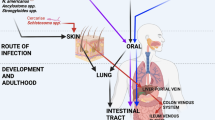
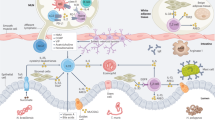
Abstract
Type 2 immune responses operate under varying conditions in distinct tissue environments and are crucial for protection against helminth infections and for the maintenance of tissue homeostasis. Here we explore how different layers of heterogeneity influence type 2 immunity. Distinct insults, such as allergens or infections, can induce type 2 immune responses through diverse mechanisms, and this can have heterogeneous consequences, ranging from acute or chronic inflammation to deficits in immune regulation and tissue repair. Technological advances have provided new insights into the molecular heterogeneity of different developmental lineages of type 2 immune cells. Genetic and environmental heterogeneity also contributes to the varying magnitude and quality of the type 2 immune response during infection, which is an important determinant of the balance between pathology and disease resolution. Hence, understanding the mechanisms underlying the heterogeneity of type 2 immune responses between individuals and between different tissues will be crucial for treating diseases in which type 2 immunity is an important component.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gause, W. C., Wynn, T. A. & Allen, J. E. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. 13, 607–614 (2013).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012). This review introduces the concept of disease tolerance as a mechanism of host defence.
Kouzaki, H., Iijima, K., Kobayashi, T., O’Grady, S. M. & Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 186, 4375–4387 (2011).
Csoka, B. et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 26, 376–386 (2012).
Patel, N. et al. A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe 15, 339–350 (2014). This study establishes adenosine as an important DAMP for initiating type 2 immune responses.
Wills-Karp, M. et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J. Exp. Med. 209, 607–622 (2012).
Belle, N. M. et al. TFF3 interacts with LINGO2 to regulate EGFR activation for protection against colitis and gastrointestinal helminths. Nat. Commun. 10, 4408 (2019).
Bosurgi, L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017). This study shows that apoptotic neutrophils synergize with type 2 cytokines to activate macrophages to adopt tissue repair properties.
Chen, F. et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 15, 938–946 (2014). Trained macrophages are shown to mediate acquired resistance during helminth infection, and neutrophil signalling is required for the development of the anti-helminth macrophage phenotype.
Ritter, M. et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc. Natl Acad. Sci. USA 107, 20459–20464 (2010).
Everts, B. et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 206, 1673–1680 (2009).
Steinfelder, S. et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J. Exp. Med. 206, 1681–1690 (2009).
Everts, B. et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 209, 1753–1767 (2012).
Maizels, R. M., Smits, H. H. & McSorley, H. J. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity 49, 801–818 (2018). This review discusses how different molecules produced by parasitic helminths modulate the host immune response.
Johnston, C. J. C. et al. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 8, 1741 (2017).
Kuroda, E. et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity 34, 514–526 (2011).
McKee, A. S. et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 183, 4403–4414 (2009).
Pelka, K. & Latz, E. Getting closer to the dirty little secret. Immunity 34, 455–458 (2011).
Mishra, P. K. et al. Micrometer-sized titanium particles can induce potent Th2-type responses through TLR4-independent pathways. J. Immunol. 187, 6491–6498 (2011).
Kool, M. et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 34, 527–540 (2011).
Persson, E. K. et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 364, eaaw4295 (2019).
Chen, G. Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Eisenbarth, S. C. et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196, 1645–1651 (2002).
Franchi, L. & Nunez, G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38, 2085–2089 (2008).
Mishra, P. K. et al. Sterile particle-induced inflammation is mediated by macrophages releasing IL-33 through a Bruton’s tyrosine kinase-dependent pathway. Nat. Mater. 18, 289–297 (2019). Sterile microparticles are shown to induce type 2 immune responses, leading to fibrosis through specific kinase and programmed cell death signalling pathways.
Ng, G. et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29, 807–818 (2008).
Flach, T. L. et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 17, 479–487 (2011).
Benmerzoug, S. et al. Sterile lung inflammation induced by silica exacerbates mycobacterium tuberculosis infection via STING-dependent type 2 immunity. Cell Rep. 27, 2649–2664 (2019).
Wang, J. & Kubes, P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165, 668–678 (2016).
Gieseck, R. L. 3rd, Wilson, M. S. & Wynn, T. A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 18, 62–76 (2018).
Roan, F., Obata-Ninomiya, K. & Ziegler, S. F. Epithelial cell-derived cytokines: more than just signaling the alarm. J. Clin. Invest. 129, 1441–1451 (2019).
Han, H., Roan, F. & Ziegler, S. F. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 278, 116–130 (2017).
Afferni, C. et al. The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front. Immunol. 9, 2601 (2018).
Hardman, C. S., Panova, V. & McKenzie, A. N. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur. J. Immunol. 43, 488–498 (2013).
Minutti, C. M. et al. Epidermal growth factor receptor expression licenses type-2 helper T cells to function in a T cell receptor-independent fashion. Immunity 47, 710–722 (2017).
Humphreys, N. E., Xu, D., Hepworth, M. R., Liew, F. Y. & Grencis, R. K. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. 180, 2443–2449 (2008).
Hung, L. Y. et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc. Natl Acad. Sci. USA 110, 282–287 (2013).
Han, H. et al. IL-33 promotes gastrointestinal allergy in a TSLP-independent manner. Mucosal Immunol. 11, 394–403 (2018).
Bonilla, W. V. et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science 335, 984–989 (2012).
Bourgeois, E. et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur. J. Immunol. 39, 1046–1055 (2009).
Smithgall, M. D. et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Vainchtein, I. D. et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273 (2018).
Chen, Y. L. et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 11, eaax2945 (2019).
Chinthrajah, S. et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight https://doi.org/10.1172/jci.insight.131347 (2019).
Ito, T. et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202, 1213–1223 (2005).
Liu, Y. J. et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 25, 193–219 (2007).
Omori, M. & Ziegler, S. Induction of IL-4 expression in CD4+ T cells by thymic stromal lymphopoietin. J. Immunol. 178, 1396–1404 (2007).
Rochman, Y. et al. TSLP signaling in CD4+ T cells programs a pathogenic T helper 2 cell state. Sci. Signal. 11, eaam8858 (2018).
Allakhverdi, Z. et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204, 253–258 (2007).
Siracusa, M. C. et al. Thymic stromal lymphopoietin-mediated extramedullary hematopoiesis promotes allergic inflammation. Immunity 39, 1158–1170 (2013).
Siracusa, M. C. et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477, 229–233 (2011).
Noti, M. et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J. Allergy Clin. Immunol. 133, 1390–1399 (2014).
Kim, B. S. et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170ra116 (2013).
Massacand, J. C. et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl Acad. Sci. USA 106, 13968–13973 (2009).
Taylor, B. C. et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206, 655–667 (2009).
Corren, J. et al. Tezepelumab in adults with uncontrolled asthma. N. Engl. J. Med. 377, 936–946 (2017).
Demehri, S. et al. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J. Clin. Invest. 126, 1458–1470 (2016).
Cunningham, T. J. et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J. Clin. Invest. 127, 106–116 (2017).
Kuan, E. L. & Ziegler, S. F. A tumor-myeloid cell axis, mediated via the cytokines IL-1α and TSLP, promotes the progression of breast cancer. Nat. Immunol. 19, 366–374 (2018).
Hayes, K. S. et al. Chronic Trichuris muris infection causes neoplastic change in the intestine and exacerbates tumour formation in APC min/+ mice. PLoS Negl. Trop. Dis. 11, e0005708 (2017).
von Moltke, J., Ji, M., Liang, H. E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature 529, 221–225 (2016). This study, along with Howitt et al. (2016) and Gerbe et al. (2016), establishes tuft cells as being important in type 2 immune response initiation.
Howitt, M. R. et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016).
Gerbe, F. et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016).
Kohanski, M. A. et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 142, 460–469 (2018).
Ikeda, K. et al. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood 101, 3594–3596 (2003).
Kang, C. M. et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am. J. Respir. Cell Mol. Biol. 33, 290–296 (2005).
Fort, M. M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Corrigan, C. J. et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J. Allergy Clin. Immunol. 128, 116–124 (2011).
Angkasekwinai, P. et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204, 1509–1517 (2007).
Owyang, A. M. et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849 (2006).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Vannella, K. M. et al. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci. Transl. Med. 8, 337ra365 (2016).
Connor, L. M. et al. Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles. J. Exp. Med. 214, 125–142 (2017).
Nakajima, S. et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J. Invest. Dermatol. 134, 2122–2130 (2014).
Wang, M. et al. Immunomodulatory effects of IL-23 and IL-17 in a mouse model of allergic rhinitis. Clin. Exp. Allergy 43, 956–966 (2013).
Terrazas, C. et al. IL-17A promotes susceptibility during experimental visceral leishmaniasis caused by Leishmania donovani. FASEB J. 30, 1135–1143 (2016).
Chen, F. et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 18, 260–266 (2012). This study demonstrates that the type 2 response mediates tissue repair, establishing tolerance as a host-protective mechanism against helminths. It further shows that an initial surge in IL-17 after lung invasion by helminths triggers neutrophil recruitment, contributing to acute lung injury.
Sutherland, T. E. et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat. Immunol. 15, 1116–1125 (2014). Chitinase-like proteins in the lung are shown to drive lung γδ T cell IL-17 production, and neutrophils are shown to mediate resistance after primary helminth infection.
Nascimento, M. S. et al. Interleukin 17A acts synergistically with interferon gamma to promote protection against Leishmania infantum infection. J. Infect. Dis. 211, 1015–1026 (2015).
Xu, S. et al. IL-17A-producing γδT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J. Immunol. 185, 5879–5887 (2010).
Molofsky, A. B., Savage, A. K. & Locksley, R. M. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 42, 1005–1019 (2015).
Chan, T. K., Tan, W. S. D., Peh, H. Y. & Wong, W. S. F. Aeroallergens induce reactive oxygen species production and DNA damage and dampen antioxidant responses in bronchial epithelial cells. J. Immunol. 199, 39–47 (2017).
Willart, M. A. et al. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J. Exp. Med. 209, 1505–1517 (2012).
Bleriot, C. et al. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 42, 145–158 (2015).
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011). This study demonstrates the requirement for ILC2s in the remodelling of airways and the restoration of lung function after viral infection.
Man, S. M., Karki, R. & Kanneganti, T. D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277, 61–75 (2017).
Zaiss, M. M. et al. IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog. 9, e1003531 (2013).
Alhallaf, R. et al. The NLRP3 inflammasome suppresses protective immunity to gastrointestinal helminth infection. Cell Rep. 23, 1085–1098 (2018).
Van Dyken, S. J. & Locksley, R. M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu. Rev. Immunol. 31, 317–343 (2013).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–1099 (2018). This study demonstrates that ILC2s from different tissues have distinct properties and functions.
Schneider, C. et al. A metabolite-triggered tuft cell–ILC2 circuit drives small intestinal remodeling. Cell 174, 271–284 (2018).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Klose, C. S. N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017).
Harris, N. L. & Loke, P. Recent advances in type-2-cell-mediated immunity: insights from helminth infection. Immunity 48, 396 (2018).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Gundra, U. M. et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123, e110–e122 (2014).
Gundra, U. M. et al. Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat. Immunol. 18, 642–653 (2017). This study shows that during inflammation, inflammatory monocyte-derived macrophages can differentiate into tissue-resident macrophages via a mechanism that requires vitamin A.
Kumamoto, Y. et al. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 39, 733–743 (2013). A specific subpopulation of dendritic cells is shown to drive type 2 responses.
Kumamoto, Y. et al. CD301b+ mononuclear phagocytes maintain positive energy balance through secretion of resistin-like molecule α. Immunity 45, 583–596 (2016).
Knudsen, N. H. & Lee, C. H. Identity crisis: CD301b+ mononuclear phagocytes blur the M1–M2 macrophage line. Immunity 45, 461–463 (2016).
Fahy, J. V. Type 2 inflammation in asthma-present in most, absent in many. Nat. Rev. Immunol. 15, 57–65 (2015).
Israel, E. & Reddel, H. K. Severe and difficult-to-treat asthma in adults. N. Engl. J. Med. 377, 965–976 (2017).
Allen, J. E., Sutherland, T. E. & Ruckerl, D. IL-17 and neutrophils: unexpected players in the type 2 immune response. Curr. Opin. Immunol. 34, 99–106 (2015).
Czarnowicki, T., He, H., Krueger, J. G. & Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 143, 1–11 (2019).
Serezani, A. P. M. et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J. Allergy Clin. Immunol. 139, 142–151 (2017).
Simpson, E. L. et al. Two phase III trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 375, 2335–2348 (2016).
O’Leary, C. E., Schneider, C. & Locksley, R. M. Tuft cells—systemically dispersed sensory epithelia integrating immune and neural circuitry. Annu. Rev. Immunol. 37, 47–72 (2019).
Schneider, C., O’Leary, C. E. & Locksley, R. M. Regulation of immune responses by tuft cells. Nat. Rev. Immunol. 19, 584–593 (2019).
Gowthaman, U. et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365, eaaw6433 (2019).
Tibbitt, C. A. et al. Single-cell RNA sequencing of the T helper cell response to house dust mites defines a distinct gene expression signature in airway Th2 cells. Immunity 51, 169–184 (2019).
Vieira Braga, F. A. et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 25, 1153–1163 (2019).
Hammad, H. & Lambrecht, B. N. Barrier epithelial cells and the control of type 2 immunity. Immunity 43, 29–40 (2015).
Bouchery, T., Le Gros, G. & Harris, N. ILC2s —trailblazers in the host response against intestinal helminths. Front. Immunol. 10, 623 (2019).
Grainger, J. R. & Grencis, R. K. Neutrophils worm their way into macrophage long-term memory. Nat. Immunol. 15, 902–904 (2014).
Monticelli, L. A. et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 17, 656–665 (2016).
Chen, F. et al. B cells produce the tissue-protective protein RELMα during helminth infection, which inhibits IL-17 expression and limits emphysema. Cell Rep. 25, 2775–2783 (2018).
Seehus, C. R. et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat. Commun. 8, 1900 (2017).
Chan, P. Y. et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science 352, 99–103 (2016).
Rivera, A., Siracusa, M. C., Yap, G. S. & Gause, W. C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 17, 356–363 (2016).
Espinosa, V. et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci. Immunol. 2, eaan5357 (2017).
Voehringer, D. Recent advances in understanding basophil functions in vivo. F1000Res 6, 1464 (2017).
Inclan-Rico, J. M. & Siracusa, M. C. First responders: innate immunity to helminths. Trends Parasitol. 34, 861–880 (2018).
Haber, A. L. et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017).
Biton, M. et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175, 1307–1320 (2018).
Cliffe, L. J. & Grencis, R. K. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 57, 255–307 (2004).
Campbell, S. M. et al. Myeloid cell recruitment versus local proliferation differentiates susceptibility from resistance to filarial infection. eLife 7, e30947 (2018).
Gueders, M. M. et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 58, 845–854 (2009).
Watanabe, H., Numata, K., Ito, T., Takagi, K. & Matsukawa, A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22, 460–466 (2004).
Brown, E. M., Kenny, D. J. & Xavier, R. J. Gut microbiota regulation of T cells during inflammation and autoimmunity. Annu. Rev. Immunol. 37, 599–624 (2019).
Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
Loke, P. & Cadwell, K. Getting a taste for parasites in the gut. Immunity 49, 16–18 (2018).
Nadjsombati, M. S. et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49, 33–41 (2018).
Rapin, A. & Harris, N. L. Helminth–bacterial interactions: cause and consequence. Trends Immunol. 39, 724–733 (2018).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352, 608–612 (2016).
Zaiss, M. M. et al. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity 43, 998–1010 (2015).
Su, C. et al. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 11, 144–157 (2018).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Leung, J. M. et al. Rapid environmental effects on gut nematode susceptibility in rewilded mice. PLoS Biol. 16, e2004108 (2018).
Rosshart, S. P. et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, eaaw4361 (2019).
Suntharalingam, G. et al. Cytokine storm in a phase I trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Lin, J.-D. et al. Rewilding Nod2 and Atg16l1 mutant mice uncovers genetic and environmenal contributions to microbial responses and immune cell composition. Cell Host Microbe https://doi.org/10.1016/j.chom.2020.03.001 (2020). This study shows that environmental changes have a greater effect on interindividual variation of immune cell numbers and composition, whereas genetic mutations have a greater effect on cytokine production in response to microbial stimulation.
Yeung, F. et al. Altered immunity of laboratory mice in the natural environment is associated with fungal colonization. Cell Host Microbe https://doi.org/10.1016/j.chom.2020.02.015 (2020).
McDonald, B. & McCoy, K. D. Maternal microbiota in pregnancy and early life. Science 365, 984–985 (2019).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra265 (2014).
Elliott, A. M. et al. Helminth infection during pregnancy and development of infantile eczema. JAMA 294, 2032–2034 (2005).
Cooper, P. J. et al. Effects of maternal geohelminth infections on allergy in early childhood. J. Allergy Clin. Immunol. 137, 899–906.e892 (2016).
Jakobsson, H. E. et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 63, 559–566 (2014).
Al Nabhani, Z. et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 50, 1276–1288.e1275 (2019).
Odegaard, J. I. & Chawla, A. Type 2 responses at the interface between immunity and fat metabolism. Curr. Opin. Immunol. 36, 67–72 (2015).
Acknowledgements
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH, and NIH grants AI131634-01A1, DK113790 and AI131634 to W.C.G.; NIH grants DK103788, HL084312, AI133977 and AI130945 and DOD grant W81XWH-16-1-0256 to P.L.; and NIH grants AI089824 and CA212376 to C.R., who is a Howard Hughes Medical Institute Faculty Scholar. The authors apologize to those authors whose exemplary work they were unable to cite owing to space limitations.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Alternative activation
-
Historically, macrophages activated in vitro have been described as ‘M1 or classically activated’ or ‘M2 or alternatively activated’, depending on whether they have been activated with IFNγ and lipopolysaccharide or with IL-4 or IL-10, respectively. However, in vivo macrophages are highly specialized, dynamic and heterogeneous with regard to their lineages, phenotypes and functions, and their activation is continuously shaped by multiple matrix and cellular signals in the tissue microenvironment. Therefore, the classical (M1) versus alternatively activated (M2) classification and terminology is useful but overly simplistic, with each broadly different phenotype actually showing considerable heterogeneity.
- Charcot–Leyden crystals
-
Slender birefringent crystals composed of self-aggregating galectin-10 that is produced by human eosinophils and found in tissues associated with eosinophilic inflammation.
- Frustrated phagocytosis
-
Situation in which a myeloid cell attempts to phagocytose something, most often opsonized, that it cannot internalize, often resulting in the release of lysosomal contents, and in some cases cell death.
- Tuft cells
-
Chemosensory epithelial cells lining the intestinal wall that have tufts or brush-like microvilli on their surface. They can increase greatly following parasitic infections and are the major producer of IL-25 in the intestine, playing an essential role in driving group 2 innate lymphoid cell (ILC2) activation. They are also present in the respiratory epithelium, where they are called brush cells.
Rights and permissions
About this article
Cite this article
Gause, W.C., Rothlin, C. & Loke, P. Heterogeneity in the initiation, development and function of type 2 immunity. Nat Rev Immunol 20, 603–614 (2020). https://doi.org/10.1038/s41577-020-0301-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-0301-x
This article is cited by
-
Type I/II Immune Balance Contributes to the Protective Effect of AIF-1 on Hepatic Immunopathology Induced by Schistosoma japonicum in a Transgenic Mouse Model
Inflammation (2024)
-
TCF1–LEF1 co-expression identifies a multipotent progenitor cell (TH2-MPP) across human allergic diseases
Nature Immunology (2024)
-
The neuroimmune axis of Alzheimer’s disease
Genome Medicine (2023)
-
Graphene oxide elicits microbiome-dependent type 2 immune responses via the aryl hydrocarbon receptor
Nature Nanotechnology (2023)
-
HTR2A agonists play a therapeutic role by restricting ILC2 activation in papain-induced lung inflammation
Cellular & Molecular Immunology (2023)