Abstract
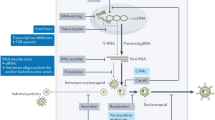
Chronic hepatitis B virus (HBV) infection is a common cause of liver disease globally, with a disproportionately high burden in South-East Asia. Vaccines and nucleoside or nucleotide drugs are available and reduce both new infection rates and the development of liver disease in HBV-positive persons who adhere to long-term suppressive treatment. Although there is still considerable value in optimizing access to virus-suppressing regimens, the scientific and medical communities have embarked on a concerted journey to identify new antiviral drugs and immune interventions aimed at curing infection. The mechanisms and drug targets being explored are diverse; however, the field universally recognizes the importance of addressing the persistence of episomal covalently closed circular DNA, the existence of integrated HBV DNA in the host genome and the large antigen load, particularly of hepatitis B surface antigen. Another major challenge is to reinvigorate the exhausted immune response within the liver microenvironment. Ultimately, combinations of new drugs will be required to cure infection. Here we critically review the recent literature that describes the rationale for curative therapies and the resulting compounds that are being tested in clinical trials for hepatitis B.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
08 January 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Nassal, M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 64, 1972–1984 (2015).
Seeger, C. & Mason, W. S. Molecular biology of hepatitis B virus infection. Virology 479–480, 672–686 (2015).
Fletcher, S. P. et al. Intrahepatic transcriptional signature associated with response to interferon-α treatment in the woodchuck model of chronic hepatitis B. PLOS Pathog. 11, e1005103 (2015).
European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 67, 370–398 (2017).
Hou, J. et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update). J. Clin. Transl Hepatol. 5, 297–318 (2017).
Durantel, D. & Zoulim, F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J. Hepatol. 64, S117–S131 (2016).
Liang, T. J. et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology 62, 1893–1908 (2015).
Terrault, N. A. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 4, 1560–1599 (2018).
Lok, A. S., Zoulim, F., Dusheiko, G. & Ghany, M. G. Hepatitis B cure: from discovery to regulatory approval. J. Hepatol. 67, 847–861 (2017).
World Health Organization. Global hepatitis report 2017 (WHO, 2017).
Cooke, G. S. et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology commission. Lancet Gastroenterol. Hepatol. 4, 135–184 (2019).
Yan, H. et al. Sodium taurocholate co-transporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1, e00049 (2012).
Li, M., Sohn, J. A. & Seeger, C. Distribution of hepatitis B virus nuclear DNA. J. Virol. 92, e01391-17 (2017).
Decorsière, A. et al. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature 531, 386–389 (2016).
Seeger, C., Ganem, D. & Varmus, H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science 232, 477–484 (1986).
Wang, J. et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J. Hepatol. 65, 700–710 (2016).
Bayliss, J. et al. Hepatitis B virus splicing is enhanced prior to development of hepatocellular carcinoma. J. Hepatol. 59, 1022–1028 (2013).
Soussan, P. et al. Expression of defective hepatitis B virus particles derived from singly spliced RNA is related to liver disease. J. Infect. Dis. 198, 218–225 (2008).
Werle-Lapostolle, B. et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126, 1750–1758 (2004).
Boyd, A. et al. Decay of ccc-DNA marks persistence of intrahepatic viral DNA synthesis under tenofovir in HIV-HBV co-infected patients. J. Hepatol. 65, 683–691 (2016).
Le Guerhier, F. et al. Characterization of the antiviral effect of 2′,3′-dideoxy-2′, 3′-didehydro-beta-L-5-fluorocytidine in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 44, 111–122 (2000).
Burdette, D. et al. Evidence for the presence of infectious virus in the serum from chronic hepatitis B patients suppressed on nucleos(t)ide therapy with detectable but not quantifiable HBV DNA [abstract PS-150]. J. Hepatol. 70, e95 (2019).
Allweiss, L. et al. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut 67, 542–552 (2018).
Schulze, A., Schieck, A., Ni, Y., Mier, W. & Urban, S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 84, 1989–2000 (2010).
Wedemeyer, H. et al. Final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferon Alpha 2a in patients with chronic HBV/HDV co-infection [abstract GS-13]. J. Hepatol. 70, e81 (2019).
Lucifora, J., Esser, K. & Protzer, U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral Res. 97, 195–197 (2013).
Watashi, K. et al. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology 59, 1726–1737 (2014).
Shimura, S. et al. Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J. Hepatol. 66, 685–692 (2017).
Li, D. et al. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. eLife 6, 213 (2017).
Zhang, T. Y. et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut 65, 658–671 (2016).
Neumann, A. U. et al. Novel mechanism of antibodies to hepatitis B virus in blocking viral particle release from cells. Hepatology 52, 875–885 (2010).
Galun, E. et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology 35, 673–679 (2002).
Freitas, N., Cunha, C., Menne, S. & Gudima, S. O. Envelope proteins derived from naturally integrated hepatitis B virus DNA support assembly and release of infectious hepatitis delta virus particles. J. Virol. 88, 5742–5754 (2014).
Königer, C. et al. Involvement of the host DNA-repair enzyme TDP2 in formation of the covalently closed circular DNA persistence reservoir of hepatitis B viruses. Proc. Natl Acad. Sci. USA 111, 4244–4253 (2014).
Long, Q. et al. The role of host DNA ligases in hepadnavirus covalently closed circular DNA formation. PLOS Pathog. 13, e1006784 (2017).
Maepa, M. B., Roelofse, I., Abdullah, E. & Patrick, A. Progress and prospects of anti-HBV gene therapy development. Int. J. Mol. Sci. 16, 17589–17610 (2015).
Bloom, K., Maepa, M. B., Ely, A. & Arbuthnot, P. Gene therapy for chronic HBV — can we eliminate cccDNA? Genes. 9, 207 (2018).
Seeger, C. & Sohn, J. A. Complete spectrum of CRISPR/Cas9-induced mutations on HBV cccDNA. Mol. Ther. 7, 1258–1266 (2016).
Wang, L. et al. A first-in-class orally available HBV cccDNA destabilizer ccc_R08 achieved sustainable HBsAg and HBV DNA suppression in the HBV circle mouse model through elimination of cccDNA-like molecules in the mouse liver [abstract PS-074]. J. Hepatol. 70, e48 (2019).
Seeger, C. Control of viral transcripts as a concept for future HBV therapies. Curr. Opin. Virol. 30, 18–23 (2018).
Rivière, L. et al. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J. Hepatol. 105, 1093–1102 (2015).
Belloni, L. et al. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc. Natl Acad. Sci. USA 106, 19975–19979 (2009).
Lucifora, J. et al. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J. Hepatol. 55, 996–1003 (2011).
Murphy, C. M. et al. Hepatitis B virus X protein promotes degradation of SMC5/6 to enhance HBV replication. Cell Rep. 16, 2846–2854 (2016).
Sekiba, K. et al. Pevonedistat, a neuronal precursor cell-expressed developmentally down-regulated protein 8-activating enzyme inhibitor, is a potent inhibitor of hepatitis B virus. Hepatology 69, 1903–1915 (2019).
Belloni, L. et al. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J. Clin. Invest. 122, 529–537 (2012).
Sekiba, K. et al. Inhibition of HBV transcription from cccDNA with nitazoxanide by targeting the HBx–DDB1 interaction. Cell. Mol. Gastroenterol. Hepatol. 7, 297–312 (2019).
Chong, C. K. et al. Role of hepatitis B core protein in HBV transcription and recruitment of histone acetyltransferases to cccDNA minichromosome. Antiviral Res. 144, 1–7 (2017).
Tropberger, P. et al. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc. Natl Acad. Sci. USA 112, E5715–E5724 (2015).
Cougot, D. et al. The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J. Biol. Chem. 282, 4277–4287 (2007).
Gilmore, S. et al. Antiviral activity of GS-5801, a liver-targeted prodrug of a lysine demethylase 5 inhibitor, in a hepatitis B virus primary human hepatocyte infection model [abstract SAT-160]. J. Hepatol. 66, S690–S691 (2017).
Maepa, M. B., Roelofse, I., Ely, A. & Arbuthnot, P. Progress and prospects of anti-HBV gene therapy development. Int. J. Mol. Sci. 16, 17589–17610 (2015).
Wooddell, C. I. et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci. Transl Med. 9, eaan0241 (2017).
Billioud, G. et al. In vivo reduction of hepatitis B virus antigenemia and viremia by antisense oligonucleotides. J. Hepatol. 64, 781–789 (2016).
Yuen, M. F. et al. Short term RNA interference therapy in chronic hepatitis B using JNJ-3989 brings majority of patients to HBsAg < 100IUml threshold [abstract PS-080]. J. Hepatol. 70, e51 (2019).
Mueller, H. et al. A novel orally available small molecule that inhibits hepatitis B virus expression. J. Hepatol. 68, 412–420 (2018).
Zhou, T. et al. HBsAg mRNA degradation induced by a dihydroquinolizinone compound depends on the HBV posttranscriptional regulatory element. Antiviral Res. 149, 191–201 (2018).
Zhang, H. et al. Safety, pharmacokinetics and anti-viral efficacy of novel core protein allosteric modifier GLS4 in patients with chronic hepatitis B: interim results from a 48 weeks phase 2a study [abstract LB-13]. Hepatology 68, 1454A–1455A (2018).
Gane, E. et al. RO7049389, a core protein allosteric modulator, demonstrates robust decline in HBV DNA and HBV RNA in chronic HBV infected patients [abstract]. J. Hepatol. 70, e491 (2019).
Katen, S. P., Chirapu, S. R., Finn, M. G. & Zlotnick, A. Trapping of hepatitis B virus capsid assembly intermediates by phenylpropenamide assembly accelerators. ACS Chem. Biol. 5, 1125–1136 (2010).
Sari, O. et al. Synthesis of sulfamoylbenzamide derivatives as HBV capsid assembly effector. Eur. J. Med. Chem. 138, 407–421 (2017).
Yuen, M. F. et al. Antiviral activity, safety, and pharmacokinetics of capsid assembly modulator NVR 3–778 in patients with chronic HBV infection. Gastroenterology 156, 1392–1403 (2019).
Zoulim, F. et al. Safety, pharmacokinetics and antiviral activity of a novel hepatitis B virus (HBV) capsid assembly modulator, JNJ-56136379, in patients with chronic hepatitis B (CHB) [abstract]. Hepatology 68, 47A (2018).
Kakuda, T. et al. JNJ64530440, a novel capsid assembly modulator: single- and multiple-ascending dose safety, tolerability and pharmacokinetics in healthy volunteers [abstract FRI-180]. J. Hepatol. 70, e469 (2019).
Eley, T. et al. Single dose safety, tolerability, and pharmacokinetics of AB-423 in healthy volunteers from the ongoing single and multiple ascending dose study AB-423-001 [abstract]. Hepatology 66, A490 (2017).
Ma, X. et al. Interim safety and efficacy results of the ABI-H0731 phase 2a program exploring the combination of ABI-H0731 with Nuc therapy in treatment-naïve and treatment-suppressed chronic hepatitis B patients [abstract LBO-06]. J. Hepatol. 70, e130 (2019).
Lahlali, T. et al. Novel potent capsid assembly modulators regulate multiple steps of the hepatitis B virus life-cycle. Antimicrob. Agents Chemother. 62, e00835 (2018).
Vaillant, A. Nucleic acid polymers: broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Res. 133, 32–40 (2016).
Bazinet, M. et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 12, 877–889 (2017).
Bazinet, M. et al. Establishment of high rates of functional cure of HBeAg negative chronic HBV infection with REP 2139-Mg based combination therapy: ongoing follow-up results from the REP 401 study [abstract FRI-210]. J. Hepatol. 70, e486 (2019).
Zhou, K., Contag, C., Whitaker, E. & Terrault, N. Spontaneous loss of surface antigen among adults living with chronic hepatitis B virus infection: a systematic review and pooled meta-analyses. Lancet Gastroenterol. Hepatol. 4, 227–238 (2019).
Bertoletti, A. & Ferrari, C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 61, 1754–1764 (2012).
Lau, G. K. et al. Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology 25, 1497–1501 (1997).
Ilan, Y. et al. Adoptive transfer of immunity to hepatitis B virus after T cell-depleted allogeneic bone marrow transplantation. Hepatology 18, 246–252 (1993).
Thimme, R. et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77, 68–76 (2003).
Yeo, W. et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J. Clin. Oncol. 27, 605–611 (2009).
Lebossé, F. et al. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J. Hepatol. 66, 897–909 (2017).
Wieland, S., Thimme, R., Purcell, R. H. & Chisari, F. V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl Acad. Sci. USA 101, 6669–6674 (2004).
Mutz, P. et al. HBV bypasses the innate immune response and does not protect HCV from antiviral activity of interferon. Gastroenterology 154, 1791–1804 (2018).
Suslov, A., Boldanova, T., Wang, X., Wieland, S. & Heim, M. H. Hepatitis B virus does not interfere with innate immune responses in the human liver. Gastroenterology 154, 1778–1790 (2018).
Gehring, A. J. & Ann D’Angelo, J. Dissecting the dendritic cell controversy in chronic hepatitis B virus infection. Cell. Mol. Immunol. 12, 283–291 (2015).
Maini, M. K. & Gehring, A. J. The role of innate immunity in the immunopathology and treatment of HBV infection. J. Hepatol. 64, S60–S70 (2016).
Gehring, A. J. & Protzer, U. Targeting innate and adaptive immune responses to cure chronic HBV infection. 156, 325–337 (2019).
Liaw, Y. F. et al. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology 126, 1024–1029 (2004).
Sagnelli, E. et al. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology 36, 1285–1291 (2002).
Sato, S. et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 42, 123–132 (2015).
Lucifora, J. et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 343, 1221–1228 (2014).
McClary, H., Koch, R., Chisari, F. V. & Guidotti, L. G. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74, 2255–2264 (2000).
Watashi, K. et al. Interleukin-1 and tumor necrosis factor-α trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID). J. Biol. Chem. 288, 31715–31727 (2013).
Puro, R. & Schneider, R. J. Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA. J. Virol. 81, 7351–7362 (2007).
Suslov, A., Wieland, S. & Menne, S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr. Opin. Virol. 30, 9–17 (2018).
Maini, M. K. & Peppa, D. NK cells: a double-edged sword in chronic hepatitis B virus infection. Front. Immunol. 4, 57 (2013).
Thimme, R. & Dandri, M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J. Hepatol. 58, 205–209 (2013).
Fisicaro, P. et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 58, 974–982 (2009).
Webster, G. J. et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32, 1117–1124 (2000).
Peppa, D. et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J. Exp. Med. 210, 99–114 (2013).
Bertoletti, A. & Gehring, A. J. Immune therapeutic strategies in chronic hepatitis B virus infection: virus or inflammation control? PLOS Pathog. 9, e1003784 (2013).
Zoulim, F., Luangsay, S. & Durantel, D. Targeting innate immunity: a new step in the development of combination therapy for chronic hepatitis B. Gastroenterology 144, 1342–1344 (2013).
Peppa, D. et al. Interferon alpha induces sustained changes in NK cell responsiveness to hepatitis B viral load suppression in vivo. PLOS Pathog. 12, e1005788 (2016).
Micco, L. et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J. Hepatol. 58, 225–233 (2013).
Xia, Y. et al. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 150, 194–205 (2016).
Chisari, F. V., Mason, W. S. & Seeger, C. Comment on “Specific and non-hepatotoxic degradation of nuclear hepatitis B virus cccDNA”. Science 344, 1237 (2014).
Ji, C. et al. Targeted delivery of interferon-α to hepatitis B virus-infected cells using T cell receptor-like antibodies. Hepatology 56, 2027–2038 (2012).
Guo, H. et al. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J. Virol. 83, 847–858 (2009).
Guo, F. et al. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob. Agents Chemother. 59, 1273–1281 (2015).
Lanford, R. E. et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 144, 1508–1517 (2013).
Colledge, D. et al. Inarigivir is a novel selective inhibitor of the HBV replicase complex in vitro [abstract]. Hepatology 68, 229A (2018).
Yuen, M. F. et al. Ascending dose cohort study of inarigivir – a novel RIG I agonist in chronic HBV patients: final results of the ACHIEVE trial [abstract GS-12]. J. Hepatol. 70, e47–e48 (2019).
Menne, S. et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J. Hepatol. 62, 1237–1245 (2015).
Gane, E. J. et al. The oral Toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J. Hepatol. 63, 320–328 (2015).
Boni, C. et al. TLR7 agonist increases responses of hepatitis B virus-specific T cells and natural killer cells in patients with chronic hepatitis B treated with nucleos(t)ide analogues. Gastroenterology 154, 1764–1777 (2018).
Jo, J. et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLOS Pathog. 10, e1004210 (2014).
Tang, X. Z. et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J. Immunol. 190, 3142–3152 (2013).
Schurich, A. et al. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLOS Pathog. 9, e1003208 (2013).
Boni, C. et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81, 4215–4225 (2007).
Fisicaro, P. et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 138, 682–693 (2010).
Salimzadeh, L. et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J. Clin. Invest. 128, 4573–4587 (2018).
Burton, A. R. et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J. Clin. Invest. 128, 4588–4603 (2018).
Verdon, D. et al. Immunological assessment of HBeAg-negative chronic hepatitis B patient responses following anti-PD-1 treatment [abstract 41]. Hepatology 66 (Suppl. 1), 23A (2017).
Michel, M. L., Pol, S., Brechot, C. & Tiollais, P. Immunotherapy of chronic hepatitis B by anti HBV vaccine: from present to future. Vaccine 19, 2395–2399 (2001).
Mancini-Bourgine, M. et al. Induction or expansion of T cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 40, 874–882 (2004).
Vandepapelière, P. et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 25, 8585–8597 (2007).
Xu, D. Z. et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J. Hepatol. 59, 450–456 (2013).
Godon, O. et al. Immunological and antiviral responses after therapeutic DNA immunization in chronic hepatitis B patients efficiently treated by analogues. Mol. Ther. 22, 675–684 (2013).
Lok, A. S. et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 65, 509–516 (2016).
Boni, C. et al. Combined GS-4774 and tenofovir therapy can improve HBV-specific T-cell responses in patients with chronic hepatitis. Gastroenterology 157, 227–241 (2019).
Liu, J. et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLOS Pathog. 10, e1003856 (2014).
Wu, W. et al. Blockade of Tim-3 signaling restores the virus-specific CD8+ T cell response in patients with chronic hepatitis B. Eur. J. Immunol. 42, 1180–1191 (2012).
Kurktschiev, P. D. et al. Dysfunctional CD8+ T cells in hepatitis B and C are characterized by a lack of antigen-specific T-bet induction. J. Exp. Med. 211, 167 (2014).
Bengsch, B., Martin, B. & Thimme, R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J. Hepatol. 61, 1212–1219 (2014).
Schurich, A. et al. Distinct metabolic requirements of exhausted and functional virus-specific CD8 T cells in the same host. Cell Rep. 16, 1243–1252 (2016).
Fisicaro, P. et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 23, 327–336 (2017).
Kah, J. et al. Lymphocytes transiently expressing virus-specific T cell receptors reduce hepatitis B virus infection. J. Clin. Invest. 127, 3177–3188 (2017).
Koh, S. et al. Non-lytic lymphocytes engineered to express virus-specific T cell receptors limit HBV infection by activating APOBEC3. Gastroenterology 155, 180–193 (2018).
Bohne, F. et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology 134, 239–247 (2008).
Gehring, A. J. et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J. Hepatol. 55, 103–110 (2011).
Krebs, K. et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology 145, 456–465 (2013).
Qasim, W. et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J. Hepatol. 62, 486–491 (2015).
Kaiser, A. D. et al. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther. 22, 72–78 (2015).
Sastry, K. S. et al. Targeting hepatitis B virus-infected cells with a T cell receptor-like antibody. J. Virol. 85, 1935–1942 (2011).
Oates, J., Hassan, N. J. & Jakobsen, B. K. ImmTACs for targeted cancer therapy: why, what, how, and which. Mol. Immunol. 67, 67–74 (2015).
Yang, H. et al. Elimination of latently HIV-infected cells from antiretroviral therapy-suppressed subjects by engineered immune-mobilizing T cell receptors. Mol. Ther. 24, 1913–1925 (2016).
Liu, F. et al. Alpha-interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLOS Pathog. 9, e1003613 (2013).
Kim, K. A. et al. Hepatic SOCS3 expression is strongly associated with non-response to therapy and race in HCV and HCV/HIV infection. J. Hepatol. 50, 705–711 (2009).
Fletcher, S. P. et al. Transcriptomic analysis of the woodchuck model of chronic hepatitis B. Hepatology 11, 820–830 (2012).
Koeberlein, B. et al. Hepatitis B virus overexpresses suppressor of cytokine signaling-3 (SOCS3) thereby contributing to severity of inflammation in the liver. Virus Res. 148, 51–59 (2010).
Tan-Garcia, A. et al. Intrahepatic CD206+ macrophages contribute to inflammation in advanced viral-related liver disease. J. Hepatol. 67, 490–500 (2017).
Beyer, M. et al. Tumor-necrosis factor impairs CD4+ T cell–mediated immunological control in chronic viral infection. Nat. Immunol. 17, 593–603 (2016).
Das, A. et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 205, 2111–2124 (2008).
Sandalova, E. et al. Increased levels of arginase in patients with acute hepatitis B suppress antiviral T cells. Gastroenterology 143, 78–87 (2012).
Pallett, L. J. et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat. Med. 21, 591–600 (2015).
Guidotti, L. G. et al. Immunosurveillance of the liver by intravascular effector CD8+ T cells. Cell 161, 486–500 (2015).
Op den Brouw, M. L. et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology 126, 280–289 (2009).
Woltman, A. M. et al. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLOS ONE 6, e15324 (2011).
Xu, Y. et al. HBsAg inhibits TLR9-mediated activation and IFN-α production in plasmacytoid dendritic cells. Mol. Immunol. 46, 2640–2646 (2009).
Shi, B. et al. HBsAg inhibits IFN-α production in plasmacytoid dendritic cells through TNF-α and IL-10 induction in monocytes. PLOS ONE 7, e44900 (2017).
Visvanathan, K. et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the pre-core protein. Hepatology 45, 102–110 (2006).
Wu, J. et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 49, 1132–1140 (2009).
Martinet, J. et al. Altered functions of plasmacytoid dendritic cells and reduced cytolytic activity of natural killer cells in patients with chronic HBV infection. Gastroenterology 143, 1586–1596 (2012).
Gehring, A. J. et al. Mobilizing monocytes to cross-present circulating viral antigen in chronic infection. J. Clin. Invest. 123, 3766–3776 (2013).
Andrade, B. B. et al. Hepatitis B infection is associated with asymptomatic malaria in the Brazilian Amazon. PLOS ONE 6, e19841 (2011).
Hong, M. et al. Trained immunity in newborn infants of HBV-infected mothers. Nat. Commun. 6, 6588 (2015).
Milich, D. R. et al. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl Acad. Sci. USA 87, 6599–6603 (1990).
Park, J. J. et al. Hepatitis B virus—specific and global T-cell dysfunction in chronic hepatitis B. Gastroenterology 150, 684–695 (2015).
Schuch, A. et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut 68, 905–915 (2019).
Hoogeveen, R. C. et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut 68, 893–904 (2019).
Bertoletti, A. & Kennedy, P. T. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell. Mol. Immunol. 12, 258–263 (2015).
Dolman, G. E., Koffas, A., Mason, W. S. & Kennedy, P. T. Why, who and when to start treatment for chronic hepatitis B virus infection. Curr. Opin. Virol. 30, 39–47 (2018).
Revill, P. A. et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol. Hepatol. 4, 545–558 (2019).
Anderson, R. T. et al. Challenges, considerations and principles to guide trials of combination therapies for chronic HBV infection. Gastroenterology 156, 529–533 (2019).
Zoulim, F. & Mason, W. S. Reasons to consider earlier treatment of chronic HBV infections. Gut 61, 333–336 (2012).
Armas, D. et al. A phase I single ascending dose study of CRV431 [abstract]. J. Hepatol. 70, e464 (2019).
Agarwal, K. et al. Bi-weekly dosing of ARB-1467 LNP siRNA in HBeAg negative, virally suppressed patients with chronic HBV infection leads to deeper declines in HBsAg and potential association with IL28b [abstract]. Hepatology 66, LB17 (2017).
Han, K. et al. Safety, tolerability and pharmacokinetics of GSK3389404, an antisense oligonucleotide for the treatment of chronic hepatitis B (CHB) infection: a randomized, double-blind, placebo-controlled, dose-escalation, first time in human study [abstract]. Hepatology 66, A490 (2017).
Arbutus Biopharma. Press release: Arbutus reports first quarter 2019 financial results and provides corporate update. Arbutus Biopharma https://investor.arbutusbio.com/news-releases/news-release-details/arbutus-reports-first-quarter-2019-financial-results-and (2019).
Gane, E. et al. TLR7 agonist RO7020531 triggers immune activation after multiple doses in chronic hepatitis B patients [abstract]. Hepatology 68, LB–33 (2018).
Reyes, M. et al. First in human study of GS-9688, an oral Toll-like receptor 8 (TLR8) agonist, in healthy volunteers: assessment of safety, tolerability, pharmacokinetics, pharmacodynamics and food effect [abstract]. Hepatology 68, 233A (2018).
Fournier, C. et al. Safety and immunogenicity of single and multiple injections of the therapeutic vaccine TG1050 in NUC-suppressed chronic hepatitis B (CHB) patients: unblinded analysis of a double-blind, placebo-controlled phase 1b study [abstract]. Hepatology 68, 252A (2018).
Hu, Y. et al. A phase 1 clinical trial of therapeutic vaccine t101 in chronic hepatitis b patients: a randomized, double-blind, placebo controlled, single and multiple injections, dose escalation study [abstract]. J. Hepatol. 70, e153 (2019).
Lim, Y. S. et al. A phase 1b evaluation of HepTcell HBV-specific immunotherapy in nuc-controlled, eAg negative chronic HBV infection [abstract]. J. Hepatol. 70, e50 (2019).
Wang, F. S., Fan, J. G., Zhang, Z., Gao, B. & Wang, H. Y. The global burden of liver disease: the major impact of China. Hepatology 60, 2099–2108 (2014).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study. Lancet 380, 2095–2128 (2010).
Xia, G. ‑L. et al. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Int. Hepatol. Comm. 5, 62–73 (1996).
Liang, X. et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J. Infect. Dis. 200, 39–47 (2009).
Ott, J. J., Stevens, G. A., Groeger, J. & Wiersma, S. T. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30, 2212–2219 (2012).
Velkov, S., Ott, J. J., Protzer, U. & Michler, T. The global hepatitis B virus genotype distribution approximated from available genotyping data. Genes 9, 495 (2018).
Rajoriya, N., Combet, C., Zoulim, F. & Janssen, H. L. A. How viral genetic variants and genotypes influence disease and treatment outcome of chronic hepatitis B. Time for an individualized approach? J. Hepatol. 67, 1281–1297 (2017).
Testoni, B., Levrero, M. & Zoulim, F. Challenges to a cure for HBV infection. Semin. Liver Dis. 37, 231–242 (2017).
Cornberg, M. et al. The role of quantitative hepatitis B surface antigen revisited. J. Hepatol. 66, 398–411 (2017).
Butler, E. K. et al. Hepatitis B virus serum DNA and RNA Levels in nucleos(t)ide analog-treated or untreated patients during chronic and acute infection. Hepatology 68, 2106–2117 (2018).
Liu, S. et al. RNA: a new potential biomarker for chronic hepatitis B virus infection. Hepatology 69, 1816–1827 (2019).
Honda, M. et al. Hepatitis B virus (HBV) core-related antigen during nucleos(t)ide analog therapy is related to intra-hepatic HBV replication and development of hepatocellular carcinoma. J. Infect. Dis. 213, 1096–1106 (2016).
Testoni, B. et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J. Hepatol. 70, 615–625 (2019).
Jilbert, A. R., Miller, D. S., Scougall, C. A., Turnbull, H. & Burrell, C. J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226, 338–345 (1996).
Chen, X. et al. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus-infected mongolians. Hepatology 66, 1739–1749 (2017).
Liao, B. et al. Epidemiological, clinical and histological characteristics of HBV/HDV co-infection: a retrospective cross-sectional study in Guangdong. PLOS ONE 9, e115888 (2014).
Lempp, F. A., Ni, Y. & Urban, S. Hepatitis delta virus: insights into a peculiar pathogen and novel treatment options. Nat. Rev. Gastroenterol. Hepatol. 13, 580–589 (2016).
Yurdaydin, C. et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: the LOWR HDV-1 study. Hepatology 67, 1224–1236 (2018).
US Food and Drug Administration. Codevelopment of two or more new investigational drugs for use in combination. FDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/codevelopment-two-or-more-new-investigational-drugs-use-combination (2013).
Lempp, F. A. et al. Sodium taurocholate co-transporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes. Hepatology 66, 703–716 (2017).
Dandri, M. & Petersen, J. Animal models of HBV infection. Best Pract. Res. Clin. Gastroenterol. 31, 273–279 (2017).
Strick-Marchand, H. et al. A novel mouse model for stable engraftment of a human immune system and human hepatocytes. PLOS ONE 10, e0119820 (2015).
Cote, P. J. et al. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 31, 190–200 (2000).
Burwitz, B. J. et al. Hepatocytic expression of human sodium-taurocholate cotransporting polypeptide enables hepatitis B virus infection of macaques. Nat. Commun. 8, 2146 (2017).
Xu, D. et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J. Immunol. 177, 739–747 (2006).
Liang, X. et al. Reprint of: Epidemiological serosurvey of hepatitis B in China — declining HBV prevalence due to hepatitis B vaccination. Vaccine 31, J21–J28 (2013).
Liu, J. et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21–49 years in rural China: a population-based, cross-sectional study. Lancet Infect. Dis. 16, 80–86 (2016).
Cui, F. et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerging Infect. Dis. 23, 765 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.C.F. is an employee of Janssen Pharmaceuticals. A.B. participates in advisory boards/consultancy on hepatitis B virus (HBV) immune therapy for Gilead, Janssen, Vir, Jiangsu Simcere Pharmaceutical and Humabs BioMed; is the scientific founder of Lion TCR Pte. Ltd.; is an advisor for Arbutus, Assembly, Gilead, Janssen, Myr Pharma, Roche, Transgene and Vir Biotech; and has received research grants from Evotec and Roche. F.Z. participates in advisory boards on HBV for AbbVie, Aligos, Gilead, Janssen, Myr Pharma, Spring Bank, Roche, Transgene and Vir Biotech; and has received research grants from Evotec and Roche. J.H. participates in advisory boards/consultancy for AbbVie, Arbutus, Bristol–Myers Squibb, Gilead Sciences, Johnson & Johnson and Roche, and has received grants from Bristol–Myers Squibb and Johnson & Johnson.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fanning, G.C., Zoulim, F., Hou, J. et al. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov 18, 827–844 (2019). https://doi.org/10.1038/s41573-019-0037-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-019-0037-0
This article is cited by
-
Leveraging stem cells to combat hepatitis: a comprehensive review of recent studies
Molecular Biology Reports (2024)
-
The Microbiome Matters: Its Impact on Cancer Development and Therapeutic Responses
Journal of Microbiology (2024)
-
CTLA4+CD4+CXCR5−FOXP3+ T cells associate with unfavorable outcome in patients with chronic HBV infection
BMC Immunology (2023)
-
Single-cell RNA sequencing reveals the mediatory role of cancer-associated fibroblast PTN in hepatitis B virus cirrhosis-HCC progression
Gut Pathogens (2023)
-
Molecular elucidation of drug-induced abnormal assemblies of the hepatitis B virus capsid protein by solid-state NMR
Nature Communications (2023)