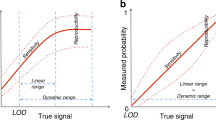
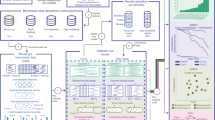
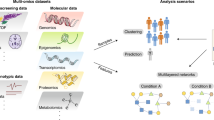
Abstract
Technological advances enabling massively parallel measurement of biological features — such as microarrays, high-throughput sequencing and mass spectrometry — have ushered in the omics era, now in its third decade. The resulting complex landscape of analytical methods has naturally fostered the growth of an omics benchmarking industry. Benchmarking refers to the process of objectively comparing and evaluating the performance of different computational or analytical techniques when processing and analysing large-scale biological data sets, such as transcriptomics, proteomics and metabolomics. With thousands of omics benchmarking studies published over the past 25 years, the field has matured to the point where the foundations of benchmarking have been established and well described. However, generating meaningful benchmarking data and properly evaluating performance in this complex domain remains challenging. In this Review, we highlight some common oversights and pitfalls in omics benchmarking. We also establish a methodology to bring the issues that can be addressed into focus and to be transparent about those that cannot: this takes the form of a spreadsheet template of guidelines for comprehensive reporting, intended to accompany publications. In addition, a survey of recent developments in benchmarking is provided as well as specific guidance for commonly encountered difficulties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Weber, L. M. et al. Essential guidelines for computational method benchmarking. Genome Biol. 20, 125 (2019). This landmark paper describes the fundamental tenets of omics benchmarking in biology, for those intending to perform benchmarking studies or to study the literature in search of guidance.
Mangul, S. et al. Systematic benchmarking of omics computational tools. Nat. Commun. 10, 1393 (2019). This landmark paper describes the fundamentals of benchmarking, with a focus on the big picture rather than the particulars of data generation.
Aniba, M. R., Poch, O. & Thompson, J. D. Issues in bioinformatics benchmarking: the case study of multiple sequence alignment. Nucleic Acids Res. 38, 7353–7363 (2010).
Mathews, D. H. How to benchmark RNA secondary structure prediction accuracy. Methods 162–163, 60–67 (2019).
Bokulich, N. A., Ziemski, M., Robeson, M. S. & Kaehler, B. D. Measuring the microbiome: best practices for developing and benchmarking microbiomics methods. Comput. Struct. Biotechnol. J. 18, 4048–4062 (2020).
Meyer, F. et al. Tutorial: assessing metagenomics software with the CAMI benchmarking toolkit. Nat. Protoc. 16, 1785–1801 (2021).
Olson, N. D. et al. Variant calling and benchmarking in an era of complete human genome sequences. Nat. Rev. Genet. 24, 464–483 (2023).
Crowell, H. L., Morillo Leonardo, S. X., Soneson, C. & Robinson, M. D. The shaky foundations of simulating single-cell RNA sequencing data. Genome Biol. 24, 62 (2023).
Escalona, M., Rocha, S. & Posada, D. A comparison of tools for the simulation of genomic next-generation sequencing data. Nat. Rev. Genet. 17, 459–469 (2016).
Milhaven, M. & Pfeifer, S. P. Performance evaluation of six popular short-read simulators. Heredity 130, 55–63 (2023).
Shakola, F., Palejev, D. & Ivanov, I. A framework for comparison and assessment of synthetic RNA-seq data. Genes 13, 2362 (2022).
Kimes, P. K. & Reyes, A. Reproducible and replicable comparisons using SummarizedBenchmark. Bioinformatics 35, 137–139 (2018).
Germain, P.-L., Sonrel, A. & Robinson, M. D. pipeComp, a general framework for the evaluation of computational pipelines, reveals performant single cell RNA-seq preprocessing tools. Genome Biol. 21, 227 (2020).
Stephens, M. DSC: dynamic statistical comparisons. GitHub https://stephenslab.github.io/dsc-wiki/overview.html (2023).
Robinson, M. Omnibenchmark: open and continuous community benchmarking. Omnibenchmark https://omnibenchmark.org (2023).
Capella-Gutierrez, S. et al. Lessons learned: recommendations for establishing critical periodic scientific benchmarking. Preprint at bioRxiv https://doi.org/10.1101/181677 (2017).
de Pico, E. M., Gelpi, J. L. & Capella-Gutiérrez, S. FAIRsoft — a practical implementation of FAIR principles for research software. Preprint at bioRxiv https://doi.org/10.1101/2022.05.04.490563 (2022).
Nakato, R. & Sakata, T. Methods for ChIP-seq analysis: a practical workflow and advanced applications. Methods 187, 44–53 (2021).
Li, Y., Ge, X., Peng, F., Li, W. & Li, J. J. Exaggerated false positives by popular differential expression methods when analyzing human population samples. Genome Biol. 23, 79 (2022).
Ison, J. et al. Tools and data services registry: a community effort to document bioinformatics resources. Nucleic Acids Res. 44, D38–D47 (2015).
Wikipedia. List of bioinformatics software. Wikipedia https://en.wikipedia.org/wiki/List_of_bioinformatics_software (2022).
Zappia, L. & Theis, F. J. Over 1000 tools reveal trends in the single-cell RNA-seq analysis landscape. Genome Biol. 22, 301 (2021).
Koch, F. C., Sutton, G. J., Voineagu, I. & Vafaee, F. Supervised application of internal validation measures to benchmark dimensionality reduction methods in scRNA-seq data. Brief. Bioinform. 22, bbab304 (2021).
Norel, R., Rice, J. J. & Stolovitzky, G. The self-assessment trap: can we all be better than average? Mol. Syst. Biol. 7, 537 (2011). This paper reviews the reported performances of new methods and calls for increased use of multiple evaluation metrics and publication of novel methods even when they do not improve performance above prior works.
Buchka, S., Hapfelmeier, A., Gardner, P. P., Wilson, R. & Boulesteix, A. L. On the optimistic performance evaluation of newly introduced bioinformatic methods. Genome Biol. 22, 152 (2021). This review compares the initial performance claims of published methods to later benchmarking of the same methods, highlighting the need for independent benchmarking.
Germain, P.-L. et al. RNAontheBENCH: computational and empirical resources for benchmarking RNAseq quantification and differential expression methods. Nucleic Acids Res. 44, 5054–5067 (2016).
Holik, A. Z. et al. RNA-seq mixology: designing realistic control experiments to compare protocols and analysis methods. Nucleic Acids Res. 45, e30 (2017). This paper demonstrates the importance of including both technical and biological variation in benchmark data, as well as one approach for including realistic biological variation when evaluating RNA-seq.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Sandve, G. K. & Greiff, V. Access to ground truth at unconstrained size makes simulated data as indispensable as experimental data for bioinformatics methods development and benchmarking. Bioinformatics 38, 4994–4996 (2022). This paper argues for nearly always including simulated data in methods evaluation in order to go beyond the limitations of experimental data with regard to factors such as sample size, knowledge of ground truth and explicit presentation of assumptions.
Maza, E., Frasse, P., Senin, P., Bouzayen, M. & Zouine, M. Comparison of normalization methods for differential gene expression analysis in RNA-seq experiments: a matter of relative size of studied transcriptomes. Commun. Integr. Biol. 6, e25849 (2013).
Szalkowski, A. M. & Schmid, C. D. Rapid innovation in ChIP-seq peak-calling algorithms is outdistancing benchmarking efforts. Brief. Bioinform. 12, 626–633 (2011).
Jelizarow, M., Guillemot, V., Tenenhaus, A., Strimmer, K. & Boulesteix, A. L. Over-optimism in bioinformatics: an illustration. Bioinformatics 26, 1990–1998 (2010). This paper emphasizes the importance of evaluating methods on ‘fresh’ validation data sets that were not used for tuning the method under evaluation.
Szikszai, M., Wise, M., Datta, A., Ward, M. & Mathews, D. H. Deep learning models for RNA secondary structure prediction (probably) do not generalize across families. Bioinformatics 38, 3892–3899 (2022).
Mehta, T., Tanik, M. & Allison, D. B. Towards sound epistemological foundations of statistical methods for high-dimensional biology. Nat. Genet. 36, 943–947 (2004).
Lin, M. H. et al. Benchmarking differential expression, imputation and quantification methods for proteomics data. Brief. Bioinform. 23, bbac138 (2022).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Lahens, N. F. et al. CAMPAREE: a robust and configurable RNA expression simulator. BMC Genomics 22, 692 (2021).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Korthauer, K. et al. A practical guide to methods controlling false discoveries in computational biology. Genome Biol. 20, 118 (2019).
Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188 (2001).
Burton, A., Altman, D. G., Royston, P. & Holder, R. L. The design of simulation studies in medical statistics. Stat. Med. 25, 4279–4292 (2006).
Madsen, L. & Birkes, D. Simulating dependent discrete data. J. Stat. Comput. Simul. 83, 677–691 (2013).
Soneson, C. & Robinson, M. D. Towards unified quality verification of synthetic count data with countsimQC. Bioinformatics 34, 691–692 (2017).
Cao, Y., Yang, P. & Yang, J. Y. H. A benchmark study of simulation methods for single-cell RNA sequencing data. Nat. Commun. 12, 6911 (2021). A benchmark of 12 single-cell RNA-seq simulation methods, including an exhaustive evaluation of simulation quality by comparison to real data sets.
Warton, D. I. & Hui, F. K. C. The central role of mean–variance relationships in the analysis of multivariate abundance data: a response to Roberts (2017). Methods Ecol. Evol. 8, 1408–1414 (2017).
Baruzzo, G. et al. Simulation-based comprehensive benchmarking of RNA-seq aligners. Nat. Methods 14, 135–139 (2017).
Yang, L. & Shami, A. On hyperparameter optimization of machine learning algorithms: theory and practice. Neurocomputing 415, 295–316 (2020). This paper reviews the common techniques used for parameter optimization in machine learning, some of which can be used in omics benchmarking for optimizing parameters of the assessed tools.
Bischl, B. et al. Hyperparameter optimization: foundations, algorithms, best practices, and open challenges. WIREs Data Min. Knowl. Discov. 13, e1484 (2023).
Lessmann, S., Stahlbock, R. & Crone, S. F. in Proc. Int. Conf. Artificial Intelligence 74–82 (ICAI, 2005).
Lorenzo, P. R., Nalepa, J., Kawulok, M., Ramos, L. S. & Pastor, J. R. in Proc. Genetic Evolutionary Computation Conf. 481–488 (ACM, 2017).
Eggensperger, K., Hutter, F., Hoos, H. & Leyton-Brown, K. in Proc. AAAI Conf. Artificial Intelligence (AAAI, 2015).
Anscombe, F. J. Graphs in statistical analysis. Am. Stat. 27, 17–21 (1973). This classic paper shows, with a now well-known example, the shortcomings of summary statistics such as mean and correlation.
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
Chen, X. & Sarkar, S. K. On Benjamini–Hochberg procedure applied to mid p-values. J. Stat. Plan. Infer. 205, 34–45 (2020).
Lyu, P., Li, Y., Wen, X. & Cao, H. JUMP: replicability analysis of high-throughput experiments with applications to spatial transcriptomic studies. Bioinformatics 39, btad366 (2023).
Soneson, C. & Robinson, M. D. iCOBRA: open, reproducible, standardized and live method benchmarking. Nat. Methods 13, 283 (2016). A widely useful library for benchmarking that performs comparisons of methods that produce ranked lists of features, particularly P values but also numerical rankings.
Sing, T., Sander, O., Beerenwinkel, N. & Lengauer, T. ROCR: visualizing classifier performance in R. Bioinformatics 21, 3940–3941 (2005).
Breheny, P., Stromberg, A. & Lambert, J. p-value histograms: inference and diagnostics. High Throughput 7, 23 (2018).
VanderWeele, T. J. & Mathur, M. B. Some desirable properties of the Bonferroni correction: is the Bonferroni correction really so bad. Am. J. Epidemiol. 188, 617–618 (2019).
Bayarri, M. J. & Berger, J. O. P values for composite null models. J. Am. Stat. Assoc. 95, 1127–1142 (2000).
Tardaguila, M. et al. SQANTI: extensive characterization of long-read transcript sequences for quality control in full-length transcriptome identification and quantification. Genome Res. 28, 396–411 (2018).
Rye, M. B., Sætrom, P. & Drabløs, F. A manually curated ChIP-seq benchmark demonstrates room for improvement in current peak-finder programs. Nucleic Acids Res. 39, e25 (2010).
Wilbanks, E. G. & Facciotti, M. T. Evaluation of algorithm performance in ChIP-seq peak detection. PLoS ONE 5, e11471 (2010).
Thomas, R., Thomas, S., Holloway, A. K. & Pollard, K. S. Features that define the best ChIP-seq peak calling algorithms. Brief. Bioinform. 18, 441–450 (2016).
de Boer, B. A. et al. OccuPeak: ChIP-seq peak calling based on internal background modelling. PLoS ONE 9, e99844 (2014).
Johnson, D. S., Mortazavi, A., Myers, R. M. & Wold, B. Genome-wide mapping of in vivo protein–DNA interactions. Science 316, 1497–1502 (2007).
Laajala, T. D. et al. A practical comparison of methods for detecting transcription factor binding sites in ChIP-seq experiments. BMC Genomics 10, 618 (2009).
Li, Q., Brown, J. B., Huang, H. & Bickel, P. J. Measuring reproducibility of high-throughput experiments. Ann. Appl. Stat. 5, 1752–1779 (2011). This paper defines the widely used irreproducible discovery rate, which measures the consistency of rankings of features to evaluate consistency across independent biological samples.
Nakato, R. & Shirahige, K. Recent advances in ChIP-seq analysis: from quality management to whole-genome annotation. Brief. Bioinform. 18, 279–290 (2016).
Huang, M. et al. SAVER: gene expression recovery for single-cell RNA sequencing. Nat. Methods 15, 539–542 (2018).
Laloum, D. & Robinson-Rechavi, M. Methods detecting rhythmic gene expression are biologically relevant only for strong signal. PLoS Comput. Biol. 16, e1007666 (2020).
Kryuchkova-Mostacci, N. & Robinson-Rechavi, M. A benchmark of gene expression tissue-specificity metrics. Brief. Bioinform. 18, 205–214 (2016).
Manni, M., Berkeley, M. R., Seppey, M. & Zdobnov, E. M. BUSCO: assessing genomic data quality and beyond. Curr. Protoc. 1, e323 (2021).
Hayer, K. E., Pizarro, A., Lahens, N. F., Hogenesch, J. B. & Grant, G. R. Benchmark analysis of algorithms for determining and quantifying full-length mRNA splice forms from RNA-seq data. Bioinformatics 31, 3938–3945 (2015).
Sonrel, A. et al. Meta-analysis of (single-cell method) benchmarks reveals the need for extensibility and interoperability. Genome Biol. 24, 119 (2023). This paper extensively reviews recent single-cell analysis method benchmarking papers and quantifies the need for documented, reproducible and extensible benchmarking.
Kryshtafovych, A., Schwede, T., Topf, M., Fidelis, K. & Moult, J. Critical assessment of methods of protein structure prediction (CASP) — round XIV. Proteins 89, 1607–1617 (2021). An important example of competition-style benchmarking, in which regularly scheduled independent, blind assessment of protein structure prediction methods is performed using novel, experimentally determined proteins as reference.
Merkel, D. Docker: lightweight Linux containers for consistent development and deployment. Linux J. 2014, 2 (2014).
Kadri, S., Sboner, A., Sigaras, A. & Roy, S. Containers in bioinformatics: applications, practical considerations, and best practices in molecular pathology. J. Mol. Diagn. 24, 442–454 (2022).
Audoux, J. et al. SimBA: a methodology and tools for evaluating the performance of RNA-seq bioinformatic pipelines. BMC Bioinformatics 18, 428 (2017).
Bansal, S. & Parmar, S. Decay of URLs citation: a case study of current science. Libr. Philos. Pract. https://digitalcommons.unl.edu/libphilprac/3582 (2020).
Edgar, R., Domrachev, M. & Lash, A. E. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 41, D991–D995 (2013).
Altenhoff, A. M. et al. The Quest for Orthologs benchmark service and consensus calls in 2020. Nucleic Acids Res. 48, W538–W545 (2020).
Conte, A. D. et al. Critical assessment of protein intrinsic disorder prediction (CAID) — results of round 2. Proteins 91, 1925–1934 (2023).
Bryce-Smith, S. et al. Extensible benchmarking of methods that identify and quantify polyadenylation sites from RNA-seq data. RNA 29, 1839–1855 (2023).
Nevers, Y. et al. The Quest for Orthologs orthology benchmark service in 2022. Nucleic Acids Res. 50, W623–W632 (2022).
Pratapa, A., Jalihal, A. P., Law, J. N., Bharadwaj, A. & Murali, T. M. Benchmarking algorithms for gene regulatory network inference from single-cell transcriptomic data. Nat. Methods 17, 147–154 (2020).
Seppey, M., Manni, M. & Zdobnov, E. M. LEMMI: a continuous benchmarking platform for metagenomics classifiers. Genome Res. 30, 1208–1216 (2020).
Kuznetsov, D. et al. OrthoDB v11: annotation of orthologs in the widest sampling of organismal diversity. Nucleic Acids Res. 51, D445–D451 (2022).
Perscheid, C. Comprior: facilitating the implementation and automated benchmarking of prior knowledge-based feature selection approaches on gene expression data sets. BMC Bioinformatics 22, 401 (2021).
Soneson, C. compcodeR — an R package for benchmarking differential expression methods for RNA-seq data. Bioinformatics 30, 2517–2518 (2014).
Teng, M. et al. A benchmark for RNA-seq quantification pipelines. Genome Biol. 17, 74 (2016).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019).
Smolander, J., Junttila, S. & Elo, L. L. Cell-connectivity-guided trajectory inference from single-cell data. Bioinformatics 39, btad515 (2023).
Wang, C. X., Zhang, L. & Wang, B. One cell at a time (OCAT): a unified framework to integrate and analyze single-cell RNA-seq data. Genome Biol. 23, 102 (2022).
Van den Berge, K. et al. Trajectory-based differential expression analysis for single-cell sequencing data. Nat. Commun. 11, 1201 (2020).
Li, R. & Quon, G. scBFA: modeling detection patterns to mitigate technical noise in large-scale single-cell genomics data. Genome Biol. 20, 193 (2019).
Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2018).
Choudhary, S. & Satija, R. Comparison and evaluation of statistical error models for scRNA-seq. Genome Biol. 23, 27 (2022).
Spies, D., Renz, P. F., Beyer, T. A. & Ciaudo, C. Comparative analysis of differential gene expression tools for RNA sequencing time course data. Brief. Bioinform. 20, 288–298 (2017).
Zhu, A., Srivastava, A., Ibrahim, J. G., Patro, R. & Love, M. I. Nonparametric expression analysis using inferential replicate counts. Nucleic Acids Res. 47, e105 (2019).
Gilis, J., Vitting-Seerup, K., Van den Berge, K. & Clement, L. satuRn: scalable analysis of differential transcript usage for bulk and single-cell RNA-sequencing applications. F1000Res 10, 374 (2021).
Wu, E. Y. et al. SEESAW: detecting isoform-level allelic imbalance accounting for inferential uncertainty. Genome Biol. 24, 165 (2023).
He, Z., Pan, Y., Shao, F. & Wang, H. Identifying differentially expressed genes of zero inflated single cell RNA sequencing data using mixed model score tests. Front. Genet. 12, 616686 (2021).
Li, Y., Mansmann, U., Du, S. & Hornung, R. Benchmark study of feature selection strategies for multi-omics data. BMC Bioinformatics 23, 412 (2022).
Herrmann, M., Probst, P., Hornung, R., Jurinovic, V. & Boulesteix, A.-L. Large-scale benchmark study of survival prediction methods using multi-omics data. Brief. Bioinform. 22, bbaa167 (2020).
Leng, D. et al. A benchmark study of deep learning-based multi-omics data fusion methods for cancer. Genome Biol. 23, 171 (2022).
Cantini, L. et al. Benchmarking joint multi-omics dimensionality reduction approaches for the study of cancer. Nat. Commun. 12, 124 (2021).
Pierre-Jean, M., Deleuze, J.-F., Le Floch, E. & Mauger, F. Clustering and variable selection evaluation of 13 unsupervised methods for multi-omics data integration. Brief. Bioinform. 21, 2011–2030 (2020).
Rappoport, N. & Shamir, R. Multi-omic and multi-view clustering algorithms: review and cancer benchmark. Nucleic Acids Res. 46, 10546–10562 (2018).
Fawcett, T. An introduction to ROC analysis. Pattern Recogn. Lett. 27, 861–874 (2006). This paper provides advice on applying the widely used receiver operating characteristic (ROC) curve, including pitfalls in interpretation when using the ROC to compare method performance.
Matthews, B. W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim. Biophys. Acta 405, 442–451 (1975).
Cramér, H. Mathematical Methods of Statistics 282 (Princeton Univ. Press, 1946).
Rand, W. M. Objective criteria for the evaluation of clustering methods. J. Am. Stat. Assoc. 66, 846–850 (1971).
Fowlkes, E. B. & Mallows, C. L. A method for comparing two hierarchical clusterings. J. Am. Stat. Assoc. 78, 553–569 (1983).
Ekstrom, C. T., Gerds, T. A. & Jensen, A. K. Sequential rank agreement methods for comparison of ranked lists. Biostatistics 20, 582–598 (2019).
Fijorek, K., Fijorek, D., Wisniowska, B. & Polak, S. BDTcomparator: a program for comparing binary classifiers. Bioinformatics 27, 3439–3440 (2011).
Knight, C. H. et al. IBRAP: integrated benchmarking single-cell RNA-sequencing analytical pipeline. Brief. Bioinform. 24, bbad061 (2023).
Tantasatityanon, P. & Wichadakul, D. in Proc. 15th Int. Conf. Computer Modeling Simulation 84–91 (ACM, 2023).
Sang-aram, C., Browaeys, R., Seurinck, R. & Saeys, Y. Spotless: a reproducible pipeline for benchmarking cell type deconvolution in spatial transcriptomics. eLife 12, RP88431 (2023).
Virshup, I. et al. The scverse project provides a computational ecosystem for single-cell omics data analysis. Nat. Biotechnol. 41, 604–606 (2023).
Huber, W. et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121 (2015).
Amezquita, R. A. et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 17, 137–145 (2020).
Lähnemann, D. et al. Eleven grand challenges in single-cell data science. Genome Biol. 21, 31 (2020).
Jiang, R., Sun, T., Song, D. & Li, J. J. Statistics or biology: the zero-inflation controversy about scRNA-seq data. Genome Biol. 23, 31 (2022).
Silverman, J. D., Roche, K., Mukherjee, S. & David, L. A. Naught all zeros in sequence count data are the same. Comput. Struct. Biotechnol. J. 18, 2789–2798 (2020).
Gatto, L. et al. Initial recommendations for performing, benchmarking and reporting single-cell proteomics experiments. Nat. Methods 20, 375–386 (2023).
Valecha, M. & Posada, D. Somatic variant calling from single-cell DNA sequencing data. Comput. Struct. Biotechnol. J. 20, 2978–2985 (2022).
Baker, E. A. G., Schapiro, D., Dumitrascu, B., Vickovic, S. & Regev, A. In silico tissue generation and power analysis for spatial omics. Nat. Methods 20, 424–431 (2023).
Zappia, L., Phipson, B. & Oshlack, A. Splatter: simulation of single-cell RNA sequencing data. Genome Biol. 18, 174 (2017).
Li, B. et al. Benchmarking spatial and single-cell transcriptomics integration methods for transcript distribution prediction and cell type deconvolution. Nat. Methods 19, 662–670 (2022).
Raimundo, F., Prompsy, P., Vert, J.-P. & Vallot, C. A benchmark of computational pipelines for single-cell histone modification data. Genome Biol. 24, 143 (2023).
Yuan, H. & Kelley, D. R. scBasset: sequence-based modeling of single-cell ATAC-seq using convolutional neural networks. Nat. Methods 19, 1088–1096 (2022).
Ashuach, T., Reidenbach, D. A., Gayoso, A. & Yosef, N. PeakVI: a deep generative model for single-cell chromatin accessibility analysis. Cell Rep. Methods 2, 100182 (2022).
Liu, Z., Sun, D. & Wang, C. Evaluation of cell–cell interaction methods by integrating single-cell RNA sequencing data with spatial information. Genome Biol. 23, 218 (2022).
Long, B., Miller, J. & the SpaceTx Consortium. SpaceTx: a roadmap for benchmarking spatial transcriptomics exploration of the brain. Preprint at arXiv https://doi.org/10.48550/arXiv.2301.08436 (2023).
Zhang, Y. et al. Reference-based cell type matching of in situ image-based spatial transcriptomics data on primary visual cortex of mouse brain. Sci. Rep. 13, 9567 (2023).
Bullard, J. H., Purdom, E., Hansen, K. D. & Dudoit, S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics 11, 94 (2010).
Yoshihara, K. et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 34, 4845–4854 (2015).
Lataretu, M. & Hölzer, M. RNAflow: an effective and simple RNA-seq differential gene expression pipeline using Nextflow. Genes 11, 1487 (2020).
Tarazona, S., Garcia-Alcalde, F., Dopazo, J., Ferrer, A. & Conesa, A. Differential expression in RNA-seq: a matter of depth. Genome Res. 21, 2213–2223 (2011).
Costa-Silva, J., Domingues, D. & Lopes, F. M. RNA-seq differential expression analysis: an extended review and a software tool. PLoS ONE 12, e0190152 (2017).
Yang, E. W., Girke, T. & Jiang, T. Differential gene expression analysis using coexpression and RNA-seq data. Bioinformatics 29, 2153–2161 (2013).
Zhang, Z. H. et al. A comparative study of techniques for differential expression analysis on RNA-seq data. PLoS ONE 9, e103207 (2014).
Rajkumar, A. P. et al. Experimental validation of methods for differential gene expression analysis and sample pooling in RNA-seq. BMC Genomics 16, 548 (2015).
Das, A., Das, D. & Panda, A. C. Validation of circular RNAs by PCR. Methods Mol. Biol. 2392, 103–114 (2022).
Rai, M. F., Tycksen, E. D., Sandell, L. J. & Brophy, R. H. Advantages of RNA-seq compared to RNA microarrays for transcriptome profiling of anterior cruciate ligament tears. J. Orthop. Res. 36, 484–497 (2018).
Beck, T. F., Mullikin, J. C., Program, N. C. S. & Biesecker, L. G. Systematic evaluation of Sanger validation of next-generation sequencing variants. Clin. Chem. 62, 647–654 (2016).
Zheng, J. et al. A comprehensive assessment of next-generation sequencing variants validation using a secondary technology. Mol. Genet. Genom. Med. 7, e00748 (2019).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Griebel, T. et al. Modelling and simulating generic RNA-seq experiments with the flux simulator. Nucleic Acids Res. 40, 10073–10083 (2012).
Frazee, A. C., Jaffe, A. E., Langmead, B. & Leek, J. T. Polyester: simulating RNA-seq datasets with differential transcript expression. Bioinformatics 31, 2778–2784 (2015).
Franklin, J. M., Schneeweiss, S., Polinski, J. M. & Rassen, J. A. Plasmode simulation for the evaluation of pharmacoepidemiologic methods in complex healthcare databases. Comput. Stat. Data Anal. 72, 219–226 (2014).
Acknowledgements
The authors thank the reviewers and editors for their helpful comments and suggestions. This work was funded by the National Center for Advancing Translational Sciences (grant 5UL1TR000003).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Yvan Saeys and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Open Problems in Single-Cell Analysis: https://openproblems.bio
SpaceTx: https://spacetx.github.io
Updates and domain-specific templates: https://github.com/itmat/OmicsBenchmarkReport
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brooks, T.G., Lahens, N.F., Mrčela, A. et al. Challenges and best practices in omics benchmarking. Nat Rev Genet 25, 326–339 (2024). https://doi.org/10.1038/s41576-023-00679-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-023-00679-6