, C. & Hamon, M. Corticosteroid–serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci. Biobehav. Rev. 32, 1174–1184 (2008).
Sui, P. et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360, eaan8546 (2018).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198.e16 (2017).
Sidler, D. et al. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene 30, 2411–2419 (2011).
Honke, N. et al. Endogenously produced catecholamines improve the regulatory function of TLR9-activated B cells. PLoS Biol. 20, e3001513 (2022).
Guida, F. et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 67, 230–245 (2018).
Monje, M. et al. Roadmap for the emerging field of cancer neuroscience. Cell 181, 219–222 (2020).
Walker, A. K. et al. Circulating epinephrine is not required for chronic stress to enhance metastasis. Psychoneuroendocrinology 99, 191–195 (2019).
Renz, B. W. et al. β2 adrenergic–neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33, 75–90.e7 (2018).
Renz, B. W. et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8, 1458–1473 (2018).
Renz, B. W. et al. β2 adrenergic–neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 34, 863–867 (2018).
Reijmen, E., Vannucci, L., De Couck, M., De Greve, J. & Gidron, Y. Therapeutic potential of the vagus nerve in cancer. Immunol. Lett. 202, 38–43 (2018).
De Couck, M., Caers, R., Spiegel, D. & Gidron, Y. The role of the vagus nerve in cancer prognosis: a systematic and a comprehensive review. J. Oncol. 2018, 1236787 (2018).
Husby, A., Wohlfahrt, J. & Melbye, M. Vasectomy and prostate cancer risk: a 38-year nationwide cohort study. J. Natl Cancer Inst. 112, 71–77 (2020).
Webster, J. I., Tonelli, L. & Sternberg, E. M. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 20, 125–163 (2002).
Taves, M. D. & Ashwell, J. D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 18, 309–345 (2020).
Cain, D. W. & Cidlowski, J. A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 17, 233–247 (2017).
Dhabhar, F. S. & McEwen, B. S. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc. Natl Acad. Sci. USA 96, 1059–1064 (1999).
Sharma, D. & Farrar, J. D. Adrenergic regulation of immune cell function and inflammation. Semin. Immunopathol. 42, 709–717 (2020).
Hunzeker, J. T. et al. A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells. J. Immunol. 186, 183–194 (2011).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101.e15 (2019).
Franco, L. M. et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. J. Exp. Med. 216, 384–406 (2019).
Pani, L., Porcella, A. & Gessa, G. L. The role of stress in the pathophysiology of the dopaminergic system. Mol. Psychiatry 5, 14–21 (2000).
Soliman, A. et al. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology 33, 2033–2041 (2008).
Pruessner, J. C., Champagne, F., Meaney, M. J. & Dagher, A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. 24, 2825–2831 (2004).
Watanabe, Y. et al. Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. J. Immunol. 176, 848–856 (2006).
Mikulak, J. et al. Dopamine inhibits the effector functions of activated NK cells via the upregulation of the D5 receptor. J. Immunol. 193, 2792–2800 (2014).
Yan, Y. et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 (2015).
Yanagawa, Y., Matsumoto, M. & Togashi, H. Enhanced dendritic cell antigen uptake via α2 adrenoceptor-mediated PI3K activation following brief exposure to noradrenaline. J. Immunol. 185, 5762–5768 (2010).
Takenaka, M. C. et al. Norepinephrine controls effector T cell differentiation through β2-adrenergic receptor-mediated inhibition of NF-κB and AP-1 in dendritic cells. J. Immunol. 196, 637–644 (2016).
Guereschi, M. G. et al. β2-Adrenergic receptor signaling in CD4+Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur. J. Immunol. 43, 1001–1012 (2013).
Devi, S. et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230.e7 (2021).
Schiller, M., Ben-Shaanan, T. L. & Rolls, A. Neuronal regulation of immunity: why, how and where? Nat. Rev. Immunol. 21, 20–36 (2021).
Lutgendorf, S. K. et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 23, 7105–7113 (2005).
Varker, K. A. et al. Impaired natural killer cell lysis in breast cancer patients with high levels of psychological stress is associated with altered expression of killer immunoglobin-like receptors. J. Surg. Res. 139, 36–44 (2007).
Levi, B. et al. Stress impairs the efficacy of immune stimulation by CpG-C: potential neuroendocrine mediating mechanisms and significance to tumor metastasis and the perioperative period. Brain Behav. Immun. 56, 209–220 (2016).
Koide, S. S. Mifepristone. Auxiliary therapeutic use in cancer and related disorders. J. Reprod. Med. 43, 551–560 (1998).
Check, J. H., Dix, E., Cohen, R., Check, D. & Wilson, C. Efficacy of the progesterone receptor antagonist mifepristone for palliative therapy of patients with a variety of advanced cancer types. Anticancer. Res. 30, 623–628 (2010).
Check, J. H., Check, D., Wilson, C. & Lofberg, P. Long-term high-quality survival with single-agent mifepristone treatment despite advanced cancer. Anticancer. Res. 36, 6511–6513 (2016).
Cronin-Fenton, D. et al. Concurrent new drug prescriptions and prognosis of early breast cancer: studies using the Danish Breast Cancer Group clinical database. Acta Oncol. 57, 120–128 (2018). This study identifies a particular myeloid cell population in the TME that produces endogenous glucocorticoid to induce dysfunctional CD8+ T cells and failure of cancer immunotherapy.
Mulick, A. et al. Is improvement in comorbid major depression associated with longer survival in people with cancer? A long-term follow-up of participants in the SMaRT oncology-2 and 3 trials. J. Psychosom. Res. 116, 106–112 (2019).
Acharya, N. et al. Endogenous glucocorticoid signaling regulates CD8+ T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity 53, 658–671.e6 (2020).
Yi, L. & Zheng, C. The emerging roles of ZDHHCs-mediated protein palmitoylation in the antiviral innate immune responses. Crit. Rev. Microbiol. 47, 34–43 (2021).
Arbour, K. C. et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J. Clin. Oncol. 36, 2872–2878 (2018).
Maxwell, R. et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology 7, e1500108 (2018).
Aston, W. J. et al. Dexamethasone differentially depletes tumour and peripheral blood lymphocytes and can impact the efficacy of chemotherapy/checkpoint blockade combination treatment. Oncoimmunology 8, e1641390 (2019).
Papa, I. et al. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 547, 318–323 (2017).
van der Heijden, C. et al. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ. Res. 127, 269–283 (2020).
Elenkov, I. J. in NeuroImmune Biology (eds del Rey, A. et al.) Vol. 7 189–206 (Elsevier, 2007).
Arreola, R. et al. Immunomodulatory effects mediated by dopamine. J. Immunol. Res. 2016, 3160486 (2016).
Baik, J. H. Stress and the dopaminergic reward system. Exp. Mol. Med. 52, 1879–1890 (2020).
Bloomfield, M. A., McCutcheon, R. A., Kempton, M., Freeman, T. P. & Howes, O. The effects of psychosocial stress on dopaminergic function and the acute stress response. eLife 8, e46797 (2019).
Saha, B., Mondal, A. C., Basu, S. & Dasgupta, P. S. Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. Int. Immunopharmacol. 1, 1363–1374 (2001).
Figueroa, C. et al. Inhibition of dopamine receptor D3 signaling in dendritic cells increases antigen cross-presentation to CD8+ T-cells favoring anti-tumor immunity. J. Neuroimmunol. 303, 99–107 (2017).
Hoeppner, L. H. et al. Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells. Mol. Oncol. 9, 270–281 (2015).
Wu, J. et al. Dopamine inhibits the function of Gr-1+CD115+ myeloid-derived suppressor cells through D1-like receptors and enhances anti-tumor immunity. J. Leukoc. Biol. 97, 191–200 (2015).
Myers, S. A., Eriksson, N., Burow, R., Wang, S. C. & Muscat, G. E. β-Adrenergic signaling regulates NR4A nuclear receptor and metabolic gene expression in multiple tissues. Mol. Cell Endocrinol. 309, 101–108 (2009).
Liu, X. et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 567, 525–529 (2019).
Daher, C. et al. Blockade of β-adrenergic receptors improves CD8+ T-cell priming and cancer vaccine efficacy. Cancer Immunol. Res. 7, 1849–1863 (2019).
Nissen, M. D., Sloan, E. K. & Mattarollo, S. R. β-Adrenergic signaling impairs antitumor CD8+ T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol. Res. 6, 98–109 (2018).
Qiao, G. et al. β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. 68, 11–22 (2019).
Qin, J. F. et al. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 48, 295–300 (2015).
Sloan, E. K. et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 (2010).
Mohammadpour, H. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest. 129, 5537–5552 (2019).
Cheng, Y. et al. Depression-induced neuropeptide Y secretion promotes prostate cancer growth by recruiting myeloid cells. Clin. Cancer Res. 25, 2621–2632 (2019).
Kokolus, K. M. et al. β blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7, e1405205 (2018).
Bucsek, M. J. et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 77, 5639–5651 (2017).
Chen, M. et al. Adrenergic stress constrains the development of anti-tumor immunity and abscopal responses following local radiation. Nat. Commun. 11, 1821 (2020).
Botta, F. & Maestroni, G. J. Adrenergic modulation of dendritic cell cancer vaccine in a mouse model: role of dendritic cell maturation. J. Immunother. 31, 263–270 (2008).
Hiller, J. G. et al. Pre-operative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin. Cancer Res. 26, 1803–1811 (2019).
Shaashua, L. et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 23, 4651–4661 (2017).
De Giorgi, V. et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 4, e172908 (2018).
Phadke, S. & Clamon, G. β blockade as adjunctive breast cancer therapy: a review. Crit. Rev. Oncol. Hematol. 138, 173–177 (2019).
Zhang, S. et al. Neuroendocrine regulation of stress-induced T cell dysfunction during lung cancer immunosurveillance via the Kisspeptin/GPR54 signaling pathway. Adv. Sci. 9, e2104132 (2022).
Hill, M. N. et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl Acad. Sci. USA 107, 9406–9411 (2010).
Xiong, X. et al. Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal. Transduct. Target. Ther. 7, 99 (2022).
Won, E. & Kim, Y. K. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr. Neuropharmacol. 14, 665–673 (2016).
Gao, F. G., Wan da, F. & Gu, J. R. Ex vivo nicotine stimulation augments the efficacy of therapeutic bone marrow-derived dendritic cell vaccination. Clin. Cancer Res. 13, 3706–3712 (2007).
Dubeykovskaya, Z. et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat. Commun. 7, 10517 (2016).
Liu, L. et al. Hippocampal metabolic differences implicate distinctions between physical and psychological stress in four rat models of depression. Transl. Psychiatry 8, 4 (2018).
Czeh, B. et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl Acad. Sci. USA 98, 12796–12801 (2001).
Peckett, A. J., Wright, D. C. & Riddell, M. C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 60, 1500–1510 (2011).
Jia, H. M. et al. Chronic unpredictive mild stress leads to altered hepatic metabolic profile and gene expression. Sci. Rep. 6, 23441 (2016).
Rodrigues Mantuano, N. et al. Hyperglycemia enhances cancer immune evasion by inducing alternative macrophage polarization through increased O-GlcNAcylation. Cancer Immunol. Res. 8, 1262–1272 (2020). This study explores how hyperglycaemia drives tumour progression, by increasing O-GlcNAcylation in TAMs to switch on the M2-like phenotype and favour cancer immune evasion.
Brzozowski, B. et al. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain–gut axis. Curr. Neuropharmacol. 14, 892–900 (2016).
Patel, C. H., Leone, R. D., Horton, M. R. & Powell, J. D. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat. Rev. Drug. Discov. 18, 669–688 (2019).
Voss, K. et al. A guide to interrogating immunometabolism. Nat. Rev. Immunol. 21, 637–652 (2021).
Nonogaki, K. & Iguchi, A. Stress, acute hyperglycemia, and hyperlipidemia role of the autonomic nervous system and cytokines. Trends Endocrinol. Metab. 8, 192–197 (1997).
Maduka, I. C., Neboh, E. E. & Ufelle, S. A. The relationship between serum cortisol, adrenaline, blood glucose and lipid profile of undergraduate students under examination stress. Afr. Health Sci. 15, 131–136 (2015).
Mohammadpour, H., MacDonald, C. R., McCarthy, P. L., Abrams, S. I. & Repasky, E. A. β2-Adrenergic receptor signaling regulates metabolic pathways critical to myeloid-derived suppressor cell function within the TME. Cell Rep. 37, 109883 (2021).
Muthuswamy, R. et al. Epinephrine promotes COX-2-dependent immune suppression in myeloid cells and cancer tissues. Brain Behav. Immun. 62, 78–86 (2017).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Picard, M. & McEwen, B. S. Psychological stress and mitochondria: a systematic review. Psychosom. Med. 80, 141–153 (2018).
Qiao, G. et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol. Res. 9, 651–664 (2021).
Tokunaga, A. et al. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J. Exp. Med. 216, 2701–2713 (2019).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Perego, M. et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci. Transl. Med. 12, eaab5817 (2020).
Bresnick, A. R., Weber, D. J. & Zimmer, D. B. S100 proteins in cancer. Nat. Rev. Cancer 15, 96–109 (2015).
Diskin, C., Ryan, T. A. J. & O’Neill, L. A. J. Modification of proteins by metabolites in immunity. Immunity 54, 19–31 (2021).
Barth, E. et al. Glucose metabolism and catecholamines. Crit. Care Med. 35, S508–S518 (2007).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Chang, Y. H., Weng, C. L. & Lin, K. I. O-GlcNAcylation and its role in the immune system. J. Biomed. Sci. 27, 57 (2020).
Xiu, F., Stanojcic, M., Diao, L. & Jeschke, M. G. Stress hyperglycemia, insulin treatment, and innate immune cells. Int. J. Endocrinol. 2014, 486403 (2014).
Picard, M., Juster, R. P. & McEwen, B. S. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 10, 303–310 (2014).
Jiang, H. et al. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev. 118, 919–988 (2018).
Wild, A. R. et al. Exploring the expression patterns of palmitoylating and de-palmitoylating enzymes in the mouse brain using the curated RNA-seq database BrainPalmSeq. eLife 11, e75804 (2022).
Zareba-Koziol, M. et al. Stress-induced changes in the S-palmitoylation and S-nitrosylation of synaptic proteins. Mol. Cell Proteom. 18, 1916–1938 (2019).
Yao, H. et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng. 3, 306–317 (2019).
Cryan, J. F. et al. The microbiota–gut–brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Morais, L. H., Schreiber, H. L. T. & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255 (2021).
Mittal, R. et al. Neurotransmitters: the critical modulators regulating gut–brain axis. J. Cell Physiol. 232, 2359–2372 (2017).
Wu, M. et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 10, 350 (2020).
Jiang, H. et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194 (2015).
Jiang, H. Y. et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 104, 130–136 (2018).
Hollins, S. L. & Hodgson, D. M. Stress, microbiota, and immunity. Curr. Opin. Behav. Sci. 28, 66–71 (2019).
Gao, X. et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl Acad. Sci. USA 115, E2960–E2969 (2018).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Yu, L. X. & Schwabe, R. F. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat. Rev. Gastroenterol. Hepatol. 14, 527–539 (2017).
Lebeer, S., Vanderleyden, J. & De Keersmaecker, S. C. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8, 171–184 (2010).
Ochoa-Reparaz, J. et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185, 4101–4108 (2010).
Zitvogel, L., Ayyoub, M., Routy, B. & Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020). This study reports that naturally processed cancer antigens and microbial peptides may share cross-reactive T cell epitopes, which can be targeted for cancer immunotherapy.
Ruff, W. E. & Kriegel, M. A. Autoimmune host–microbiota interactions at barrier sites and beyond. Trends Mol. Med. 21, 233–244 (2015).
Ait-Belgnaoui, A. et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37, 1885–1895 (2012).
Ait-Belgnaoui, A. et al. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic–pituitary–adrenal axis modulation. J. Neurogastroenterol. Motil. 24, 138–146 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Merchak, A. & Gaultier, A. Microbial metabolites and immune regulation: new targets for major depressive disorder. Brain Behav. Immun. Health 9, 100169 (2020).
Sittipo, P., Choi, J., Lee, S. & Lee, Y. K. The function of gut microbiota in immune-related neurological disorders: a review. J. Neuroinflammation 19, 154 (2022).
Kim, C. H. Immune regulation by microbiome metabolites. Immunology 154, 220–229 (2018).
Zhao, L. et al. TGR5 deficiency activates antitumor immunity in non-small cell lung cancer via restraining M2 macrophage polarization. Acta Pharm. Sin. B 12, 787–800 (2022).
You, W. et al. Farnesoid X receptor constructs an immunosuppressive microenvironment and sensitizes FXRhighPD-L1low NSCLC to anti-PD-1 immunotherapy. Cancer Immunol. Res. 7, 990–1000 (2019).
Kenison, J. E. et al. The aryl hydrocarbon receptor suppresses immunity to oral squamous cell carcinoma through immune checkpoint regulation. Proc. Natl Acad. Sci. USA 118, e2012692118 (2021).
Bachem, A. et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297 e285 (2019). This study shows that microbiota-derived metabolites, SCFAs, can guide the metabolic rewiring that increases the memory potential of antigen-specific CD8+ T cells.
Botticelli, A. et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J. Transl. Med. 18, 49 (2020).
Nomura, M. et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw. Open. 3, e202895 (2020).
Schneider, M. A. et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci. Transl. Med. 13, eabc8188 (2021).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011).
Kienzl, M., Kargl, J. & Schicho, R. The immune endocannabinoid system of the tumor microenvironment. Int J. Mol. Sci. 21, 8929 (2020).
Huang, D. et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat. Cell Biol. 24, 230–241 (2022).
Zhang, B. et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature 599, 471–476 (2021).
Luqman, A., Nega, M., Nguyen, M. T., Ebner, P. & Gotz, F. SadA-expressing staphylococci in the human gut show increased cell adherence and internalization. Cell Rep. 22, 535–545 (2018).
Williams, B. B. et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16, 495–503 (2014).
Bauknecht, P. & Jekely, G. Ancient coexistence of norepinephrine, tyramine, and octopamine signaling in bilaterians. BMC Biol. 15, 6 (2017).
Connil, N. et al. Identification of the Enterococcus faecalis tyrosine decarboxylase operon involved in tyramine production. Appl. Env. Microbiol. 68, 3537–3544 (2002).
Lameris, T. W. et al. Catecholamine handling in the porcine heart: a microdialysis approach. Am. J. Physiol. 277, H1562–H1569 (1999).
Weis, W. I. & Kobilka, B. K. The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 87, 897–919 (2018).
Kepp, O., Marabelle, A., Zitvogel, L. & Kroemer, G. Oncolysis without viruses — inducing systemic anticancer immune responses with local therapies. Nat. Rev. Clin. Oncol. 17, 49–64 (2020).
Foster, J. A., Rinaman, L. & Cryan, J. F. Stress & the gut–brain axis: regulation by the microbiome. Neurobiol. Stress. 7, 124–136 (2017).
Vuong, H. E., Yano, J. M., Fung, T. C. & Hsiao, E. Y. The microbiome and host behavior. Annu. Rev. Neurosci. 40, 21–49 (2017).
Sidler, D. et al. Colon cancer cells produce immunoregulatory glucocorticoids. Oncoimmunology 1, 529–530 (2012).
Verhoeven, G. T. et al. Glucocorticoids hamper the ex vivo maturation of lung dendritic cells from their low autofluorescent precursors in the human bronchoalveolar lavage: decreases in allostimulatory capacity and expression of CD80 and CD86. Clin. Exp. Immunol. 122, 232–240 (2000).
Rea, D. et al. Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood 95, 3162–3167 (2000).
Kim, K. D., Choe, Y. K., Choe, I. S. & Lim, J. S. Inhibition of glucocorticoid-mediated, caspase-independent dendritic cell death by CD40 activation. J. Leukoc. Biol. 69, 426–434 (2001).
Herold, M. J., McPherson, K. G. & Reichardt, H. M. Glucocorticoids in T cell apoptosis and function. Cell Mol. Life Sci. 63, 60–72 (2006).
Talaber, G. et al. Mitochondrial translocation of the glucocorticoid receptor in double-positive thymocytes correlates with their sensitivity to glucocorticoid-induced apoptosis. Int. Immunol. 21, 1269–1276 (2009).
Lowenberg, M. et al. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood 106, 1703–1710 (2005).
Wu, Y. et al. The disbalance of LRP1 and SIRPα by psychological stress dampens the clearance of tumor cells by macrophages. Acta Pharm. Sin. B 12, 197–209 (2022).
Xie, Y. et al. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis. Model. Mech. 12, dmm037887 (2019).
Lu, Y. et al. Glucocorticoid receptor promotes the function of myeloid-derived suppressor cells by suppressing HIF1α-dependent glycolysis. Cell Mol. Immunol. 15, 618–629 (2018).
Xiang, Z. et al. Dexamethasone suppresses immune evasion by inducing GR/STAT3 mediated downregulation of PD-L1 and IDO1 pathways. Oncogene 40, 5002–5012 (2021).
Eddy, J. L., Krukowski, K., Janusek, L. & Mathews, H. L. Glucocorticoids regulate natural killer cell function epigenetically. Cell Immunol. 290, 120–130 (2014).
Quatrini, L. et al. Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat. Immunol. 19, 954–962 (2018).
Cavalcanti, D. M. et al. Endogenous glucocorticoids control neutrophil mobilization from bone marrow to blood and tissues in non-inflammatory conditions. Br. J. Pharmacol. 152, 1291–1300 (2007).
Nadkarni, S. et al. Investigational analysis reveals a potential role for neutrophils in giant-cell arteritis disease progression. Circ. Res. 114, 242–248 (2014).
Walther, A., Riehemann, K. & Gerke, V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol. Cell 5, 831–840 (2000).
Saffar, A. S., Ashdown, H. & Gounni, A. S. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr. Drug. Targets 12, 556–562 (2011).
Staedtke, V. et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature 564, 273–277 (2018).
Cao, M. et al. Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through β-adrenergic-activated CXCL5–CXCR2–Erk signaling cascades. Int. J. Cancer 149, 460–472 (2021).
Karvonen, H. et al. Glucocorticoids induce differentiation and chemoresistance in ovarian cancer by promoting ROR1-mediated stemness. Cell Death Dis. 11, 790 (2020).
Hara, M. R. et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 477, 349–353 (2011).
Sood, A. K. et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J. Clin. Invest. 120, 1515–1523 (2010).
Dwyer, A. R. et al. Glucocorticoid receptors drive breast cancer cell migration and metabolic reprogramming via PDK4. Endocrinology 164, bqad083 (2023).
Karra, A. G. et al. Increased expression of the mitochondrial glucocorticoid receptor enhances tumor aggressiveness in a mouse xenograft model. Int. J. Mol. Sci. 24, 3740 (2023).
Tiwari, R. K. et al. Epinephrine facilitates the growth of T cell lymphoma by altering cell proliferation, apoptosis, and glucose metabolism. Chem. Biol. Interact. 369, 110278 (2023).
Talaber, G., Jondal, M. & Okret, S. Local glucocorticoid production in the thymus. Steroids 103, 58–63 (2015).
Croft, A. P. et al. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res. 1238, 12–22 (2008).
Cima, I. et al. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 200, 1635–1646 (2004).
Nagashima, H. et al. Neuropeptide CGRP limits group 2 innate lymphoid cell responses and constrains type 2 inflammation. Immunity 51, 682–695.e6 (2019).
Reigstad, C. S. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403 (2015).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015). This study highlights that certain bacterial species can act as important modulators of host 5-HT biosynthesis and its downstream bioactivities.
Finocchiaro, L. M. et al. Serotonin and melatonin synthesis in peripheral blood mononuclear cells: stimulation by interferon-γ as part of an immunomodulatory pathway. J. Interferon Res. 8, 705–716 (1988).
Kushnir-Sukhov, N. M., Brown, J. M., Wu, Y., Kirshenbaum, A. & Metcalfe, D. D. Human mast cells are capable of serotonin synthesis and release. J. Allergy Clin. Immunol. 119, 498–499 (2007).
Leon-Ponte, M., Ahern, G. P. & O’Connell, P. J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109, 3139–3146 (2007).
Brenner, B. et al. Plasma serotonin levels and the platelet serotonin transporter. J. Neurochem. 102, 206–215 (2007).
Cox, M. A. et al. Beyond neurotransmission: acetylcholine in immunity and inflammation. J. Intern. Med. 287, 120–133 (2019).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Kiecolt-Glaser, J. K. et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J. Clin. Oncol. 32, 1040–1049 (2014).
Stagl, J. M. et al. A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast Cancer Res. Treat. 154, 319–328 (2015).
Teo, I., Krishnan, A. & Lee, G. L. Psychosocial interventions for advanced cancer patients: a systematic review. Psychooncology 28, 1394–1407 (2019).
Dethlefsen, C. et al. Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 77, 4894–4904 (2017).
Huang, C. W. et al. Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. FASEB J. 34, 9678–9693 (2020).
Sarkar, D. K., Murugan, S., Zhang, C. & Boyadjieva, N. Regulation of cancer progression by β-endorphin neuron. Cancer Res. 72, 836–840 (2012).
Harber, V. J. & Sutton, J. R. Endorphins and exercise. Sports Med. 1, 154–171 (1984).
Hojman, P. et al. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Endocrinol. Metab. 301, E504–E510 (2011).
Hamy, A. S. et al. Comedications influence immune infiltration and pathological response to neoadjuvant chemotherapy in breast cancer. Oncoimmunology 9, 1677427 (2020).
Acknowledgements
Y.M. is supported by Science and Technology Innovation 2030 Major Project (STI2030-Major Projects 2022ZD0205700), Natural Science Foundation of China (NSFC; grant No. 81972701), CAMS Innovation Fund for Medical Sciences (CIFMS; 2021-I2M-1-074, 2022-I2M-2-004), National special support plan for high-level talents, Suzhou Municipal Key Laboratory (SZS2023005) and Innovative and Entrepreneurial Team Program (Jiangsu Province). G.K. is supported by the Ligue contre le Cancer (équipe labellisée), Agence National de la Recherche (ANR)–Projets blancs, AMMICa US23/CNRS UMS3655, Association pour la recherche sur le cancer (ARC), Cancéropôle Ile-de-France, Fondation pour la Recherche Médicale (FRM), Equipex Onco-Pheno-Screen, European Joint Programme on Rare Diseases, the European Union Horizon 2020 Projects Oncobiome and Crimson, Institut National du Cancer (INCa), Institut Universitaire de France, LabEx Immuno-Oncology (ANR-18-IDEX-0001), High-end Foreign Expert Program in China (GDW20171100085), the RHU Immunolife, Seerave Foundation, SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE) and SIRIC Cancer Research and Personalized Medicine (CARPEM).
Author information
Authors and Affiliations
Contributions
All authors researched data for this Review. All authors contributed substantially to discussion of the content. Y.M. wrote and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Guillermo Armaiz-Pena, Hubert Hondermark and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Kroemer, G. The cancer-immune dialogue in the context of stress. Nat Rev Immunol 24, 264–281 (2024). https://doi.org/10.1038/s41577-023-00949-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00949-8