Abstract
Hepatitis B virus (HBV) is a non-cytopathic, hepatotropic virus with the potential to cause a persistent infection, ultimately leading to cirrhosis and hepatocellular carcinoma. Over the past four decades, the basic principles of HBV gene expression and replication as well as the viral and host determinants governing infection outcome have been largely uncovered. Whereas HBV appears to induce little or no innate immune activation, the adaptive immune response mediates both viral clearance as well as liver disease. Here, we review our current knowledge on the immunobiology and pathogenesis of HBV infection, focusing in particular on the role of CD8+ T cells and on several recent breakthroughs that challenge current dogmas. For example, we now trust that HBV integration into the host genome often serves as a relevant source of hepatitis B surface antigen (HBsAg) expression during chronic infection, possibly triggering dysfunctional T cell responses and favouring detrimental immunopathology. Further, the unique haemodynamics and anatomy of the liver — and the changes they frequently endure during disease progression to liver fibrosis and cirrhosis — profoundly influence T cell priming, differentiation and function. We also discuss why therapeutic approaches that limit the intrahepatic inflammatory processes triggered by HBV-specific T cells might be surprisingly beneficial for patients with chronic infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Guidotti, L. G. & Chisari, F. V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. Mech. Dis. 1, 23–61 (2006).
Locarnini, S., Hatzakis, A., Chen, D.-S. & Lok, A. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J. Hepatol. 62, S76–S86 (2015).
Yuen, M.-F. et al. Hepatitis B virus infection. Nat. Rev. Dis. Primers 4, 18035 (2018).
Revill, P. A. et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol. Hepatol. 4, 545–558 (2019).
Udompap, P. & Kim, W. R. Development of hepatocellular carcinoma in patients with suppressed viral replication: changes in risk over time. Clin. Liver Dis. 15, 85–90 (2020).
Levrero, M., Testoni, B. & Zoulim, F. HBV cure: why, how, when? Curr. Opin. Virol. 18, 135–143 (2016).
Fanning, G. C., Zoulim, F., Hou, J. & Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat. Rev. Drug Discov. 18, 827–844 (2019).
Rehermann, B., Ferrari, C., Pasquinelli, C. & Chisari, F. V. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 2, 1104–1108 (1996).
Kim, C. Y. & Tilles, J. G. Purification and biophysical characterization of hepatitis B antigen. J. Clin. Invest. 52, 1176–1186 (1973).
Seeger, C. & Mason, W. S. Molecular biology of hepatitis B virus infection. Virology 479, 672–686 (2015).
Bertoletti, A. & Ferrari, C. Adaptive immunity in HBV infection. J. Hepatol. 64, S71–S83 (2016).
Guidotti, L. G., Isogawa, M. & Chisari, F. V. Host–virus interactions in hepatitis B virus infection. Curr. Opin. Immunol. 36, 61–66 (2015).
Tu, T. et al. Integration occurs early in the viral life cycle in an in vitro infection model via sodium taurocholate cotransporting polypeptide-dependent uptake of enveloped virus particles. J. Virol. 92, e02007–e02017 (2018). This study shows that HBV DNA integration occurs early upon infection in an in vitro infection model.
Summers, J. et al. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc. Natl Acad. Sci. USA 100, 11652–11659 (2003).
Yang, W. & Summers, J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor. J. Virol. 73, 9710–9717 (1999).
Wooddell, C. I. et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci. Transl Med. 9, eaan0241 (2017). This paper reveals integrated HBV DNA as a relevant source of HBsAg in patients and chimpanzees with chronic infection.
Simon, T. G. et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N. Engl. J. Med. 382, 1018–1028 (2020). This manuscript represents one of a large number of meta-analyses describing an association between low-dose aspirin treatment and reduced HCC incidence.
Sitia, G. et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc. Natl Acad. Sci. USA 109, E2165–E2172 (2012). This preclinical study shows that anti-platelet therapy reduces liver fibrosis and prevents HCC in mouse models of CHB.
Iannacone, M., Sitia, G., Narvaiza, I., Ruggeri, Z. M. & Guidotti, L. G. Antiplatelet drug therapy moderates immune-mediated liver disease and inhibits viral clearance in mice infected with a replication-deficient adenovirus. Clin. Vaccine Immunol. 14, 1532–1535 (2007).
Jilbert, A. R., Miller, D. S., Scougall, C. A., Turnbull, H. & Burrell, C. J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226, 338–345 (1996).
Asabe, S. et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J. Virol. 83, 9652–9662 (2009).
Wisse, E., Jacobs, F., Topal, B., Frederik, P. & Geest, B. D. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 15, 1193–1199 (2008).
Vollmar, B. & Menger, M. D. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 89, 1269–1339 (2009).
Whalley, S. A. et al. Kinetics of acute hepatitis B virus infection in humans. J. Exp. Med. 193, 847–854 (2001).
Guidotti, L. G., Matzke, B., Schaller, H. & Chisari, F. V. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69, 6158–6169 (1995).
Guidotti, L. G. et al. Viral clearance without destruction of infected cells during acute HBV infection. Science 284, 825–829 (1999).
Wieland, S. F., Spangenberg, H. C., Thimme, R., Purcell, R. H. & Chisari, F. V. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc. Natl Acad. Sci. USA 101, 2129–2134 (2004).
Wieland, S., Thimme, R., Purcell, R. H. & Chisari, F. V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl Acad. Sci. USA 101, 6669–6674 (2004).
Suslov, A. et al. Virus does not interfere with innate immune responses in the human liver. Gastroenterology 154, 1778–1790 (2018).
Tsui, L. V., Guidotti, L. G., Ishikawa, T. & Chisari, F. V. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc. Natl Acad. Sci. USA 92, 12398–12402 (1995).
Heise, T., Guidotti, L. G., Cavanaugh, V. J. & Chisari, F. V. Hepatitis B virus RNA-binding proteins associated with cytokine-induced clearance of viral RNA from the liver of transgenic mice. J. Virol. 73, 474–481 (1999).
Heise, T., Guidotti, L. G. & Chisari, F. V. La autoantigen specifically recognizes a predicted stem-loop in hepatitis B virus RNA. J. Virol. 73, 5767–5776 (1999).
McClary, H., Koch, R., Chisari, F. V. & Guidotti, L. G. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74, 2255–2264 (2000).
Wieland, S. F., Guidotti, L. G. & Chisari, F. V. Intrahepatic induction of α/β interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 74, 4165–4173 (2000).
Kimura, K., Kakimi, K., Wieland, S., Guidotti, L. G. & Chisari, F. V. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J. Immunol. 169, 5188–5195 (2002).
Vilarinho, S., Ogasawara, K., Nishimura, S., Lanier, L. L. & Baron, J. L. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc. Natl Acad. Sci. USA 104, 18187–18192 (2007).
Isogawa, M., Robek, M. D., Furuichi, Y. & Chisari, F. V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 79, 7269–7272 (2005).
Suslov, A., Wieland, S. & Menne, S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr. Opin. Virol. 30, 9–17 (2018).
Iwasaki, A. A virological view of innate immune recognition. Annu. Rev. Microbiol. 66, 177–196 (2012).
Webster, G. J. M. et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32, 1117–1124 (2000).
Thimme, R. et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77, 68–76 (2003).
Hoofnagle, J. H., Gerety, R. J. & Barker, L. F. Antibody to hepatitis-B-virus core in man. Lancet 302, 869–873 (1973).
Maini, M. K. & Burton, A. R. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat. Rev. Gastroenterol. Hepatol. 16, 662–675 (2019).
Guidotti, L. G. et al. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl Acad. Sci. USA 91, 3764–3768 (1994).
Guidotti, L. G. et al. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4, 25–36 (1996).
Wong, Y. C., Tay, S. S., McCaughan, G. W., Bowen, D. G. & Bertolino, P. Immune outcomes in the liver: is CD8 T cell fate determined by the environment? J. Hepatol. 63, 1005–1014 (2015).
Isogawa et al. CD40 activation rescues antiviral CD8+ T cells from PD-1-mediated exhaustion. PLoS Pathog. 9, e1003490 (2013).
Bénéchet, A. P. et al. Dynamics and genomic landscape of CD8+ T cells undergoing hepatic priming. Nature 574, 200–205 (2019). This paper reveals that hepatocellular priming leads to a T cell dysfunction that is refractory to checkpoint inhibition but responds to IL-2.
Bertolino, P. et al. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 11, 1225–1238 (1999).
Pol et al. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 217, 2261 (2020).
Blattman, J. N. et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9, 540–547 (2003).
West, E. E. et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 123, 2604–2615 (2013).
Kuipery, A., Gehring, A. J. & Isogawa, M. Mechanisms of HBV immune evasion. Antivir. Res. 179, 104816 (2020).
Kennedy, P. T. F. et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 143, 637–645 (2012).
Shimizu, Y., Guidotti, L. G., Fowler, P. & Chisari, F. V. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J. Immunol. 161, 4520–4529 (1998).
Kakimi, K., Isogawa, M., Chung, J., Sette, A. & Chisari, F. V. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J. Virol. 76, 8609–8620 (2002).
Ishak, K. et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 22, 696–699 (1995).
Fisicaro, P. et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 23, 327–336 (2017). This article suggests a central role for reactive oxygen species in T cell exhaustion during CHB, thus providing novel potential therapeutic targets.
Wieland, S. F. The chimpanzee model for hepatitis B virus infection. CSH Perspect. Med. 5, a021469 (2015).
Chen, M. T. et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl Acad. Sci. USA 101, 14913–14918 (2004).
Chen, M. et al. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 79, 3016–3027 (2005).
Tian, Y., Kuo, C., Akbari, O. & Ou, J. J. Maternal-derived hepatitis B virus e antigen alters macrophage function in offspring to drive viral persistence after vertical transmission. Immunity 44, 1204–1214 (2016).
Publicover, J. et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J. Clin. Invest. 123, 3728–3739 (2013).
Brunetto, M. R. et al. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl Acad. Sci. USA 88, 4186–4190 (1991).
Rivino, L. et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J. Clin. Invest. 128, 668–681 (2018).
Schuch, A. et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut 68, 905–915 (2019).
Fumagalli, V. et al. Serum HBsAg clearance has minimal impact on CD8+ T cell responses in mouse models of HBV infection. J. Exp. Med. 217, e20200298 (2020). This study shows that circulating HBsAg clearance does not improve HBV-specific CD8+ T cell responses.
Bert, N. L. et al. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection. Gastroenterology 159, 652–664 (2020).
Li et al. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. eLife 6, e26738 (2017).
Zhang, T.-Y. et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut 65, 658 (2015).
Neumann et al. Novel mechanism of antibodies to hepatitis B virus in blocking viral particle release from cells. Hepatology 52, 875–885 (2010).
Galun, E. et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology 35, 673–679 (2002).
Bertoletti, A. et al. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J. Exp. Med. 180, 933–943 (1994).
Bertoletti, A. et al. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature 369, 407–410 (1994).
Maini, M. K. et al. T cell receptor usage of virus-specific CD8 cells and recognition of viral mutations during acute and persistent hepatitis B virus infection. Eur. J. Immunol. 30, 3067–3078 (2000).
Bertoletti, A. & Kennedy, P. T. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol. Immunol. 12, 258–263 (2015).
Fisicaro, P. et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front. Immunol. 11, 849 (2020).
Burton, A. R. et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J. Clin. Invest. 128, 4588–4603 (2018).
Salimzadeh, L. et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J. Clin. Invest. 128, 4573–4587 (2018). Together with Burton et al. (2018), this paper detects and characterizes dysfunctional HBsAg-specific B cell responses in patients with chronic HBV infection.
Tian, C. et al. Use of ELISpot assay to study HBs-specific B cell responses in vaccinated and HBV infected humans. Emerg. Microbes Infec 7, 16 (2018).
Xu, X. et al. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol. Immunol. 12, 309–316 (2015).
Bert, N. L. et al. Comparative characterization of B cells specific for HBV nucleocapsid and envelope proteins in patients with chronic hepatitis B. J. Hepatol. 72, 34–44 (2019).
Vanwolleghem, T. et al. Hepatitis B core-specific memory B cell responses associate with clinical parameters in patients with chronic HBV. J. Hepatol. 73, 52–61 (2020).
Milich, D. & McLachlan, A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 234, 1398–1401 (1986).
Guidotti, L. G. & Iannacone, M. Effector CD8 T cell trafficking within the liver. Mol. Immunol. 55, 94–99 (2013).
Iannacone, M. Hepatic effector CD8+ T-cell dynamics. Cell Mol. Immunol. 12, 269–272 (2015).
Inverso, D. & Iannacone, M. Spatiotemporal dynamics of effector CD8+ T cell responses within the liver. J. Leukoc. Biol. 99, 51–55 (2016).
Benechet, A. P. & Iannacone, M. Determinants of hepatic effector CD8+ T cell dynamics. J. Hepatol. 66, 228–233 (2017).
Guidotti, L. G. et al. Immunosurveillance of the liver by intravascular effector CD8+ T cells. Cell 161, 486–500 (2015). This manuscript reports that effector CD8+ T cells can recognize and kill antigen-expressing hepatocytes without extravasating by extending cytoplasmic protrusions through endothelial fenestration.
Sironi, L. et al. In vivo flow mapping in complex vessel networks by single image correlation. Sci. Rep. 4, 7341 (2014).
Warren, A. et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 44, 1182–1190 (2006).
Guidotti, L. G. The role of cytotoxic T cells and cytokines in the control of hepatitis B virus infection. Vaccine 20, A80–A82 (2002).
Fioravanti, J. et al. Effector CD8+ T cell-derived interleukin-10 enhances acute liver immunopathology. J. Hepatol. 67, 543–548 (2017).
Iannacone, M. & Guidotti, L. G. Mouse models of hepatitis B virus pathogenesis. CSH Perspect. Med. 5, a021477 (2015).
Guidotti, L. G., McClary, H., Loudis, J. M. & Chisari, F. V. Nitric oxide inhibits hepatitis b virus replication in the livers of transgenic mice. J. Exp. Med. 191, 1247–1252 (2000).
Wieland, S. F., Eustaquio, A., Whitten-Bauer, C., Boyd, B. & Chisari, F. V. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc. Natl Acad. Sci. USA 102, 9913–9917 (2005).
Robek, M. D., Wieland, S. F. & Chisari, F. V. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J. Virol. 76, 3570–3574 (2002).
Xia, Y. et al. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 150, 194–205 (2016).
Michalak, T. I., Pasquinelli, C., Guilhot, S. & Chisari, F. V. Hepatitis B virus persistence after recovery from acute viral hepatitis. J. Clin. Invest. 93, 230–239 (1994).
Pallett, L. J. et al. IL-2high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 214, 1567–1580 (2017). This paper characterizes tissue-resident memory T cells in the liver of patients chronically infected by HBV.
Ando, K. et al. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152, 3245–3253 (1994).
Nakamoto, Y., Guidotti, L. G., Pasquetto, V., Schreiber, R. D. & Chisari, F. V. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J. Immunol. 158, 5692–5697 (1997).
Sitia, G. et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 7, e1002061 (2011).
Sitia et al. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J. Leukoc. Biol. 81, 100–107 (2007).
Sitia, G. et al. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl Acad. Sci. USA 99, 13717–13722 (2002).
Sitia, G. et al. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Invest. 113, 1158–1167 (2004).
Kakimi, K. et al. Blocking chemokine responsive to γ-2/interferon (IFN)-γ inducible protein and monokine induced by IFN-γ activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 194, 1755–1766 (2001).
Maini, M. K. et al. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191, 1269–1280 (2000).
Reignat, S. et al. Escaping high viral load exhaustion CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 195, 1089–1101 (2002).
Webster, G. J. M. et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78, 5707–5719 (2004).
Boni, C. et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81, 4215–4225 (2007).
Hoogeveen, R. C. et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut 68, 893–904 (2018).
Nakamoto, Y., Guidotti, L. G., Kuhlen, C. V., Fowler, P. & Chisari, F. V. Immune pathogenesis of hepatocellular carcinoma. J. Exp. Med. 188, 341–350 (1998).
Isogawa, M., Furuichi, Y. & Chisari, F. V. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity 23, 53–63 (2005).
Khakpoor, A. et al. Spatiotemporal differences in presentation of CD8 T cell epitopes during hepatitis B virus infection. J. Virol. 93, e01457-18 (2018).
Nakamoto, Y., Suda, T., Momoi, T. & Kaneko, S. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 64, 3326–3333 (2004).
Tang, L. S. Y., Covert, E., Wilson, E. & Kottilil, S. Chronic hepatitis B infection: a review. JAMA 319, 1802–1813 (2018).
Buendia, M.-A. & Neuveut, C. Hepatocellular carcinoma. CSH Perspect. Med. 5, a021444 (2015).
Levrero, M. & Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 64, S84–S101 (2016).
Bisceglie, A. M. D. Hepatitis B and hepatocellular carcinoma. Hepatology 49, S56–S60 (2009).
Schuppan, D. & Afdhal, N. H. Liver cirrhosis. Lancet 371, 838–851 (2008).
Bataller, R. & Brenner, D. A. Liver fibrosis. J. Clin. Invest. 115, 209–218 (2005).
Friedman, S. L. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastr 1, 98–105 (2004).
Iannacone, M. et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat. Med. 11, 1167–1169 (2005). This study establishes platelets as critical mediators of liver damage through their capacity to promote liver homing of effector CD8+ T cells.
Ornelas, A. et al. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metast Rev. 36, 289–303 (2017).
Haemmerle, M., Stone, R. L., Menter, D. G., Afshar-Kharghan, V. & Sood, A. K. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell 33, 965–983 (2018).
Lee, P.-C. et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann. Surg. Oncol. 23, 874–883 (2016).
Hwang, I. C., Chang, J., Kim, K. & Park, S. M. Aspirin use and risk of hepatocellular carcinoma in a national cohort study of Korean adults. Sci. Rep. 8, 4968 (2018).
Simon, T. G. et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 4, 1683 (2018).
Lee, T.-Y. et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern. Med. 179, 633–640 (2019).
Wang, S. et al. Association of aspirin therapy with risk of hepatocellular carcinoma: a systematic review and dose–response analysis of cohort studies with 2.5 million participants. Pharmacol. Res. 151, 104585 (2019).
Liao, Y.-H. et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study. BMC Gastroenterol. 20, 6 (2020).
Bosetti, C., Santucci, C., Gallus, S., Martinetti, M. & Vecchia, C. L. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann. Oncol. 31, 558–568 (2020).
Hayashi, T. et al. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers 12, 3215 (2020).
Guidotti, L. G., Vecchia, C. L. & Colombo, M. Is it time to recommend low-dose aspirin treatment for the prevention of hepatocellular carcinoma? Gastroenterology 159, 1988–1990 (2020).
Martinez, M. G., Villeret, F., Testoni, B. & Zoulim, F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. 40, 27–34 (2020).
Hillis, W. D. Viral hepatitis associated with sub-human primates. Transfusion 3, 445–454 (1963).
Walter, E., Keist, R., Niederöst, B., Pult, I. & Blum, H. E. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology 24, 1–5 (1996).
Schulze, A., Gripon, P. & Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46, 1759–1768 (2007).
Sureau, C. & Salisse, J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus A-determinant. Hepatology 57, 985–994 (2013).
Roskams, T. et al. Heparan sulfate proteoglycan expression in normal human liver. Hepatology 21, 950–958 (1995).
Yan, H. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1, e00049 (2012).
Döring, B., Lütteke, T., Geyer, J. & Petzinger, E. The SLC10 carrier family: transport functions and molecular structure. Curr. Top. Membr. 70, 105–168 (2012).
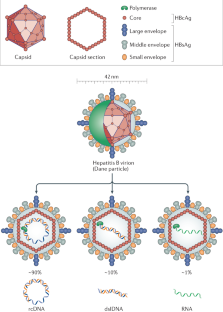
Hu, J. & Liu, K. Complete and incomplete hepatitis B virus particles: formation, function, and application. Viruses 9, 56 (2017).
Seitz, S., Habjanič, J., Schütz, A. K. & Bartenschlager, R. The hepatitis B virus envelope proteins: molecular gymnastics throughout the viral life cycle. Ann. Rev. Virol. 7, 1–26 (2020).
Wisse, E., de Zanger, R. B., Charels, K., Van Der Smissen, P. & McCuskey, R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of disse. Hepatology 5, 683–692 (1985).
Ficht, X. & Iannacone, M. Immune surveillance of the liver by T cells. Sci. Immunol. 5, eaba2351 (2020).
Iwakiri, Y. The lymphatic system: a new frontier in hepatology. Hepatology 64, 706–707 (2016).
Jenne, C. N. & Kubes, P. Immune surveillance by the liver. Nat. Immunol. 14, 996–1006 (2013).
Horst, A. K., Neumann, K., Diehl, L. & Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol. Immunol. 13, 277–292 (2016).
Wong, Y. C., McCaughan, G. W., Bowen, D. G. & Bertolino, P. The CD8 T-cell response during tolerance induction in liver transplantation. Clin. Transl Immunol. 5, e102 (2016).
Mason, W. S. et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 151, 986–998.e4 (2016).
Tu, T., Budzinska, M. A., Shackel, N. A. & Urban, S. HBV DNA integration: molecular mechanisms and clinical implications. Viruses 9, 75 (2017).
Budzinska, M. A., Shackel, N. A., Urban, S. & Tu, T. Cellular genomic sites of hepatitis B virus DNA integration. Genes 9, 365 (2018).
Huang, Z. M. & Yen, T. S. Dysregulated surface gene expression from disrupted hepatitis B virus genomes. J. Virol. 67, 7032–7040 (1993).
Dienes, H. P. et al. Hepatic expression patterns of the large and middle hepatitis B virus surface proteins in viremic and nonviremic chronic hepatitis B. Gastroenterology 98, 1017–1023 (1990).
Chisari, F. V. et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 59, 1145–1156 (1989).
Su, I., Wang, H., Wu, H. & Huang, W. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection. J. Gastroen Hepatol. 23, 1169–1174 (2008).
Hadziyannis, S., Gerber, M. A., Vissoulis, C. & Popper, H. Cytoplasmic hepatitis B antigen in “ground-glass” hepatocytes of carriers. Arch. Pathol. 96, 327–330 (1973).
Tu, T. et al. Clonal expansion of hepatocytes with a selective advantage occurs during all stages of chronic hepatitis B virus infection. J. Viral Hepat. 22, 737–753 (2015).
Acknowledgements
The authors thank M. Silva for secretarial assistance, F. Andreata for help with figure preparation and the members of the Iannacone and Guidotti laboratories for helpful discussions. They apologize to all authors whose work they could not cite due to space constraints. M.I. is supported by the European Research Council (ERC) Consolidator Grant 725038, ERC Proof of Concept Grant 957502, Italian Association for Cancer Research (AIRC) Grants 19891 and 22737, Italian Ministry of Health (MoH) Grants RF-2018-12365801 and COVID-2020-12371617, Lombardy Foundation for Biomedical Research (FRRB) Grant 2015-0010, the European Molecular Biology Organization Young Investigator Program and a Funded Research Agreement from Gilead Sciences. L.G.G. is supported by the AIRC Grant 22737, Lombardy Open Innovation Grant 229452, PRIN Grant 2017MPCWPY from the Italian Ministry of Education, University and Research, and Funded Research Agreements from Gilead Sciences, Avalia Therapeutics and CNCCS SCARL.
Author information
Authors and Affiliations
Contributions
M.I. and L.G.G. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
M.I. participates in advisory boards/consultancies for Gilead Sciences, Roche, Third Rock Ventures, Amgen, Asher Bio and Allovir. L.G.G is a member of the board of directors at Genenta Science and Epsilon Bio and participates in advisory boards/consultancies for Gilead Sciences, Roche and Arbutus Biopharma. M.I. and L.G.G. are inventors on patents filed, owned and managed by San Raffaele Scientific Institute, Vita-Salute San Raffaele University and Telethon Foundation on technology related to work discussed in this manuscript (WO2020/016434, WO2020/016427, WO2020/030781, WO2020/234483, EU patent applications n. 19211249.8 and n. 20156716.1, and UK patent application n. 1907493.9).
Additional information
Peer review information
Nature Reviews Immunology thanks Anna Lok, Antonio Bertoletti and Mala Maini for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Cirrhosis
-
The final stage of fibrosis in which fibrous septa surrounding nodules of regenerating hepatocytes induce profound architectural distortion of the liver and functional insufficiency.
- Cross-priming
-
A functional outcome of cross-presentation (the presentation of extracellular antigens on MHC class I molecules), whereby antigen-specific naive CD8+ T cells are activated by antigen-presenting cells to become effector cells.
- Checkpoint inhibitor therapy
-
A form of cancer immunotherapy targeting immune checkpoints (for example, PD1, CTLA4).
- SSB/La-dependent mechanism
-
T cell-induced cytokines such as IFNγ and TNF have been shown to induce the post-transcriptional downregulation of hepatitis B virus (HBV) RNAs in vivo. This process appears to rely on the degradation of the full-length SSB/La protein, which normally functions as a HBV RNA stabilizer in the nucleus of the hepatocyte.
- sALT values
-
The serum concentrations of the liver enzyme alanine aminotransferase. Commonly measured clinically as a biomarker for liver damage.
- Space of Disse
-
(Also referred to as perisinusoidal space). The space that lies between the hepatocytes and the sinusoids.
Rights and permissions
About this article
Cite this article
Iannacone, M., Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol 22, 19–32 (2022). https://doi.org/10.1038/s41577-021-00549-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-021-00549-4
This article is cited by
-
The combination of Schisandrin C and Luteolin synergistically attenuates hepatitis B virus infection via repressing HBV replication and promoting cGAS-STING pathway activation in macrophages
Chinese Medicine (2024)
-
Phosphorylation of RGS16 at Tyr168 promote HBeAg-mediated macrophage activation by ERK pathway to accelerate liver injury
Journal of Molecular Medicine (2024)
-
Insights into the impact of hepatitis B virus on hepatic stellate cell activation
Cell Communication and Signaling (2023)
-
CTLA4+CD4+CXCR5−FOXP3+ T cells associate with unfavorable outcome in patients with chronic HBV infection
BMC Immunology (2023)
-
Interferon stimulated immune profile changes in a humanized mouse model of HBV infection
Nature Communications (2023)