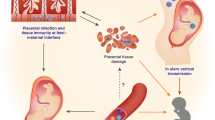
Abstract
The role of the maternal immune system in reproductive success in humans remains controversial. Here we focus on the events that occur in the maternal decidua during the first few weeks of human pregnancy, because this is the site at which maternal leukocytes initially interact with and can recognize fetal trophoblast cells, potentially involving allorecognition by both T cells and natural killer (NK) cells. NK cells are the dominant leukocyte population in first-trimester decidua, and genetic studies point to a role of allorecognition by uterine NK cells in establishing a boundary between the mother and the fetus. By contrast, definitive evidence that allorecognition by decidual T cells occurs during the first trimester is lacking. Thus, our view is that during the crucial period when the placenta is established, damaging T cell-mediated adaptive immune responses towards placental trophoblast are minimized, whereas NK cell allorecognition contributes to successful implantation and healthy pregnancy.
Similar content being viewed by others
Introduction
The functional relationship formed through the close apposition of the fetal placenta and the maternal uterus is essential for successful pregnancy. Following on from his studies of skin grafts, Peter Medawar (1915–1987) pointed out that pregnancy involves two genetically distinct individuals coexisting throughout gestation1, which later led to the emergence of the concept that maternal T cell tolerance must be essential for successful mammalian pregnancy. This view of pregnancy as being ‘Nature’s transplant’ has had a marked influence on studies investigating how the maternal immune system recognizes and responds to the fetus and placenta in pregnancy2. Much of the research focus so far has been on genetic differences, particularly of major histocompatibility complex (MHC) genes, between the mother and her fetus. Multiple mechanisms have been discovered that help to avoid potentially damaging allorecognition responses involving maternal T cells during pregnancy. By contrast, there is now ample evidence that allorecognition by natural killer (NK) cells that are unique to the uterus does occur, although the mechanisms by which this affects reproductive outcomes are still being investigated.
Here we present an overview of maternal immune cells at the placental–uterine interface in early human pregnancy, discuss the controversies and outstanding questions in the field, and make suggestions as to how these issues might be resolved. Many of the major clinical problems in human pregnancy, such as pre-eclampsia, although classically presenting in the third trimester, have their origins in the first trimester when maternal–fetal interactions determine placental development and access to the maternal blood supply3,4. Thus, we focus exclusively here on the dialogue between the human uterine immune system and trophoblast early in gestation. Some studies of decidua in the second and third trimesters have failed to separate uterine leukocytes from maternal or fetal blood leukocytes and thus do not provide definitive evidence of local interactions in this tissue microenvironment. Furthermore, it is hard to distinguish cause from effect for systemic immune changes observed during pregnancy complications at term. We therefore think it is important to separately discuss events that occur early in pregnancy at the tissue site of maternal–fetal interactions, with a focus on human data rather than animal models.
The human placental–uterine interface
The immunology of human pregnancy needs to be viewed in light of the anatomy of placental development3. Apart from the great apes, no other species uses a similar strategy for pregnancy, particularly with respect to the extent of placental invasion into the uterus, arterial transformation by trophoblast and the differentiation of non-pregnant endometrium to decidua before implantation occurs.
Development of the human placenta
Trophoblast cells are the earliest extra-embryonic cells to differentiate in the mammalian embryo, and they form the interface between the fetus and the mother throughout pregnancy. First, the conceptus embeds into the endometrium of the uterus5, and then villous cytotrophoblast cells rapidly proliferate to form branching villi covered by a multinucleated syncytiotrophoblast (SCT) layer that is the site of nutrient and oxygen exchange with maternal blood. Extravillous trophoblast (EVT) cells arise from the villi attached to the decidua and invade towards the uterine spiral arteries. EVT transforms these normally thick walled arteries into low-resistance vessels capable of high conductance. Thus, following implantation, there are two main sites of contact between maternal leukocytes and fetal trophoblast cells: all maternal decidual cells, in particular stromal cells and immune cells, are in contact with EVT, whereas maternal blood in the intervillous space flows over the SCT layer. One source of confusion in reproductive immunology is the failure to distinguish between local uterine responses and systemic immune responses (Box 1). A third site of contact between fetal trophoblast cells and maternal immune cells in the decidua parietalis is not present until after the first trimester when the uterine cavity is obliterated, so is of less relevance to the early maternal–fetal interactions that determine placental development. In addition, the fetus itself is never in contact with maternal blood or decidua, with the placenta always functioning as a barrier between the two individuals.
Role of the decidua in placentation
The human uterine endometrium begins to differentiate into decidua before implantation, during the secretory phase of the menstrual cycle, in response to progesterone secreted following ovulation. This spontaneous decidualization necessitates the cyclical menstrual shedding and renewal that occur only in simian primates6. All elements of the uterine mucosa (blood vessels and epithelial, stromal and immune cells) undergo marked changes during decidualization, including the proliferation of a specialized population of uterine NK (uNK) cells. Decidualization and uNK cells are seen only in species such as humans, rats, mice and non-human primates that have forms of haemochorial placentation whereby uterine invasion by trophoblast occurs7,8. Furthermore, in these species, the depth of trophoblast penetration correlates with the extent of decidualization, with both of these being greatest in humans. From the findings taken together, it is clear that successful pregnancy requires a mutually beneficial dialogue between the decidua and the placenta, involving trophoblast invasion that seems to be modified in some way by the decidua (Fig. 1).
Successful pregnancy requires that the boundary between placental trophoblast cells and uterine tissues be formed in the correct place. In normal pregnancy (centre panel), to establish optimal blood flow to the fetoplacental unit, extravillous trophoblast (EVT) arises from the placental villi attached to the decidua and invades the decidua (as interstitial EVT), eventually reaching and destroying the media of maternal spiral arteries. EVT with a spidery appearance becomes embedded in the fibrinoid material that replaces the media of these arteries. Endovascular trophoblast then moves down the spiral arteries, partially replacing the endothelium. EVT thus transforms these normally thick-walled arteries into low-resistance vessels capable of high conductance. If the invasion of the decidua by trophoblast is defective (left panel), part of the deeper portions of the maternal spiral arteries remain thick-walled, leading to turbulent blood flow into the intervillous space. As a result, the villous placenta develops abnormally and eventually becomes stressed and dysfunctional. This is associated with pregnancy disorders such as pre-eclampsia and fetal growth restriction. If the trophoblast cells invade maternal tissue too deeply (right panel), which usually occurs where decidua is absent at the site of a previous caesarean section, the fetal–maternal boundary is formed beyond the decidua and into the myometrium or beyond. Interstitial EVT invades deeply into the myometrium, with no formation of placental bed giant cells. These conditions, termed ‘placenta accreta syndrome’, have a high risk of maternal and fetal morbidity.
The effects of disrupting this dialogue can be seen from pathological pregnancies in humans. When a blastocyst implants at a site in the uterus where decidua is absent, typically on a caesarean section scar, excessive trophoblast invasion occurs, even penetrating through to the peritoneum in some cases (Fig. 1). Limited infiltration of EVT is also problematic as the transformation of maternal arteries mediated by trophoblast is essential to increase and maintain fetoplacental blood flow until the end of pregnancy9,10. Inadequate transformation of the uterine spiral arteries occurs in a range of disorders, including pre-eclampsia, preterm labour, fetal growth restriction and unexplained stillbirth (Fig. 1). These conditions, known collectively as the great obstetric syndromes, affect at least 10% of first pregnancies, and all have their origins in the first trimester4,11,12. Decidua is thus the tissue site where the boundary is formed between two genetically distinct individuals, and if the boundary is drawn in the correct place, then the outcome is successful for both the mother and the baby. A central question therefore is how do the decidua and placenta recognize and cooperate with each other to establish this successful boundary?
A role for the immune system in regulating placentation first emerged from studies of the epidemiology of pre-eclampsia, a disorder that is unique to humans. The syndrome arises from maternal endothelial cell dysfunction triggered by factors released by SCT that becomes stressed as a result of disordered uterine blood flow into the intervillous space12,13. Pre-eclampsia occurs more commonly in first pregnancies than in subsequent pregnancies14, which suggests the involvement of some kind of memory response. Furthermore, there is a clear genetic contribution to the risk of pre-eclampsia associated with particular fathers15,16, although the interbirth interval is a confounding effect17. Because memory and specificity are both characteristics of adaptive immune responses, this gave rise to the idea that immune ‘maladaptation’ is the underlying cause of pre-eclampsia18. However, mechanistic explanations for these epidemiological findings in pregnancy are lacking. A focus on the decidual leukocytes and immune responses that are present early in pregnancy when EVT invades the spiral arteries has provided some answers.
The early decidual immune response
The leukocyte population in endometrium and decidua in the first few weeks of human pregnancy is dominated by distinctive NK cells specific to this location. In addition, despite the intrusive nature of human placentation deep into the uterine mucosa, an inflammatory response is not seen.
Decidual leukocytes
The composition of leukocytes changes in the endometrium throughout the menstrual cycle and in the decidua throughout gestation19,20,21,22,23,24,25. Following ovulation in the secretory phase of the menstrual cycle, ~20% of CD45+ cells in the non-pregnant endometrium are macrophages and ~10% are T cells. Only 1% of CD45+ cells are dendritic cells, and sparse B cells are present in basal lymphoid aggregates. The main population of CD45+ cells in endometrium and early decidua are specialized CD56bright uNK cells, which account for ~70% of leukocytes21. By contrast, at term, uNK cells account for less than 50% of leukocytes in the endometrium, with the number of T cells having increased proportionally26,27. Thus, during early pregnancy when the boundary between the decidua and the placenta forms, uNK cells and macrophages, rather than adaptive immune cells, dominate the decidual immune landscape, although their relative importance seems to decline as gestation proceeds. A recent detailed study by mass cytometry described the decidual leukocytes that are present throughout gestation, excluding blood leukocytes26, which is particularly important when one is interpreting studies of cell isolates from term decidua, as contaminating maternal and fetal blood leukocytes will inevitably be present. In addition, samples taken after delivery are affected by the inflammatory events characteristic of labour and birth. By contrast, there is little evidence that a classic inflammatory response occurs early in pregnancy during placentation despite the large numbers of innate immune cells.
Is there an inflammatory response in early decidua?
Breaching a mucosal barrier, as occurs during implantation and trophoblast invasion of the decidua, would be predicted to activate an inflammatory response. The endometrium is receptive to implantation during a window of only 3–6 days in the mid-secretory phase, and a common view is that the receptive endometrium is an inflammatory environment28,29. However, mast cells, which are a key trigger of inflammatory responses, are not present in the functional layer of the endometrium or decidua, although they do populate the myometrium30. Despite some reports to the contrary, neutrophils, which would be the hallmark of an acute inflammatory response and are easily and definitively identified by histology alone31,32, are present only when the endometrium breaks down at menstruation and miscarriage33,34. In decidua, neutrophils are confined to the narrow zone of necrosis — Nitabuch’s layer — at the boundary between the anchoring villi and superficial decidua basalis. Macrophages are constantly present in the endometrium and decidua, and a recent report using imaging mass cytometry has defined several subsets of macrophages (including an HLA-DR-negative subset present in first-trimester decidua basalis) and their distribution throughout gestation35.
Features of granulation tissue that characterize the progression from inflammation to wound healing — angiogenesis, influx of macrophages and other inflammatory cells, and deposition of collagen by fibroblasts — are also not seen during pregnancy, and the total loss of the uterine mucosa each month during menstruation does not result in fibrosis unless the basal layer is lost. Indeed, the wound healing response is suppressed in murine decidua by the histone methyltransferase EZH2 (ref.36). The classical angiogenesis response typical of granulation tissue in wound healing is also not a feature of human decidua. Angiogenesis is responsible for the increased uterine blood flow to the placenta in species with epitheliochorial placentation, where the uterine epithelium remains intact (large domestic animals and pro-simian primates)37. By contrast, during early pregnancy in humans, increased blood flow is achieved mainly by trophoblast-mediated arterial transformation, involving destruction of the media by interstitial EVT and, subsequently, endothelial replacement by endovascular EVT8. In vitro assays that have assessed the functions of uNK cells using angiogenesis as a readout do not reflect this in vivo situation38,39. Similarly, the change in the spiral arteries during the non-pregnant secretory phase in humans occurs by non-sprouting angiogenesis40, which is unlike the angiogenesis seen in wound healing.
Furthermore, clinical trials of a procedure used in assisted reproduction to enhance local inflammation, whereby the endometrium is ‘scratched’ in the mid-luteal phase — before implantation but when the endometrium has already begun to differentiate into decidua —have shown no beneficial effects with regard to embryo implantation rates41. All of these features indicate that inflammation is not required for successful implantation.
So, what exactly has been meant by ‘inflammatory’ in the context of the uterine endometrium and decidua42? Perhaps the difficulty here is semantic. Genes that are characteristically expressed by a receptive endometrium have been described as ‘inflammatory’ in other environments, including those encoding IL-15, granulysin, granzymes and IL-6 receptor. In the uterus, however, these gene products function as part of a physiological process involving the active proliferation of NK cells during the secretory phase. Numerous anti-inflammatory factors are present during this phase, including IL-17A, which inhibits neutrophil recruitment by decidual stromal cells, abundant prostaglandin E2 (PGE2) and transforming growth factor-β (TGFβ) derived from EVT, and annexin A1 (ANXA1), which is expressed by a subset of uNK cells (uNK2 cells)43,44,45. Indeed, a compelling argument has been made that modification of the inflammatory response elicited by trophoblast invasion was essential for placental evolution in eutherian mammals46.
Inflammation is present in decidual tissue examined after a spontaneous miscarriage. However, this is an effect rather than a cause of the failing pregnancy because the decidua is usually not shed until several days after the loss of the fetal heartbeat. The changes that occur after miscarriage resemble the influx of neutrophils and macrophages seen at menstruation that restore the mucosa. Therefore, comparisons of gene expression data from decidua from spontaneous miscarriages compared with therapeutic surgical terminations of pregnancy are uninformative, as the changes in miscarriage samples reflect only these secondary inflammatory events and not the underlying pathogenesis47,48,49.
So, given the dominance of uNK cells in first trimester but the lack of a classical inflammatory immune response, what is the role of these cells in recognizing and responding to fetal trophoblast cells and potentially other maternal cells in the uterus, and are they involved in responding to infection (Box 2)?
Uterine NK cells
Uterine lymphoid cells with distinctive granules were first described early in the twentieth century50 and are now recognized as members of the innate lymphoid cell (ILC) family51. Uterine ILCs are dominated by a population closely resembling ILC1s — the CD56bright NK cells — that are unique to the uterine mucosa, being hormonally regulated and proliferating in response to progesterone secreted by the corpus luteum, which induces IL-15 production by stromal cells52,53,54. uNK cells differentiate within the uterine microenvironment throughout the menstrual cycle, sequentially acquiring expression of killer cell immunoglobulin-like receptors (KIRs), LILRB1 and NKG2C, which regulate NK cell function55. A small population of decidual ILC3s (CD127+CD117+) is also present, but no ILC2s are present56,57.
uNK cells are CD56brightCD94+NKG2A+CD16−CD57− cells that express the tissue residence markers CD49a, CD9 and CD69. They have only few, but large, cytoplasmic granules (compared with peripheral blood NK cells), which might have a role in cytokine production as uNK cells in the first trimester are poorly cytolytic owing to an inability to polarize the microtubule-organizing centre58,59,60. Single-cell transcriptomic and mass cytometry studies have defined three main subsets of uNK cells: uNK1, uNK2 and uNK3 cells (with an additional cycling population)43,59. The major group of uNK1 cells express NK receptors for HLA class I ligands present on EVT — namely, KIRs (receptors for HLA-C), LILRB1 (receptor for HLA-G, which is specific to EVT), CD94–NKG2A (inhibitory receptor for HLA-E) and CD94–NKG2C (activating receptor for HLA-E) (Fig. 2). They are large cells defined by expression of eomesodermin and CD39. Higher levels of uNK1 cells are found in the decidua of women who have had more than one pregnancy61, with a possible confounding effect of cytomegalovirus infection status62. Perhaps uNK1 cells are ‘trained’ by a first pregnancy and thereafter expand more rapidly in subsequent pregnancies, which might partially explain the increased frequency of pre-eclampsia and lower birthweight that are observed in first pregnancies14,63. uNK2 cells express anti-inflammatory ANXA1 and ITGB2. The minor population of CD103+T-bet+ uNK3 cells closely resemble intraepithelial ILC1s and express CCL5, KLRB1 and TIGIT (which bind CCR1, CLEC2D and PVR, respectively, all of which are expressed by EVT). uNK3 cells are small and agranular, with uNK2 cells lying between uNK1 and uNK3 subsets in terms of their size and granularity. Further details regarding the functional and phenotypic characteristics of endometrial and decidual uNK cell subsets have recently been published59,64. The uNK1 and uNK2 subsets dominate early in pregnancy, but by term, most cells of the remaining uNK cell population are uNK3 cells64.
Extravillous trophoblast (EVT) does not express HLA class II molecules or the HLA class I molecules HLA-A and HLA-B. Different combinations of the highly polymorphic maternal killer cell immunoglobulin-like receptors (KIRs; both activating and inhibitory) expressed by uterine natural killer (uNK) cells and fetal HLA-C variants expressed by EVT are associated with disorders of placentation, and result in altered secretion of cytokines and chemokines by EVT. The effect of HLA-E expressed by EVT engaging inhibitory CD94–NKG2A or activating CD94–NKG2C receptors on uNK cells is not known. LILRB1, which is expressed by both uNK cells (mainly the uNK1 subset) and maternal decidual antigen-presenting cells (APCs), interacts with a dimer of HLA-G molecules. This deviates APCs towards a tolerogenic phenotype. Potential pathways for the indirect T cell-mediated recognition of paternal HLA-C or other alloantigens expressed by EVT are shown. However, there is no evidence that activation of decidual T cells occurs, and there are many mechanisms in the decidua to maintain T cell tolerance, as described in the main text and in Box 3. TCR, T cell receptor.
Heterogeneity between uNK cell subsets associated with their location, menstrual phase and stage of gestation is likely. Whether uNK cells are derived from resident or circulating progenitors has been partially resolved by studies of uterine transplants, in which uNK cells are derived from the recipient and not the donor, suggesting a source of circulating progenitors55. However, further work is needed as both mouse and human studies suggest that tissue-resident progenitors may also contribute to the uNK cell population65.
Allorecognition of trophoblast by uNK cells
Recognition of allogeneic cells depends on the detection of highly diverse HLA molecules by T cells or NK cells. Owing to the extreme polymorphism of HLA genes, the fetus usually inherits different variants from each parent. There is general agreement about the expression of HLA molecules by trophoblast66. HLA class II molecules are not expressed by trophoblast even after exposure to interferon-γ. Villous cytotrophoblast and SCT also express no HLA class I allotypes. By contrast, EVT, which is in direct contact with all maternal decidual cells, including immune cells, expresses the HLA class I molecules HLA-C, HLA-E and HLA-G, but not class I HLA-A or HLA-B molecules, which are the major T cell receptor ligands. HLA-E and HLA-G are non-classical class I molecules with only minimal polymorphism, so the only major polymorphic classical HLA alloantigen on EVT that could potentially be recognized by uterine T cells and uNK cells is paternally derived HLA-C (Fig. 2).
The effector function of NK cells depends on a balance between the signals received by activating and inhibitory receptors67. Normally, the functional inhibition of NK cells is mediated either by CD94–NKG2A binding to HLA-E or by inhibitory members of the diverse KIR family that bind to HLA class I molecules68. Activating receptors — CD94–NKG2C, activating KIRs and other NK receptors (such as NKG2D) — override this inhibition when their ligands are present on target cells, and this is likely to be affected by the peptide bound to the HLA class I molecule. KIRs bind to four major HLA epitopes: two groups of HLA-C allotypes (C1+ and C2+), HLA-Bw4 and HLA-A11 (ref.68). Thus, given the absence of HLA-A and HLA-B on trophoblast, KIRs expressed by uNK cells can recognize only the HLA-C allotypes expressed by EVT.
Evidence from multiple functional and genetic studies supports the view that uNK cells can recognize and respond to EVT through KIR–HLA-C interactions: higher proportions of uNK cells express KIRs for HLA-C compared with peripheral blood NK cells in the first trimester; KIR tetramers bind to HLA-C molecules expressed by trophoblast, and HLA-C tetramers specifically bind to uNK cells; and the activation of KIRs on uNK cells results in the secretion of cytokines (GM-CSF and XCL1) whose receptors are expressed by EVT and which seem to affect EVT invasion69,70,71,72,73,74. In addition, genetic studies in the UK and Uganda of pregnancy disorders arising from defective placentation, such as pre-eclampsia, show that specific combinations of maternal KIR and fetal HLA-C variants are reproducibly found in women experiencing pre-eclampsia — in particular, two copies of the strongly inhibitory KIR2DL1 (found on the KIR A haplotype) together with the presence of C2+ HLA-C in the fetus75,76,77 (Fig. 3). Conversely, protection from pre-eclampsia and higher birthweights are associated with the presence of KIR2DS1 (found on the KIR B haplotype), which is an activating receptor for C2+ HLA-C72,78,79. Thus, it seems that this NK cell allorecognition system has a physiological role in striking a trade-off between the fetal developmental requirements of adequate nutrition and oxygenation and the mother’s need to remain healthy to nurture her child, survive and reproduce again.
Maternal killer cell immunoglobulin-like receptor (KIR) AA haplotypes, which encode two copies of KIR2DL1 (a potent inhibitory receptor for C2+ HLA-C epitopes), result in strong inhibition of uterine natural killer (uNK) cells when C2+ HLA-C is inherited paternally and expressed on extravillous trophoblast (EVT). This is associated with low birthweight, increased risk of pre-eclampsia and recurrent miscarriage, probably secondary to reduced remodelling of the maternal vasculature and poor placentation. The presence of the activating KIR KIR2DS1, encoded in women with KIR AB or BB haplotypes, in combination with paternal C2+ HLA-C results in less uNK cell inhibition and increased secretion of cytokines such as CCL4, XCL1 and GM-CSF. This is associated with a greater frequency of large for gestational age infants. Increased birthweight is associated with maternal and neonatal complications, including dysfunctional labour, shoulder dystocia, maternal trauma and post-partum haemorrhage. Human birthweight is thus an example of balancing selection partially dependent on KIR and HLA gene families69.
How do uNK cells affect EVT?
uNK cells are usually thought to function by regulating EVT invasion, but it remains unclear how this might be mediated38,39,80,81,82,83,84, with results in vitro seeming to depend on the particular invasion assays and trophoblast cells that are used85. Indeed, a fundamental unanswered question is whether uNK cells facilitate or impede trophoblast invasion. Although EVT needs to transform the maternal arteries for successful pregnancy, this process must also be regulated to avoid the risk of excessive invasion. Most trophoblast invasion assays have not considered how the genetic studies relating to maternal KIR–fetal HLA-C combinations might translate into the functional effects that uNK cells exert on EVT as they move through the decidua84. It is also unknown how EVT phenotype and functions change as these cells move deeper into the decidua, or how EVT cells stop invading and fuse to become placental bed giant cells in the myometrium. To study this will require samples taken from pregnant hysterectomies, which are rare operations in early pregnancy.
Other aspects relating to interactions between NK receptors and trophoblast HLA molecules also remain unresolved. KIR2DL1, KIR2DL2, KIR2DL3 and KIR2DS1 all bind to trophoblast HLA-C allotypes, but it is still controversial whether KIR2DL4 recognizes trophoblast-specific HLA-G. KIR2DL4 is expressed in late endosomes in peripheral blood NK cells (although this has not yet been demonstrated for uNK cells), and its triggering by a soluble HLA-G construct results in upregulated expression of cytokines86. However, the crystal structure of KIR2DL4 suggests that binding to HLA class I molecules is precluded, and no direct binding to HLA-G was shown by surface plasmon resonance87. In addition, there is no known association of any KIR2DL4 alleles with the risk of pre-eclampsia88. By contrast, there is agreement that HLA-G dimers bind the inhibitory receptor LILRB1, which is expressed by uNK1 cells as well as myeloid cells89,90,91. Whether LILRB1 might also function as an activating receptor for peripheral blood NK cells or uNK cells in some contexts is controversial, with contradictory reports even from the same group38,61,92,93,94.
HLA-E expressed by EVT is the ligand for the inhibitory receptor CD94–NKG2A, which is expressed at high levels by almost all uNK cells (like other tissue NK cells) and has a role in NK cell education and pregnancy success95,96. An unusual feature of uNK1 cells is that they co-express the activating receptor NKG2C, which also recognizes HLA-E39,55,61,97. The leader sequence of HLA-G, which binds to and is presented by HLA-E, has higher affinity for both NKG2A and NKG2C than peptides derived from any other HLA class I molecules98,99. This means that uNK cells receive different signals from infiltrating EVT cells (which express HLA-G) compared with surrounding maternal cells (which do not express HLA-G), which has unknown functional consequences.
Another important issue in attempting to elucidate the functions of uNK cells is that the assays traditionally used to study peripheral blood NK cells involve cytotoxicity or interferon-γ production, which are not features of most freshly isolated human uNK cells. Preactivation with IL-2 or IL-15 increases the cytotoxicity of freshly isolated uNK cells52,58,100,101 but without preactivation, interferon-γ is made only by minor uNK cell subsets and not by the main populations of uNK1 or uNK2 cells38,43,59,61. Furthermore, secreted products that have been attributed to uNK cells in some studies may in fact be mostly derived from other decidual cells, particularly macrophages and stromal cells, that are invariably present in uNK cell isolates. For example, VEGFA and SPP1, which were previously thought to be produced by uNK cells61,93, have been shown in more recent single-cell analyses to be produced by macrophages43. Similarly, pleiotrophin and osteoglycin are secreted by stromal cells43,93. The cytokines and chemokines that are produced by the main uNK cell populations in vivo, whose receptors are expressed by EVT cells, include GM-CSF, CSF1, CCL4 and XCL1 (refs.43,59,72,73,102,103). However, it has not been rigorously studied how these factors affect trophoblast function, and few functional studies have genotyped uNK cells for KIR expression. Experiments are required that reflect the normal in utero environment and can systematically determine how responses generated by specific uNK cell receptors regulate trophoblast cells.
Do uNK cells affect other uterine maternal cells?
In addition to their interactions with fetal trophoblast cells, uNK cells could also have effects on other maternal cells in the decidua, including maternal immune cells such as macrophages and arterial, glandular or stromal cells. Conversely, other uterine maternal cells might affect the functions of uNK cells, although very little is known about this possibility. A controversial view is that uNK cells can eliminate decidual stromal cells104. A subset of senescent pro-inflammatory stromal cells is said to be normally removed by uNK cells105, although standard NK cell cytotoxicity assays were not used in that study and the uNK cells were preactivated with IL-15, which increases the otherwise low levels of cytotoxicity that are observed in the first trimester106. Another difficulty is that the markers used to define senescence in vitro (β-galactosidase and CDKN2) are not specific to this process107,108. A possible explanation is that these ‘senescent’ cells were identified after culturing of stromal cells in vitro with the standard decidualization cocktail of progesterone and cAMP, which induces stromal cells that express higher levels of senescence-associated genes than those seen with a more physiological stimulus of progesterone and PGE2 (ref.109). In vivo, stromal cells become non-proliferative, terminally differentiated, large cells in the superficial decidua and secrete abundant prolactin and IGFBP1, but not CDKN2A43. Furthermore, as both uNK cells and stromal cells are routinely shed at menstruation, there would not seem to be a rationale for uNK cells being required to remove stromal cells in vivo.
If pregnancy does not occur during the postovulatory secretory phase, the first morphological sign that menstruation rather than further decidualization will occur is the nuclear fragmentation of uNK cells, which precedes mucosal breakdown110,111. This feature is also seen as the first sign of a failed pregnancy in the first trimester, but never as a feature of a continuing pregnancy. It is, therefore, possible that uNK cells might prevent breakdown of the uterine mucosa by secreting factors that stabilize the maternal spiral arteries, although there is no clear evidence of this as yet.
Uterine T cells
The tolerance of decidual T cells to fetal alloantigens (especially HLA-C allotypes) expressed by EVT is clearly required for a successful pregnancy. The uterine mucosa is not a privileged site, as can be seen from the adaptive T cell and B cell responses that are made in response to infecting organisms. For example, abundant plasma cells are present in endometritis, and granulomas are seen in systemic tuberculosis112,113. Furthermore, HLA-C-restricted T cells have been described in clinical situations including cytomegalovirus infection, kidney allografts and tumours114. Here we discuss how decidual T cells might recognize trophoblast and how such allorecognition is prevented by a range of mechanisms.
Allorecognition of trophoblast by T cells
Because SCT, which is in contact with maternal blood, does not express HLA, it is invisible to systemic immune recognition by HLA-restricted T cells. Similarly, the lack of HLA class II expression by all trophoblast cells means that direct recognition by maternal CD4+ T cells cannot occur, although indirect recognition by CD4+ T cells via antigen presentation on HLA class II-expressing decidual myeloid cells is possible. To generate such CD4+ T cell responses, decidual HLA class II-expressing antigen-presenting cells would need to take up paternal HLA-C or other alloantigens from EVT and migrate to the draining lymph nodes to initiate a T cell response, and then activated CD4+ T cells would need to migrate back into the decidua. EVT does not express the classical HLA class I molecules HLA-A and HLA-B, and thus can be recognized by decidual CD8+ T cells only through HLA-C. Are paternal HLA-C-restricted effector T cells ever generated in early decidua and, if so, what might be the functional consequences? Although responses to paternal HLA-A and HLA-B antigens expressed by fetal somatic cells can be demonstrated in maternal peripheral blood and term decidua115,116, our view is that convincing evidence for the presence of T cells directed to alloantigens (including HLA-C) expressed by EVT in early decidua in humans is still lacking. In mice, the lack of decidual lymphatics impedes dendritic cell migration to the lymph nodes117, and it is still controversial whether human decidua has lymphatic drainage118,119. Furthermore, epigenetic silencing in murine decidual stromal cells of key cytokines (such as CXCL9 and CXCL10) that are required for T cell recruitment limits the return of T cells into the decidua120. Lymph nodes draining the uterus have not been studied in humans, but there are many tolerogenic features of the decidua itself that would suppress allorecognition by T cells (Box 3).
Mechanisms for tolerance of decidual T cells to EVT
The advantage of local allogeneic responses being inhibited directly by invading EVT only where fetal and maternal cells are in close proximity in decidual tissue is that there is no systemic immune suppression that could, for example, increase susceptibility to infection. The profile of HLA class I molecules on EVT is central to this, specifically the lack of HLA-A and HLA-B expression and the increased expression of HLA-G as it moves deeper into the decidua. All HLA class I molecules bind to the inhibitory receptors LILRB1 and LILRB2 expressed by decidual myeloid cells. However, only HLA-G forms dimers that are associated with β2-microglobulin, which bind with greatly increased avidity to LILRB1 (ref.90). This has a negative effect on antigen presentation by myeloid cells, resulting in their deviation towards a tolerogenic rather than an immunogenic phenotype89. Ligation of LILRB1 is clearly an effective strategy for immune suppression as it is also used by pathogens — for example, UL18 proteins from cytomegalovirus and dengue virus and RIFIN proteins from Plasmodium spp. all bind LILRB1 (refs.121,122,123).
T cells are not the dominant leukocyte population in human decidua (~10–15% of total leukocytes) and have many features suggesting that they are exhausted24,25,27, including expression of the immune checkpoint protein PD1, the ligands for which (PDL1 and PDL2) are highly expressed by EVT43,124,125,126. PDL2 has a higher affinity than PDL1 for PD1 and emerged in placental mammals127. Other B7 family molecules involved in the regulation of immune responses, such as B7H3 (also known as CD276), are also expressed by EVT and inhibit T cell proliferation128. Furthermore, EVT expresses high levels of TGFβ, which has a central role in immune evasion by tumours by inducing the differentiation of regulatory T cells (Treg cells) and the expression of indoleamine 2,3-dioxygenase (IDO) by dendritic cells129. As well as increased proportions of classical FOXP3+ Treg cells in decidua compared with blood130, the decidua also contains two FOXP3− populations of CD4+ T cells expressing the regulatory molecules PD1 and TIGIT131. CD8+ decidual T cells also express inhibitory PD1, TIM3, CTLA4 and LAG3 but are not irreversibly suppressed25,132. Other parallels with the immunosuppressive tumour environment include the COX2–PGE2 pathway, which is induced by human chorionic gonadotropin in decidua133,134 (Box 3).
There is also a potential role for NK cells in the avoidance of T cell responses in the decidua as the main uNK1 population expresses CD39, which is characteristic of Treg cells in tumours and the small number of Treg cells in decidua128. In combination with CD73, which is expressed by EVT and stromal cells, CD39 can convert extracellular ATP to adenosine, which suppresses effector T cells and activates Treg cells135, although there are no functional data yet to show that adenosine is generated by CD39–CD73 in the decidua. Another immunomodulatory molecule, TIGIT, is expressed by uNK3 cells, and its ligand, PVR, is expressed by EVT.
Thus, there are multiple mechanisms in the decidua to dampen potentially damaging T cell responses to EVT. This redundancy means that it is highly unlikely they would all fail together. Indeed, invasive haemochorial placentation, the primordial form in eutherian mammals, could not have evolved without mechanisms in place to avoid damaging adaptive maternal immune responses136.
Do decidual T cells cause pregnancy disorders?
Reproductive immunology articles frequently invoke Medawar’s ‘immunological paradox of pregnancy’1, but the evidence for ‘breakdown of maternal tolerance’ and T cell-mediated rejection causing pregnancy disorders such as miscarriage and pre-eclampsia is circumstantial137,138, and clear mechanisms for how this could happen have not been described. Many reports have focused on the decidual Treg cell subsets that seem to be induced by EVT and that suppress T cell proliferation through IL-10 production139,140. Reduced numbers of decidual Treg cells in pregnancy disorders such as miscarriage and pre-eclampsia compared with normal pregnancy have been described141,142,143. However, these differences were detected only after the disorder became obvious clinically, and it is not clear whether they have any role in the primary pathogenesis. Another unresolved issue is that the main regulator of Treg cell survival and function, IL-2, is absent from the decidua43,144,145,146, excluding the tiny amounts that might be produced by small numbers of decidual T cells themselves.
Because of the difficulties in studying early human pregnancy, there has been a reliance on mouse models, the relevance of which to pregnancy disorders in humans remains unclear (Box 4). Human studies have focused on data from peripheral blood or term decidua rather than considering decidual T cells in early pregnancy, when maternal–fetal interactions set the stage for a successful outcome. Both human and mouse studies have been expertly summarized, highlighting many common problems147. These include a failure to distinguish between antigens expressed by fetal somatic cells or extra-embryonic trophoblast; the focus on systemic and not decidual responses; little exploration of possible effector mechanisms for poor pregnancy outcomes; and lack of appreciation of the bystander inflammatory effects that can result from experimental manipulations. It has also never been explained how a breakdown in T cell tolerance could result in the primary defect of failure of placentation in the first trimester, in particular EVT-mediated transformation of the spiral arteries. Any systemic changes seen in T cell populations in peripheral blood probably reflect the response to syncytial stress that is characteristic of the later stages of pregnancy and that is amplified in pre-eclampsia148. Indeed, systemic inflammation or even stress such as loud noise can lead to fetal resorption in mice (miscarriage does not occur in mice)149,150.
In summary, many questions remain with regard to the role of T cells in pregnancy: does early decidua contain T cells with T cell receptor specificities that bind to HLA-C molecules expressed by EVT; what is the nature of the specific peptides presented by HLA-C on EVT; if CD8+ T cells specific for HLA-C are present in the decidua, what are their effector functions, and in particular how can they affect EVT invasion and pregnancy outcome? EVT is resistant to killing by freshly isolated uNK cells but can it be killed by T cells? Tissue Treg cells in fat, muscle and skin have distinct characteristics depending on their microenvironment, with roles in the physiological regulation of non-lymphoid progenitor cells151. A role for decidual Treg cells in tissue homeostasis rather than pathology is therefore also possible.
The way forward
Research over the past 20 years has reached a consensus regarding the importance of balanced EVT invasion during human placental development, the unique profile of decidual immune cells, expression of HLA molecules by trophoblast subpopulations, the uNK cell-mediated allorecognition system dependent on KIR–HLA-C interactions and the need for T cell tolerance to trophoblast. However, as outlined in the preceding discussion, there are still many questions regarding the dialogue between the uterine immune system and trophoblast cells in human pregnancy that historically have been difficult to tackle, partly owing to ethical and practical issues surrounding access to human tissues and the limitations of animal models.
In light of these controversies and unresolved issues in the field, use of new research technologies will be crucial to better understand the role of the immune system in human placentation and reproductive success. Single-cell RNA sequencing and single-nucleus RNA sequencing will continue to be important tools as they can highlight novel cell populations, genetic markers or potential functional pathways for further validation43,152,153. In the future, spatial transcriptomic methods and multiplex antibodies to visualize the dynamic changes occurring in the placenta in the first weeks of human pregnancy will also be informative152. The considerable differences between human and mouse placentation remain a problem (as exemplified by the direct role played by uNK cells in remodelling of the spiral arteries in mice but not humans) (Box 4). Placentation in higher primates is more similar to that in humans, but interstitial invasion by EVT occurs only in the great apes and no animal model is perfect154.
Experimenting with human trophoblast cells in vitro has so far been problematic because many of the cell lines used do not have the defining features of first-trimester trophoblast in vivo: namely, a unique HLA profile, ELF5 methylation and expression of characteristic genes such as TFAP2C and GATA3 and the chromosome 19 microRNA complex155. So far, there has been too much reliance on the use of choriocarcinoma lines, such as JEG-3 and BeWo, which have little resemblance to normal invasive EVT156. Microfluidic methods to study trophoblast invasion will be beneficial because bulk placental cell isolates always also contain non-trophoblast cells74,84. Human trophoblast stem cell lines157,158 and trophoblast organoids159,160 can be induced to differentiate to SCT or invasive EVT and are powerful tools to study placental development and interaction with maternal cells. Several recent reports showing that human embryonic stem cells can be differentiated to trophoblast will enable the generation and study of trophoblast cells from individuals (either mother or child) who have had abnormal pregnancies161,162,163,164,165.
Despite much evidence to suggest that uNK cells regulate human placentation, their precise functions still remain largely unknown. The necessity to use primary uNK cells from ongoing first-trimester pregnancies to study these functions has obvious ethical and logistical limitations, and no representative uNK cell lines exist. Although genetic studies show that particular combinations of maternal KIR and fetal HLA-C variants are associated with increased risk of pre-eclampsia and other pregnancy disorders, the functional mechanisms remain unresolved. New models of EVT derived from trophoblast stem cell lines and trophoblast organoids, which can be biobanked and typed for HLA-C alleles, will be useful to study interactions between KIR+ uNK cells and EVT. Unlike human trophoblast stem cell lines grown in two dimensions, which continue to express classical HLA-A and HLA-B molecules, EVT derived from trophoblast organoids does have the unique HLA profile of primary EVT in vivo163,166. One feature that is likely to affect NK cell and T cell responses both in the uterus and systemically is the altered pattern of protein sialylation for trophoblast compared with fetal cells from the same conceptus167. The glycosylation of trophoblast proteins should be a focus of future research into maternal–fetal immune interactions168.
Another issue relating to the current immunogenetic studies of maternal KIR–fetal HLA-C combinations is that they are typically small and restricted mainly to European populations, so replication in larger, clinically well characterized cohorts is needed to detect the effects of particular alleles of both KIR2DL1 and its HLA-C ligands169. The impact of maternal HLA-C groups and a variant in the leader sequence of HLA-B (dimorphism at position −21) that affects the education of peripheral blood NK cells also needs further study95,170. Studying pregnancies resulting from oocyte or embryo donation, in which the conceptus is entirely ‘non-self’ in relation to the mother, will also be informative as the risk of pre-eclampsia increases to ~25% in these cases171. We suggest that this increased risk might associate with surrogate mothers who are of the KIR AA genotype, which contains two copies of KIR2DL1 (a potent inhibitory receptor for C2+ HLA-C epitopes), and who are pregnant with a conceptus that has two non-self C2+ HLA-C alleles.
Given these many uncertainties regarding the functions of decidual leukocytes in placentation, there is no current rationale for the treatments offered in infertility or recurrent miscarriage clinics that purport to beneficially affect the maternal immune system. Disappointingly, there have been no translational impacts from studying human decidua–trophoblast interactions in the first few weeks of pregnancy that can prevent or predict women at risk of spontaneous fetal loss, stillbirth, fetal growth restriction, pre-eclampsia or any other pregnancy disorder (Box 5), which highlights the urgent need for further research in this area.
Conclusions
Medawar was correct to point out that, in mammals, pregnancy is a unique time when two genetically different individuals coexist. However, unlike any similarities drawn to the artificial example of organ transplantation, a crucial feature of the specialized mucosal barrier where the placenta implants is the need to define a balanced boundary between the mother and the fetus so that both survive and thrive. Evolutionary history can provide insight into how humans have developed a system whereby pregnancy success seems to depend mainly on NK cells mediating this boundary, with a still questionable role for T cells. In primates, and particularly the great apes, the emergence of KIRs and C2+ MHC-C alleles and the extent of decidualization concomitant with the specialization of uNK cells all correlate with increasing brain size and the need for a longer gestation period and better fetoplacental blood supply. Conflicting selective pressures affecting childbirth emerged in large-brained humans for whom the impact of bipedalism constrained the size of the pelvic inlet, which has likely tempered this evolution of increased decidualization, highlighting the need for EVT invasion to be carefully balanced69. Adaptive immune responses by T cells are avoided in the decidua, and there is still no convincing evidence that T cells directly recognize EVT or affect its function. By contrast, a role for an allorecognition system dependent on maternal KIRs interacting with HLA-C in mediating balanced trophoblast invasion is clear.
References
Medawar, P. B. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7, 320–338 (1953).
Male, V. Medawar and the immunological paradox of pregnancy: in context. Oxf. Open Immunol. 2, iqaa006 (2021).
Turco, M. Y. & Moffett, A. Development of the human placenta. Development 146, dev163428 (2019). This article provides an overview of the development of the human placenta, describing the phenotypes and functions of the trophoblast subpopulations.
Smith, G. C. S. First-trimester determination of complications of late pregnancy. JAMA 303, 561–562 (2010).
Kim, S.-M. & Kim, J.-S. A review of mechanisms of implantation. Dev. Reprod. 21, 351 (2017).
Finn, C. A. Menstruation: a nonadaptive consequence of uterine evolution. Q. Rev. Biol. 73, 163–173 (1998).
Mess, A. & Carter, A. M. Evolutionary transformations of fetal membrane characters in Eutheria with special reference to Afrotheria. J. Exp. Zool. B Mol. Dev. Evol. 306, 140–163 (2006).
Pijnenborg, R., Vercruysse, L. & Hanssens, M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27, 939–958 (2006). This article provides a detailed discussion of how interstitial and endovascular trophoblast function together to transform the uterine spiral arteries into high-conductance vessels.
Brosens, I. A., Robertson, W. B. & Dixon, H. G. The role of the spiral arteries in the pathogenesis of pre-eclampsia. J. Pathol. 101, 171–191 (1970).
Khong, T. Y., Liddell, H. S. & Robertson, W. B. Defective haemochorial placentation as a cause of miscarriage; a preliminary study. BJOG . Int. J. Obstet. Gynaecol. 94, 649–655 (1987).
Chappell, L. C., Cluver, C. A., Kingdom, J. & Tong, S. Pre-eclampsia. Lancet 398, 341–354 (2021).
Burton, G. J., Redman, C. W., Roberts, J. M. & Moffett, A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 366, l2381 (2019).
Pijnenborg, R., Vercruysse, L., Hanssens, M. & Brosens, I. Endovascular trophoblast and preeclampsia: a reassessment. Pregnancy Hypertens. 1, 66–71 (2011).
Luo, Z. et al. The effects and mechanisms of primiparity on the risk of pre-eclampsia: a systematic review. Paediatr. Perinat. Epidemiol. 21, 36–45 (2007).
Esplin, M. S. et al. Paternal and maternal components of the predisposition to preeclampsia. N. Engl. J. Med. 344, 867–872 (2001).
Cnattingius, S., Reilly, M., Pawitan, Y. & Lichtenstein, P. Maternal and fetal genetic factors account for most of familial aggregation of preeclampsia: a population-based Swedish cohort study. Am. J. Med. Genet. A 130, 365–371 (2004).
Wikström, A.-K., Gunnarsdóttir, J. & Cnattingius, S. The paternal role in pre-eclampsia and giving birth to a small for gestational age infant; a population-based cohort study. BMJ Open 2, e001178 (2012).
Dekker, G. A., Robillard, P. Y. & Hulsey, T. C. Immune maladaptation in the etiology of preeclampsia: a review of corroborative epidemiologic studies. Obstet. Gynecol. Surv. 53, 377–382 (1998).
Starkey, P. M., Clover, L. M. & Rees, M. C. P. Variation during the menstrual cycle of immune cell populations in human endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 39, 203–207 (1991).
Bulmer, J. N., Longfellow, M. & Ritson, A. Leukocytes and resident blood cells in endometrium. Ann. N. Y. Acad. Sci. 622, 57–68 (1991).
King, A., Balendran, N., Wooding, P., Carter, N. P. & Loke, Y. W. CD3-leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev. Immunol. 1, 169–190 (1991). Starkey et al. (1991), Bulmer et al. (1991) and King et al. (1991) are the first reports of unique NK cells present in the uterus during implantation and placentation.
Williams, P. J., Searle, R. F., Robson, S. C., Innes, B. A. & Bulmer, J. N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 82, 24–31 (2009).
Bartmann, C. et al. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am. J. Reprod. Immunol. 71, 109–119 (2014).
Feyaerts, D. et al. Human uterine lymphocytes acquire a more experienced and tolerogenic phenotype during pregnancy. Sci. Rep. 7, 1–10 (2017).
Van Der Zwan, A. et al. Mixed signature of activation and dysfunction allows human decidual CD8+ T cells to provide both tolerance and immunity. Proc. Natl Acad. Sci. USA 115, 385–390 (2017).
de Mendonça Vieira, R. et al. Human term pregnancy decidual NK cells generate distinct cytotoxic responses. J. Immunol. 204, 3149–3159 (2020).
Van der Zwan, A. et al. Visualizing dynamic changes at the maternal-fetal interface throughout human pregnancy by mass cytometry. Front. Immunol. 11, 571300 (2020). This article provides a detailed review of the phenotypes and functions of ILC populations in human decidua.
Altmäe, S. et al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci. Rep. 7, 1–15 (2017).
Robertson, S. A. et al. Corticosteroid therapy in assisted reproduction – immune suppression is a faulty premise. Hum. Reprod. 31, 2164–2173 (2016).
De Leo, B., Esnal-Zufiaurre, A., Collins, F., Critchley, H. O. D. & Saunders, P. T. K. Immunoprofiling of human uterine mast cells identifies three phenotypes and expression of ERβ and glucocorticoid receptor. F1000Research 6, 667 (2017).
Amsalem, H. et al. Identification of a novel neutrophil population: proangiogenic granulocytes in second-trimester human decidua. J. Immunol. 193, 3070–3079 (2014).
Croxatto, D. et al. Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal Immunol. 9, 1372–1383 (2016).
Critchley, H. O. D. et al. Uterus and endometrium: sex steroid regulation of leukocyte traffic in human decidua. Hum. Reprod. 11, 2257–2262 (1996).
Armstrong, G. M. et al. Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Sci. Rep. 7, 17416 (2017).
Krop, J. et al. Imaging mass cytometry reveals the prominent role of myeloid cells at the maternal-fetal interface. iScience 25, 104648 (2022).
Osokine, I. et al. Gene silencing by EZH2 suppresses TGF-β activity within the decidua to avert pregnancy-adverse wound healing at the maternal-fetal interface. Cell Rep. 38, 110329 (2022).
Johnson, G. A., Bazer, F. W. & Seo, H. The early stages of implantation and placentation in the pig. Adv. Anat. Embryol. Cell Biol. 234, 61–89 (2021).
Hanna, J. et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12, 1065–1074 (2006).
Zhang, J., Dunk, C. E. & Lye, S. J. Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum. Reprod. 28, 3026–3037 (2013).
Gambino, L. S. et al. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum. Reprod. 17, 1199–1206 (2002).
Wong, T. Y. et al. Effect of endometrial scratching on unassisted conception for unexplained infertility: a randomized controlled trial. Fertil. Steril. 117, 612–619 (2022).
Medzhitov, R. The spectrum of inflammatory responses. Science 374, 1070–1075 (2021).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563, 347–353 (2018). This article describes a single-cell RNA analysis of all the placental leukocytes, as well as maternal blood and decidual leukocytes, in the first trimester of pregnancy, defining three major subsets of uNK cells.
Chavan, A. R., Griffith, O. W. & Wagner, G. P. The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr. Opin. Genet. Dev. 47, 24–32 (2017).
Chavan, A. R. et al. Evolution of embryo implantation was enabled by the origin of decidual stromal cells in Eutherian mammals. Mol. Biol. Evol. 38, 1060–1074 (2021).
Stadtmauer, D. J. & Wagner, G. P. Cooperative inflammation: the recruitment of inflammatory signaling in marsupial and eutherian pregnancy. J. Reprod. Immunol. 137, 102626 (2020).
Wang, F. et al. Single-cell immune landscape of human recurrent miscarriage. Genomics Proteom. Bioinforma. 19, 208–222 (2021).
Chen, P. et al. The immune atlas of human deciduas with unexplained recurrent pregnancy loss. Front. Immunol. 12, 689019 (2021).
Du, L. et al. Single-cell transcriptome analysis reveals defective decidua stromal niche attributes to recurrent spontaneous abortion. Cell Prolif. 54, e13125 (2021).
Weill, P. Etudes sur les leucocytes. I. Les cellules granuleuses des muqueuses intestinal et uterine. Archs. Anat. Microsc. 17, 77 (1920).
Huhn, O. et al. How do uterine natural killer and innate lymphoid cells contribute to successful pregnancy? Front. Immunol. 12, 1964 (2021).
Verma, S., Hiby, S. E., Loke, Y. W. & King, A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol. Reprod. 62, 959–968 (2000).
Kitaya, K. et al. IL-15 expression at human endometrium and decidua. Biol. Reprod. 63, 683–687 (2000).
Wilkens, J. et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J. Immunol. 191, 2226–2235 (2013).
Strunz, B. et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci. Immunol. 6, eabb7800 (2021).
Male, V. et al. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J. Immunol. 185, 3913–3918 (2010).
Vacca, P. et al. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol. 8, 254–264 (2015).
Kopcow, H. D. et al. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc. Natl Acad. Sci. USA 102, 15563–15568 (2005).
Huhn, O. et al. Distinctive phenotypes and functions of innate lymphoid cells in human decidua during early pregnancy. Nat. Commun. 11, 1–14 (2020). This article describes phenotypic and functional analysis by cytometry by time of flight and flow cytometry of the three main decidual NK cell subsets in the first trimester.
Goodridge, J. P. et al. Remodeling of secretory lysosomes during education tunes functional potential in NK cells. Nat. Commun. 10, 1–15 (2019).
Gamliel, M. et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity 48, 951–962 (2018).
Feyaerts, D., van der Meer, A., Joosten, I. & van der Molen, R. G. Selective expansion and CMV-dependency in pregnancy trained human endometrial NK cells. Cell. Mol. Immunol. 16, 410–411 (2019).
Falcão, I. R. et al. Factors associated with low birth weight at term: a population-based linkage study of the 100 million Brazilian cohort. BMC Pregnancy Childbirth 20, 1–11 (2020).
Whettlock, E. et al. Dynamic changes in uterine NK cell subset frequency and function over the menstrual cycle and pregnancy. Front. Immunol. 13, 880438 (2022).
Sojka, D. K. et al. Cutting edge: local proliferation of uterine tissue-resident NK cells during decidualization in mice. J. Immunol. 201, 2551–2556 (2018).
Apps, R. et al. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127, 26–39 (2009). This study provides definitive identification of the unusual HLA class I expression by primary trophoblast cells.
Quatrini, L. et al. Human NK cells, their receptors and function. Eur. J. Immunol. 51, 1566–1579 (2021).
Djaoud, Z. & Parham, P. HLAs, TCRs, and KIRs, a triumvirate of human cell-mediated immunity. Annu. Rev. Biochem. 89, 717–739 (2020).
Moffett, A. & Colucci, F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol. Rev. 267, 283–297 (2015). Djaoud and Parham (2020) and Moffett and Colucci (2015) describe the extreme polymorphism of KIR and HLA genes in humans and the implications this has for evolution of human reproduction.
Sharkey, A. M. et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J. Immunol. 181, 39–46 (2008).
Marlin, R. et al. Dynamic shift from CD85j/ILT-2 to NKG2D NK receptor expression pattern on human decidual NK during the first trimester of pregnancy. PLoS ONE 7, e30017 (2012).
Xiong, S. et al. Maternal uterine NK cell–activating receptor KIR2DS1 enhances placentation. J. Clin. Invest. 123, 4264–4272 (2013).
Kennedy, P. R. et al. Activating KIR2DS4 is expressed by uterine NK cells and contributes to successful pregnancy. J. Immunol. 197, 4292–4300 (2016).
Abbas, Y. et al. A microfluidics assay to study invasion of human placental trophoblast cells. J. R. Soc. Interface 14, 20170131 (2017).
Hiby, S. E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004).
Hiby, S. E. et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120, 4102–4110 (2010).
Nakimuli, A. et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc. Natl Acad. Sci. USA 112, 845–850 (2015).
Clark, M. M. et al. Human birth weight and reproductive immunology: testing for interactions between maternal and offspring KIR and HLA-C genes. Hum. Hered. 81, 181–193 (2016).
Hiby, S. E. et al. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J. Immunol. 192, 5069–5073 (2014).
Hu, Y. et al. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-γ. J. Immunol. 177, 8522–8530 (2006).
Jia, N. & Li, J. Human uterine decidual NK cells in women with a history of early pregnancy enhance angiogenesis and trophoblast invasion. Biomed. Res. Int. 2020, 6247526 (2020).
Lash, G. E. et al. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum. Reprod. 25, 1137–1145 (2010).
De Oliveira, L. G. et al. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta 31, 595–601 (2010).
Park, J. Y. et al. A microphysiological model of human trophoblast invasion during implantation. Nat. Commun. 13, 1–18 (2022).
Abbas, Y., Turco, M. Y., Burton, G. J. & Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update 26, 501–513 (2020).
Rajagopalan, S. & Long, E. O. KIR2DL4 (CD158d): an activation receptor for HLA-G. Front. Immunol. 3, 258 (2012).
Moradi, S. et al. The structure of the atypical killer cell immunoglobulin-like receptor, KIR2DL4. J. Biol. Chem. 290, 10460–10471 (2015).
Witt, C. S. et al. Alleles of the KIR2DL4 receptor and their lack of association with pre-eclampsia. Eur. J. Immunol. 32, 18–29 (2001).
Apps, R., Gardner, L., Sharkey, A. M., Holmes, N. & Moffett, A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur. J. Immunol. 37, 1924–1937 (2007).
Shiroishi, M. et al. Efficient leukocyte Ig-like receptor signalling and crystal structure of disulfide-linked HLA-G dimer. J. Biol. Chem. 15, 10439–10447 (2006).
Kuroki, K. et al. Structural and functional basis for LILRB immune checkpoint receptor recognition of HLA-G isoforms. J. Immunol. 203, 3386–3394 (2019).
Li, C., Houser, B. L., Nicotra, M. L. & Strominger, J. L. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl Acad. Sci. USA 106, 5767–5772 (2009).
Fu, B. et al. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity 47, 1100–1113 (2017).
Saverino, D. et al. Specific recognition of the viral protein UL18 by CD85j/LIR-1/ILT2 on CD8+ T cells mediates the non-MHC-restricted lysis of human cytomegalovirus-infected cells. J. Immunol. 172, 5629–5637 (2004).
Shreeve, N. et al. The CD94/NKG2A inhibitory receptor educates uterine NK cells to optimize pregnancy outcomes in humans and mice. Immunity 54, 1231–1244 (2021).
King, A. et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 30, 1623–1631 (2000).
Kusumi, M. et al. Expression patterns of lectin-like natural killer receptors, inhibitory CD94/NKG2A, and activating CD94/NKG2C on decidual CD56bright natural killer cells differ from those on peripheral CD56dim natural killer cells. J. Reprod. Immunol. 70, 33–42 (2006).
Llano, M. et al. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur. J. Immunol. 28, 2854–2863 (1998).
Heatley, S. L. et al. Polymorphism in human cytomegalovirus UL40 impacts on recognition of human leukocyte antigen-E (HLA-E) by natural killer cells. J. Biol. Chem. 288, 8679–8690 (2013).
King, A., Birkby, C. & Loke, Y. W. Early human decidual cells exhibit NK activity against the K562 cell line but not against first trimester trophoblast. Cell. Immunol. 118, 337–344 (1989).
King, A. & Loke, Y. W. Human trophoblast and JEG choriocarcinoma cells are sensitive to lysis by IL-2-stimulated decidual NK cells. Cell. Immunol. 129, 435–448 (1990).
Saito, S. et al. Cytokine production by CD16–CD56bright natural killer cells in the human early pregnancy decidua. Int. Immunol. 5, 559–563 (1993).
Engert, S. et al. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am. J. Reprod. Immunol. 58, 129–137 (2007).
Lucas, E. S. et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 3, 1–14 (2020).
Kong, C. S. et al. Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation. FASEB J. 35, e21336 (2021).
Brighton, P. J. et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife 6, e31274 (2017).
De-Carvalho, D. P., Jacinto, A. & Saúde, L. The right time for senescence. Elife 10, e72449 (2021).
de Mera-Rodríguez, J. A. et al. Is senescence-associated β-galactosidase a reliable in vivo marker of cellular senescence during embryonic development? Front. Cell Dev. Biol. 9, 623175 (2021).
Stadtmauer, D. J. et al. Single-cell analysis of prostaglandin E2-induced human decidual cell in vitro differentiation: a minimal ancestral deciduogenic signal. Biol. Reprod. 106, 155–172 (2022).
Hamperl, H. & Hellweg, G. Granular endometrial stromal cells. Obstet. Gynecol. 11, 379–387 (1958).
Bulmer, J. N. & Sunderland, C. A. Bone-marrow origin of endometrial granulocytes in the early human placental bed. J. Reprod. Immunol. 5, 383–387 (1983).
Smith, M., Hagerty, K. A., Skipper, B. & Bocklage, T. Chronic endometritis: a combined histopathologic and clinical review of cases from 2002 to 2007. Int. J. Gynecol. Pathol. 29, 44–50 (2010).
Legro, R., Hurtado, R., Kilcoyne, A. & Roberts, D. Case records of the Massachusetts General Hospital. Case 28-2016: a 31-year-old woman with infertility. N. Engl. J. Med. 375, 1069–1077 (2016).
Tran, E. et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350, 1387–1390 (2015).
Masson, E. et al. Incidence and risk factors of anti-HLA immunization after pregnancy. Hum. Immunol. 74, 946–951 (2013).
Powell, R. M. et al. Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J. Immunol. 199, 3406–3417 (2017).
Collins, M. K., Tay, C. S. & Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 119, 2062–2073 (2009).
Volchek, M. et al. Lymphatics in the human endometrium disappear during decidualization. Hum. Reprod. 25, 2455–2464 (2010).
Windsperger, K. et al. Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions. Hum. Reprod. 32, 1208–1217 (2017).
Nancy, P. et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336, 1317–1321 (2012).
Yu, K. et al. LILRB1 polymorphisms influence posttransplant HCMV susceptibility and ligand interactions. J. Clin. Invest. 128, 1523–1537 (2018).
Harrison, T. E. et al. Structural basis for RIFIN-mediated activation of LILRB1 in malaria. Nature 587, 309–312 (2020).
Chan, K. R. et al. Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue. Proc. Natl Acad. Sci. USA 111, 2722–2727 (2014).
Holets, L. M., Hunt, J. S. & Petroff, M. G. Trophoblast CD274 (B7-H1) is differentially expressed across gestation: influence of oxygen concentration. Biol. Reprod. 74, 352–358 (2006).
Veras, E., Kurman, R. J., Wang, T. L. & Shih, I. M. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int. J. Gynecol. Pathol. 36, 146 (2017).
Petroff, M. G. et al. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol. Reprod. 68, 1496–1504 (2003).
Philips, E. A. et al. The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals. J. Biol. Chem. 295, 4372–4380 (2020).
Petroff, M. G., Kharatyan, E., Torry, D. S. & Holets, L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am. J. Pathol. 167, 465–473 (2005).
Batlle, E. & Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Mjösberg, J., Berg, G., Jenmalm, M. C. & Ernerudh, J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol. Reprod. 82, 698–705 (2010).
Salvany-Celades, M. et al. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 27, 2537–2547 (2019).
Morita, K. et al. Analysis of TCR repertoire and PD-1 expression in decidual and peripheral CD8+ T cells reveals distinct immune mechanisms in miscarriage and preeclampsia. Front. Immunol. 11, 1082 (2020).
Han, S. W., Lei, Z. M. & Rao, C. V. Treatment of human endometrial stromal cells with chorionic gonadotropin promotes their morphological and functional differentiation into decidua. Mol. Cell. Endocrinol. 147, 7–16 (1999).
Schiavon, V. et al. Microenvironment tailors nTreg structure and function. Proc. Natl Acad. Sci. USA 116, 6298–6307 (2019).
Moesta, A. K., Li, X. Y. & Smyth, M. J. Targeting CD39 in cancer. Nat. Rev. Immunol. 20, 739–755 (2020).
Wildman, D. E. et al. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc. Natl Acad. Sci. USA 103, 3203–3208 (2006).
Piccinni, M. P., Robertson, S. A. & Saito, S. Editorial: adaptive immunity in pregnancy. Front. Immunol. 12, 770242 (2021).
Deshmukh, H. & Way, S. S. Immunological basis for recurrent fetal loss and pregnancy complications. Annu. Rev. Pathol. 14, 185–210 (2019).
Tilburgs, T. et al. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc. Natl Acad. Sci. USA 112, 7219–7224 (2015).
Svensson-Arvelund, J. et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 194, 1534–1544 (2015).
Sasaki, Y. et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10, 347–353 (2004).
Inada, K., Shima, T., Ito, M., Ushijima, A. & Saito, S. Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J. Reprod. Immunol. 107, 10–19 (2015).
Steinborn, A. et al. Distinct subsets of regulatory T cells during pregnancy: is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin. Immunol. 129, 401–412 (2008).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Furtado, G. C., De Curotto Lafaille, M. A., Kutchukhidze, N. & Lafaille, J. J. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J. Exp. Med. 196, 851–857 (2002).
Jokhi, P. P., King, A. & Loke, Y. W. Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface. Cytokine 9, 126–137 (1997).
Rizzuto, G. & Erlebacher, A. In Paul’s Fundamental Immunology (eds. Flajnik, M., Singh, N. & Holland, S.) (Wolters Kluwer, 2022). This is a scholarly review of maternal immunity during pregnancy in both mice and humans.
Redman, C. W., Staff, A. C. & Roberts, J. M. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am. J. Obstet. Gynecol. 226, S907–S927 (2022).
Kondoh, E. et al. Stress affects uterine receptivity through an ovarian-independent pathway. Hum. Reprod. 24, 945–953 (2009).
Jafari, Z. et al. The adverse effects of auditory stress on mouse uterus receptivity and behaviour. Sci. Rep. 7, 1–11 (2017).
Muñoz-Rojas, A. R. & Mathis, D. Tissue regulatory T cells: regulatory chameleons. Nat. Rev. Immunol. 21, 597–611 (2021).
Garcia-Alonso, L. et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 53, 1698–1711 (2021).
Dong, C. et al. A genome-wide CRISPR-Cas9 knockout screen identifies essential and growth-restricting genes in human trophoblast stem cells. Nat. Commun. 13, 1–16 (2022).
Carter, A. M. Animal models of human pregnancy and placentation: alternatives to the mouse. Reproduction 160, R129–R143 (2020). This article reviews the different placental strategies used by eutherian mammals in relation to their validity as models for human reproduction.
Lee, C. Q. E. et al. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 6, 257–272 (2016).
Apps, R. et al. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta 32, 33–43 (2011).
Okae, H. et al. Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63 (2018). This article provides the first description of human trophoblast stem cells derived from first-trimester placentas and blastocysts.
Wei, Y. et al. Efficient derivation of human trophoblast stem cells from primed pluripotent stem cells. Sci. Adv. 7, 4416–4427 (2021).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 564, 263–267 (2018).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 11, 537–551 (2018). Turco et al. (2018) and Haider et al. (2018) describe the generation of trophoblast organoids derived from first-trimester placentas.
De Santis, R. & Brivanlou, A. H. The treasure inside human naive pluripotency, generation of trophectoderm and blastoids. Cell Stem Cell 28, 985–987 (2021).
Pera, M. F. & Rossant, J. The exploration of pluripotency space: charting cell state transitions in peri-implantation development. Cell Stem Cell 28, 1896–1906 (2021).
Karvas, R. M. et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell 29, 810–825 (2022).
Jang, Y. J., Kim, M., Lee, B.-K., Kim, J. & Roberts, R. Induction of human trophoblast stem-like cells from primed pluripotent stem cells. Proc. Natl Acad. Sci. USA 119, e2115709119 (2022).
Soncin, F. et al. Derivation of functional trophoblast stem cells from primed human pluripotent stem cells. Stem Cell Rep. 17, 1303–1317 (2022).
Sheridan, M. A. et al. Characterization of primary models of human trophoblast. Development 148, dev199749 (2021).
Whyte, A. & Loke, Y. W. Increased sialylation of surface glycopeptides of human trophoblast compared with fetal cells from the same conceptus. J. Exp. Med. 148, 1087–1092 (1978).
Rizzuto, G. & Erlebacher, A. Trophoblast antigens, fetal blood cell antigens, and the paradox of fetomaternal tolerance. J. Exp. Med. 219, e20211515 (2022).
Huhn, O. et al. High-resolution genetic and phenotypic analysis of KIR2DL1 alleles and their association with pre-eclampsia. J. Immunol. 201, 2593–2601 (2018).
Sharkey, A. M. et al. Tissue-specific education of decidual NK cells. J. Immunol. 195, 3026–3032 (2015).
Chih, H. J., Elias, F. T. S., Gaudet, L. & Velez, M. P. Assisted reproductive technology and hypertensive disorders of pregnancy: systematic review and meta-analyses. BMC Pregnancy Childbirth 21, 1–20 (2021).
Abu-Raya, B., Michalski, C., Sadarangani, M. & Lavoie, P. M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 11, 575197 (2020).
Clarke, A. G. & Kendall, M. D. The thymus in pregnancy: the interplay of neural, endocrine and immune influences. Immunol. Today 15, 545–552 (1994).
Aghaeepour, N. et al. An immune clock of human pregnancy. Sci. Immunol. 2, eaan2946 (2017).
De Clippel, D. et al. Screening for HLA antibodies in plateletpheresis donors with a history of transfusion or pregnancy. Transfusion 54, 3036–3042 (2014).
Lissauer, D., Piper, K., Goodyear, O., Kilby, M. D. & Moss, P. A. H. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 189, 1072–1080 (2012).
Hönger, G. et al. Frequency and determinants of pregnancy-induced child-specific sensitization. Am. J. Transplant. 13, 746–753 (2013).
Porrett, P. M. Biologic mechanisms and clinical consequences of pregnancy alloimmunization. Am. J. Transplant. 18, 1059–1067 (2018).
Bianchi, D. W. Fetomaternal cell traffic, pregnancy-associated progenitor cells, and autoimmune disease. Best. Pract. Res. Clin. Obstet. Gynaecol. 18, 959–975 (2004).
Rizzuto, G. et al. Establishment of fetomaternal tolerance through glycan-mediated B cell suppression. Nature 603, 497–502 (2022).
Vousden, N. et al. Management and implications of severe COVID-19 in pregnancy in the UK: data from the UK obstetric surveillance system national cohort. Acta Obstet. Gynecol. Scand. 101, 461–470 (2022).
Cervantes, O. et al. Role of hormones in the pregnancy and sex-specific outcomes to infections with respiratory viruses. Immunol. Rev. 308, 123–148 (2022).
Keestra, S. M., Male, V. & Salali, G. D. Out of balance: the role of evolutionary mismatches in the sex disparity in autoimmune disease. Med. Hypotheses 151, 110558 (2021).
Förger, F. & Villiger, P. M. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat. Rev. Rheumatol. 16, 113–122 (2020).
Holstein, R. et al. Characteristics and outcomes of hospitalized pregnant women with influenza, 2010 to 2019. Ann. Intern. Med. 175, 149–158 (2022).
Stock, S. J. et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 28, 504–512 (2022).
Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 22, 277–282 (2022).
Megli, C. J. & Coyne, C. B. Infections at the maternal–fetal interface: an overview of pathogenesis and defence. Nat. Rev. Microbiol. 20, 67–82 (2021).
Thomas, J. R. et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 218, e20200891 (2020).
Thomas, J. R., Naidu, P., Appios, A. & McGovern, N. The ontogeny and function of placental macrophages. Front. Immunol. 12, 771054 (2021).
Weisblum, Y. et al. APOBEC3A is upregulated by human cytomegalovirus (HCMV) in the maternal-fetal interface, acting as an innate anti-HCMV effector. J. Virol. 91, 1296–1313 (2017).
Siewiera, J. et al. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog. 9, e1003257 (2013).
Crespo, Â. C., van der Zwan, A., Ramalho-Santos, J., Strominger, J. L. & Tilburgs, T. Cytotoxic potential of decidual NK cells and CD8+ T cells awakened by infections. J. Reprod. Immunol. 119, 85–90 (2017).
Crespo, Â. C. et al. Decidual NK cells transfer granulysin to selectively kill bacteria in trophoblasts. Cell 182, 1125–1139 (2020).
Santara, S. S. et al. Decidual NK cells kill Zika virus-infected trophoblasts. Proc. Natl Acad. Sci. USA 118, e2115410118 (2021).
Sojka, D. K., Yang, L. & Yokoyama, W. M. Uterine natural killer cells. Front. Immunol. 10, 960 (2019).
Doisne, J.-M. et al. Composition, development, and function of uterine innate lymphoid cells. J. Immunol. 195, 3937–3945 (2015).
Filipovic, I. et al. Molecular definition of group 1 innate lymphoid cells in the mouse uterus. Nat. Commun. 9, 1–13 (2018).
Marsh, B. & Blelloch, R. Single nuclei RNA-seq of mouse placental labyrinth development. Elife 9, 1–27 (2020).
Depierreux, D. M. et al. Beyond maternal tolerance: education of uterine natural killer cells by maternal MHC drives fetal growth. Front. Immunol. 13, 808227 (2022).
Lensen, S. et al. In vitro fertilization add-ons for the endometrium: it doesn’t add-up. Fertil. Steril. 112, 987–993 (2019).
Moffett, A. & Shreeve, N. First do no harm: uterine natural killer (NK) cells in assisted reproduction. Hum. Reprod. 30, 1519–1525 (2015).
Von Woon, E. et al. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: a systematic review and meta-analysis. Hum. Reprod. Update 28, 548–582 (2022).
Lash, G. E. et al. Standardisation of uterine natural killer (uNK) cell measurements in the endometrium of women with recurrent reproductive failure. J. Reprod. Immunol. 116, 50–59 (2016).
Moffett, A., Chazara, O., Colucci, F. & Johnson, M. H. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: too early for clinical intervention. Reprod. Biomed. Online 33, 763–769 (2016).
Keukens, A., Van Wely, M., Van Der Meulen, C. & Mochtar, M. H. Pre-eclampsia in pregnancies resulting from oocyte donation, natural conception or IVF: a systematic review and meta-analysis. Hum. Reprod. 37, 586–599 (2022).
Alecsandru, D. et al. Parental human leukocyte antigen-C allotypes are predictive of live birth rate and risk of poor placentation in assisted reproductive treatment. Fertil. Steril. 114, 809–817 (2020).
Acknowledgements
The authors are grateful for helpful comments from G. Burton, V. Male, N. McGovern, K. Okkenhaug, P. Porrett, A. Sharkey, J. Trowsdale and G. Wagner. They also thank H. Anderson, L. Gardner and M. Turco. A.M. is in receipt of a Wellcome Trust Investigator Award, and N.S. is in receipt of an AMS Clinical Lecturer Grant.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks M. Eikmans, T. Tilburgs and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Pre-eclampsia
-
A systemic disorder of endothelial cells that usually presents with hypertension and proteinuria in late gestation. It is triggered by stress of the syncytiotrophoblast covering the placental villi, resulting from disordered blood flow into the intervillous space of the placenta.
- Trophoblast
-
The definitive epithelial cells of the placenta at the interface between the mother and the fetus. They are derived from trophectoderm surrounding the blastocyst.
- Decidua
-
The specialized uterine mucosa of pregnancy formed from endometrium under the influence of progesterone.
- Decidua parietalis
-
The area of the uterine mucosa that is away from the site of implantation and placentation.
- Haemochorial placentation
-
A type of placentation in which the trophoblast cells of the placenta are in direct contact with maternal blood with no intervening maternal epithelial, stromal or endothelial cells.
- Epitheliochorial placentation
-
A type of placentation in which the trophoblast cells of the placenta remain in contact throughout pregnancy with the uterine epithelium, which remains intact.
- Corpus luteum
-
A structure formed each month from the postovulatory follicle when the granulosa and thecal cells produce progesterone. It regresses if pregnancy does not occur.
- Placental bed giant cells
-
Giant cells found in the deeper layers of the decidua and inner myometrium that are the terminal end point of the differentiation pathway of interstitial extravilloustrophoblast.
- Endometritis
-
Inflammation of the endometrium, the uterine mucosa, that is usually caused by bacterial infection and is characterized by the presence of plasma cells.
- Glycogen cells
-
A specialized trophoblast cell subtype found in the junctional zone of the mouse placenta that interacts with decidua and accumulates glycogen.
- Spongiotrophoblast
-
A trophoblast subtype present in the placental junctional zone in mice, the main function of which is the production of hormones.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moffett, A., Shreeve, N. Local immune recognition of trophoblast in early human pregnancy: controversies and questions. Nat Rev Immunol 23, 222–235 (2023). https://doi.org/10.1038/s41577-022-00777-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-022-00777-2